Published online Apr 6, 2025. doi: 10.12998/wjcc.v13.i10.101647
Revised: November 19, 2024
Accepted: December 2, 2024
Published online: April 6, 2025
Processing time: 84 Days and 9.1 Hours
Nitric oxide (NO) is a gaseous molecule produced by 3 different NO synthase (NOS) isoforms: Neural/brain NOS (nNOS/bNOS, type 1), endothelial NOS (eNOS, type 3) and inducible NOS (type 2). Type 1 and 3 NOS are constitutively expressed. NO can serve different purposes: As a vasoactive molecule, as a neurotransmitter or as an immunomodulator. It plays a key role in cerebral ischemia/reperfusion injury (CIRI). Hypoxic episodes simulate the production of oxygen free radicals, leading to mitochondrial and phospholipid damage. Upon reperfusion, increased levels of oxygen trigger oxide synthases; whose products are associated with neuronal damage by promoting lipid peroxidation, nitrosylation and excitotoxicity. Molecular pathways in CIRI can be altered by NOS. Neuroprotective effects are observed with eNOS activity. While nNOS interplay is prone to endothelial inflammation, oxidative stress and apoptosis. Therefore, nNOS appears to be detrimental. The interaction between NO and other free radicals develops peroxynitrite; which is a cytotoxic agent. It plays a main role in the likelihood of hemorrhagic events by tissue plasminogen activator (t-PA). Peroxynitrite scavengers are currently being studied as potential targets to prevent hemorrhagic transformation in CIRI.
Core Tip: Nitric oxide (NO) plays a key role in cerebral ischemia/reperfusion injury (CIRI). Ischemic episodes lead to mitochondrial and phospholipid damage. While reperfusion is associated with neuronal damage by promoting lipid peroxidation, nitrosylation and excitotoxicity. Neural NO synthase (nNOS) interplay is prone to endothelial inflammation, oxidative stress and apoptosis. Therefore, nNOS appears to be detrimental. The interaction between NO and other free radicals develops peroxynitrite. And, limiting the negative effects of NO-derived compounds has been implemented as an important strategy to improve neurological outcomes after CIRI.
- Citation: Anaya-Prado R, Canseco-Villegas AI, Anaya-Fernández R, Anaya-Fernandez MM, Guerrero-Palomera MA, Guerrero-Palomera C, Garcia-Ramirez IF, Gonzalez-Martinez D, Azcona-Ramírez CC, Garcia-Perez C, Lizarraga-Valencia AL, Hernandez-Zepeda A, Palomares-Covarrubias JF, Blackaller-Medina JH, Soto-Hintze J, Velarde-Castillo MC, Cruz-Melendrez DA. Role of nitric oxide in cerebral ischemia/reperfusion injury: A biomolecular overview. World J Clin Cases 2025; 13(10): 101647
- URL: https://www.wjgnet.com/2307-8960/full/v13/i10/101647.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i10.101647
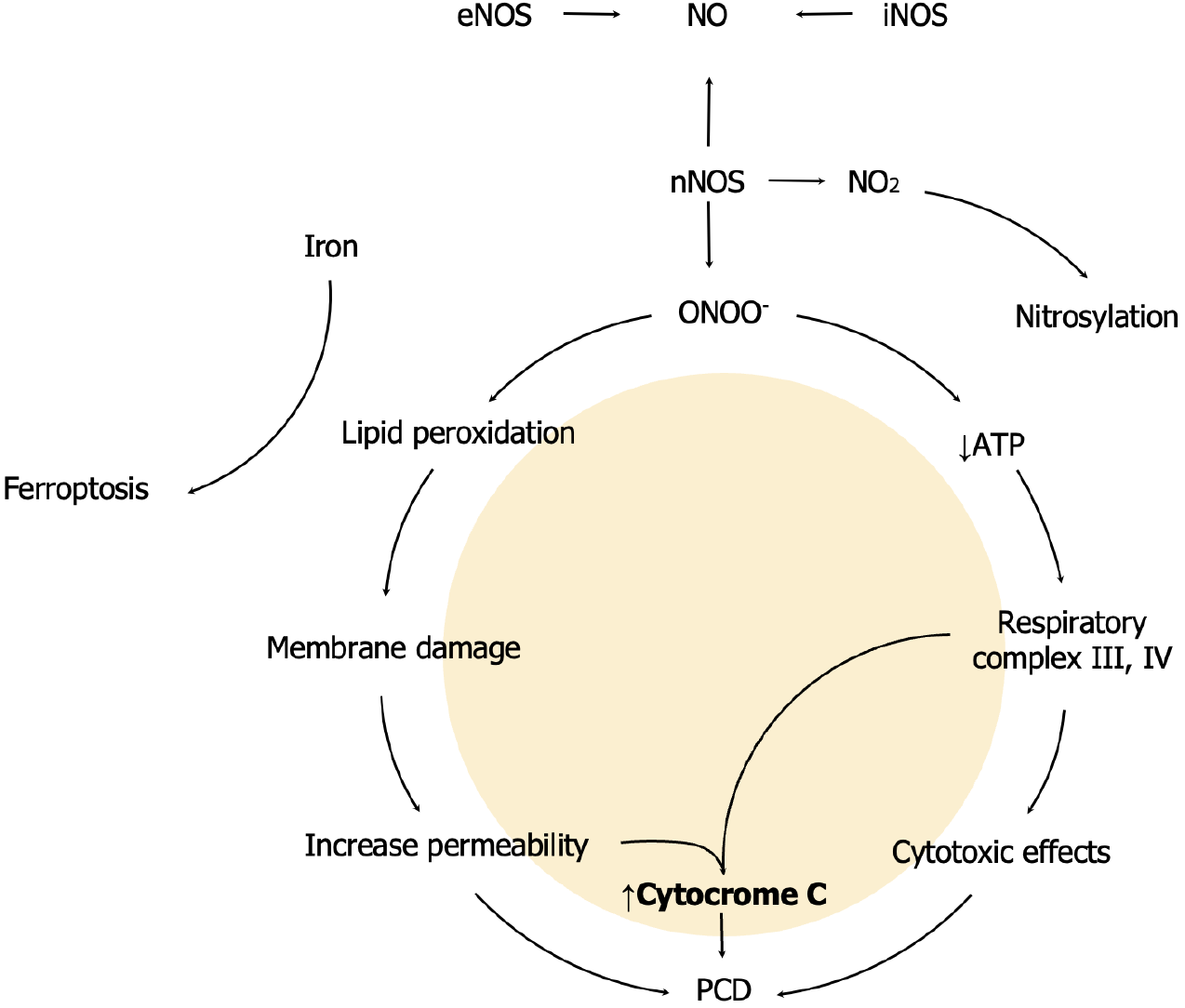
Tissue ischemia/reperfusion triggers a biomolecular response that involves reactive oxygen species. In physiological conditions, antioxidant mechanisms are sufficient to protect the cells from free radicals[1,2]. However, under oxidative stress, reactive nitrogen species (RNS) are one of the primary effectors of cerebral ischemia/reperfusion injury (CIRI), by triggering calcium-dependent nitric oxide synthases (NOS). Nitric oxide (NO) is a gaseous molecule produced by 3 different NOS isoforms: Neural/brain NOS (nNOS/bNOS, type 1), endothelial NOS (eNOS, type 3) and inducible NOS (iNOS, type 2). Type 1 and 3 NOS are constitutively expressed[3]. nNOS produces different types of RNS; such as nitrogen dioxide (NO2) and peroxynitrite (ONOO-)[4]. Specifically, this ion is highly reactive with biological molecules in the respiratory complex III and IV. These reactions result in cytotoxic effects due to a significant reduction in ATP levels. Therefore, altered resting membrane potential and osmotic moves take place. The presence of these enzymes is associated with three major events: Mitochondrial membrane damage, mitochondrial membrane permeability and protein nitrosylation[5,6].
Membrane damage is mediated by free radicals upon linoleic and arachidonic acid, a process called lipid peroxidation. As a result, there is an increased mitochondrial membrane permeability. Therefore, free radical outflow from the mitochondria into the cytoplasm ensues[7]. Excessive oxidative conditions can overcome glutathione, nicotinamide adenine dinucleotide phosphate and lysosomal iron sequestration, which are protective mechanisms against oxytosis/ferroptosis. Ferroptosis is a type of programmed cell death (PCD) mediated by iron lipid peroxidation (Figure 1). This PCD is genetically and biochemically different from other types of apoptosis[8]. NO is incorporated to another molecule through a chemical process called Nitrosylation. This usually occurs when an organic molecule “R” receives a NO molecule through a covalent bond: “R - N = O”. This reaction gives rise to nitroso compounds. And there are multiple nitroso compounds. However, the most common interaction is between sulfur and NO, which results in S-nitroso compounds. Eventually, NO2 reacts with biological molecules in a process called nitration. And Tyrosine residues, present in proteins, are prone to nitration. This reaction leads to 3-nitrotyrosine, which is a well-documented marker of NO-dependent oxidative damage[9].
It has been documented that eNOS-induced NO plays a key role as brain parenchyma blood flow regulator. Adequate blood flow reduces oxidative, procoagulant and inflammatory pathways[10,11]. Cerebrovascular accident is one of the main causes of morbidity and mortality worldwide. Pathophysiology of acute stroke can be either ischemic or hemo
NO higher levels are associated with oxidative stress, excitotoxicity and neural apoptosis[19]. With reperfusion, extracellular hydrogen excess and Calcium (Ca+) ions are moved back into the cell. It is well known that mitochondria suffer continuous swelling in order to meet metabolic demands. However, calcium excess alters mitochondrial ion homeostasis and mitochondrial matrix structure, eventually leading to pathological swelling[20,21]. This alteration results in an increased membrane permeability and cytochrome cleakage. Cytochrome c is a protein that transports electrons between Complex III and IV and triggers PCD through caspase activation[20,21]. Nitrosylation and nitration can further modify cytochrome c. There is, however, controversial information about reduced proapoptotic properties of cytochrome c, due to specific tyrosine nitration residues. Nevertheless, evidence indicates that cytochrome cnitration modifies correct electron transport[22]. Current knowledge indicates that nNOS downregulation is associated with less neurological damage[18,23,24]. While more deleterious effects are shown when iNOS reaches significant activity. This usually occurs 12 hours after reoxygenation/reperfusion process[18,24,25].
Limiting the negative effects of NO-derived compounds has been implemented as an important strategy to improve neurological outcomes. Although the specific mechanism has not yet been clarified, EdaravoneÒ is used as treatment for amyotrophic lateral sclerosis and stroke due to its antioxidant properties[3]. The use of EdaravoneÒ in mice models of CIRI has not demonstrated significant differences in nitrite (NO2-) levels, cerebral blood flow and blood pressure. However, treated animals show a significant reduction in both nNOS and hydroxyl radicals. Therefore, better outcomes observed with EdaravoneÒ obey to neuroprotective effects[26].
Tissue plasminogen activator is another drug indicated within the first 4.5 hours of ischemic stroke[27]. Hemorrhagic transformation is more likely to occur when administered after this time frame[27,28]. This effect is the result of Matrix Metalloproteinases triggering by RNS such as peroxynitrite. Baicalin is being studied as a RNS scavenger for different diseases. However, its role remains controversial[27].
RNS downregulation appears to be a promising practice to minimize CIRI. Drugs currently under study show significant antioxidant and neuroprotective effects. However, measuring the efficacy of such drugs remains a challenge.
| 1. | Warner DS, Sheng H, Batinić-Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol. 2004;207:3221-3231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 427] [Article Influence: 19.4] [Reference Citation Analysis (1)] |
| 2. | Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54:34-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 548] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 3. | Anaya-Prado R, Toledo-Pereyra LH, Lentsch AB, Ward PA. Ischemia/reperfusion injury. J Surg Res. 2002;105:248-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 153] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Landar A, Darley-Usmar VM. Nitric oxide and cell signaling: modulation of redox tone and protein modification. Amino Acids. 2003;25:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Poyton RO, Ball KA, Castello PR. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol Metab. 2009;20:332-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 374] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 6. | She R, Liu D, Liao J, Wang G, Ge J, Mei Z. Mitochondrial dysfunctions induce PANoptosis and ferroptosis in cerebral ischemia/reperfusion injury: from pathology to therapeutic potential. Front Cell Neurosci. 2023;17:1191629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 99] [Reference Citation Analysis (0)] |
| 7. | Jung JE, Kim GS, Chen H, Maier CM, Narasimhan P, Song YS, Niizuma K, Katsu M, Okami N, Yoshioka H, Sakata H, Goeders CE, Chan PH. Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol Neurobiol. 2010;41:172-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 8. | Chen Y, He W, Wei H, Chang C, Yang L, Meng J, Long T, Xu Q, Zhang C. Srs11-92, a ferrostatin-1 analog, improves oxidative stress and neuroinflammation via Nrf2 signal following cerebral ischemia/reperfusion injury. CNS Neurosci Ther. 2023;29:1667-1677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 75] [Reference Citation Analysis (0)] |
| 9. | Huang J, Chen L, Yao ZM, Sun XR, Tong XH, Dong SY. The role of mitochondrial dynamics in cerebral ischemia-reperfusion injury. Biomed Pharmacother. 2023;162:114671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 67] [Reference Citation Analysis (0)] |
| 10. | Guix FX, Uribesalgo I, Coma M, Muñoz FJ. The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol. 2005;76:126-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 504] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 11. | Phillips L, Toledo AH, Lopez-Neblina F, Anaya-Prado R, Toledo-Pereyra LH. Nitric oxide mechanism of protection in ischemia and reperfusion injury. J Invest Surg. 2009;22:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 12. | Tadi P, Lui F. Acute Stroke. 2023 Aug 17. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 13. | Chen H, He Y, Chen S, Qi S, Shen J. Therapeutic targets of oxidative/nitrosative stress and neuroinflammation in ischemic stroke: Applications for natural product efficacy with omics and systemic biology. Pharmacol Res. 2020;158:104877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 14. | Wu L, Xiong X, Wu X, Ye Y, Jian Z, Zhi Z, Gu L. Targeting Oxidative Stress and Inflammation to Prevent Ischemia-Reperfusion Injury. Front Mol Neurosci. 2020;13:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 372] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 15. | Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, Moskowitz MA. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J Cereb Blood Flow Metab. 1996;16:981-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 544] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 16. | Ito Y, Ohkubo T, Asano Y, Hattori K, Shimazu T, Yamazato M, Nagoya H, Kato Y, Araki N. Nitric oxide production during cerebral ischemia and reperfusion in eNOS- and nNOS-knockout mice. Curr Neurovasc Res. 2010;7:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Wei G, Dawson VL, Zweier JL. Role of neuronal and endothelial nitric oxide synthase in nitric oxide generation in the brain following cerebral ischemia. Biochim Biophys Acta. 1999;1455:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Liu K, Li Q, Zhang L, Zheng X. The dynamic detection of NO during stroke and reperfusion in vivo. Brain Inj. 2009;23:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Zhou L, Zhu DY. Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide. 2009;20:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 482] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 20. | Sun YY, Zhu HJ, Zhao RY, Zhou SY, Wang MQ, Yang Y, Guo ZN. Remote ischemic conditioning attenuates oxidative stress and inflammation via the Nrf2/HO-1 pathway in MCAO mice. Redox Biol. 2023;66:102852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 180] [Reference Citation Analysis (0)] |
| 21. | Wang L, Zhang X, Xiong X, Zhu H, Chen R, Zhang S, Chen G, Jian Z. Nrf2 Regulates Oxidative Stress and Its Role in Cerebral Ischemic Stroke. Antioxidants (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 196] [Reference Citation Analysis (0)] |
| 22. | Leon L, Jeannin JF, Bettaieb A. Post-translational modifications induced by nitric oxide (NO): implication in cancer cells apoptosis. Nitric Oxide. 2008;19:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Gürsoy-Ozdemir Y, Bolay H, Saribaş O, Dalkara T. Role of endothelial nitric oxide generation and peroxynitrite formation in reperfusion injury after focal cerebral ischemia. Stroke. 2000;31:1974-80; discussion 1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 148] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Zheng X, Liu K, Yang Y. Real-time measurement of murine hippocampus NO levels in response to cerebral ischemia/reperfusion. Methods Mol Biol. 2011;704:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Iadecola C, Ross ME. Molecular pathology of cerebral ischemia: delayed gene expression and strategies for neuroprotection. Ann N Y Acad Sci. 1997;835:203-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Kawasaki H, Ito Y, Kitabayashi C, Tanaka A, Nishioka R, Yamazato M, Ishizawa K, Nagai T, Hirayama M, Takahashi K, Yamamoto T, Araki N. Effects of Edaravone on Nitric Oxide, Hydroxyl Radicals and Neuronal Nitric Oxide Synthase During Cerebral Ischemia and Reperfusion in Mice. J Stroke Cerebrovasc Dis. 2020;29:104531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Wang Y, Hong F, Yang S. Roles of Nitric Oxide in Brain Ischemia and Reperfusion. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 28. | Henninger N, Fisher M. Extending the Time Window for Endovascular and Pharmacological Reperfusion. Transl Stroke Res. 2016;7:284-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/