Published online Jan 6, 2025. doi: 10.12998/wjcc.v13.i1.99938
Revised: September 18, 2024
Accepted: October 25, 2024
Published online: January 6, 2025
Processing time: 95 Days and 22.3 Hours
Malignant transformation (MT) of mature cystic teratoma (MCT) has a poor prognosis, especially in advanced cases. Concurrent chemoradiotherapy (CCRT) has an inhibitory effect on MT.
Herein, we present a case in which CCRT had a reduction effect preoperatively. A 73-year-old woman with pyelonephritis was referred to our hospital. Computed tomography revealed right hydronephrosis and a 6-cm pelvic mass. Endoscopic ultrasound-guided fine-needle biopsy (EUS-FNB) revealed squamous cell carci
EUS-FNB was useful in the diagnosis of MT of MCT; CCRT suppressed the disea
Core Tip: Malignant transformation (MT) of mature cystic teratoma (MCT) is uncommon and has a poor prognosis, especially in advanced cases. Surgery and chemotherapy are selected in accordance with ovarian cancer, but the prognosis is typically poor due to the high degree of malignancy, making treatment often challenging. We present a case of stage IV MT of MCT in which concurrent chemoradiotherapy (CCRT) had a reduction effect preoperatively. Endoscopic ultrasound-guided fine-needle biopsy (EUS-FNB) was used for diagnosis due to the patient’s poor general condition. EUS-FNB was useful in diagnosing MT of MCT; CCRT suppressed the disease and improved the patient’s quality of life.
- Citation: Kondo S, Suzuki T, Yoshiike K, Yamanaka S, Sonehara K, Nabeshima H, Oguchi O. Stage IV malignant transformation of mature cystic teratoma palliatively treated with concurrent chemoradiotherapy: A case report. World J Clin Cases 2025; 13(1): 99938
- URL: https://www.wjgnet.com/2307-8960/full/v13/i1/99938.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i1.99938
Malignant transformation (MT) of mature cystic teratoma (MCT) is uncommon, occurring in 0.17%-2% of MCTs[1]. There is no established treatment method; debulking surgery and multidrug chemotherapy are performed in accordance with epithelial ovarian cancer. However, the prognosis of advanced cancer is poor: the 2-year survival rate is approximately 30% for stage III and 0% for stage IV[2]. In some cases, due to systemic conditions or complications, adequate treatment is not available. We present a case of stage IV MT of MCT that was diagnosed using endoscopic ultrasound-guided fine-needle biopsy (EUS-FNB) and palliatively treated with concurrent chemoradiotherapy (CCRT).
A 73-year-old multiparous woman was referred to the emergency department of our hospital with fever and right lower back pain.
Symptoms started 2 weeks before presentation.
She had a medical history of cerebral infarction, hypertension, hyperlipidemia, and benign left ovarian tumor.
She denied any family history of malignancy.
On physical examination, the vital signs were as follows: Body temperature, 38.5 °C; blood pressure, 120/68 mmHg; heart rate, 82 beats per minute; respiratory rate, 20 breaths per minute. Internal examination revealed that the tumor was adhered to the pelvic wall and had poor mobility.
The serum squamous cell carcinoma antigen level was 5.7 ng/mL, carcinoembryonic antigen level was 0.9 ng/mL, CA19-9 level was 26 U/mL, and CA125 level was 10.8 U/mL. The white blood cell count was 18200/μL, C-reactive protein level was 9.62 mg/dL, serum creatine level was 1.17 mg/dL, and the estimated glomerular filtration rate was 35 mL/min.
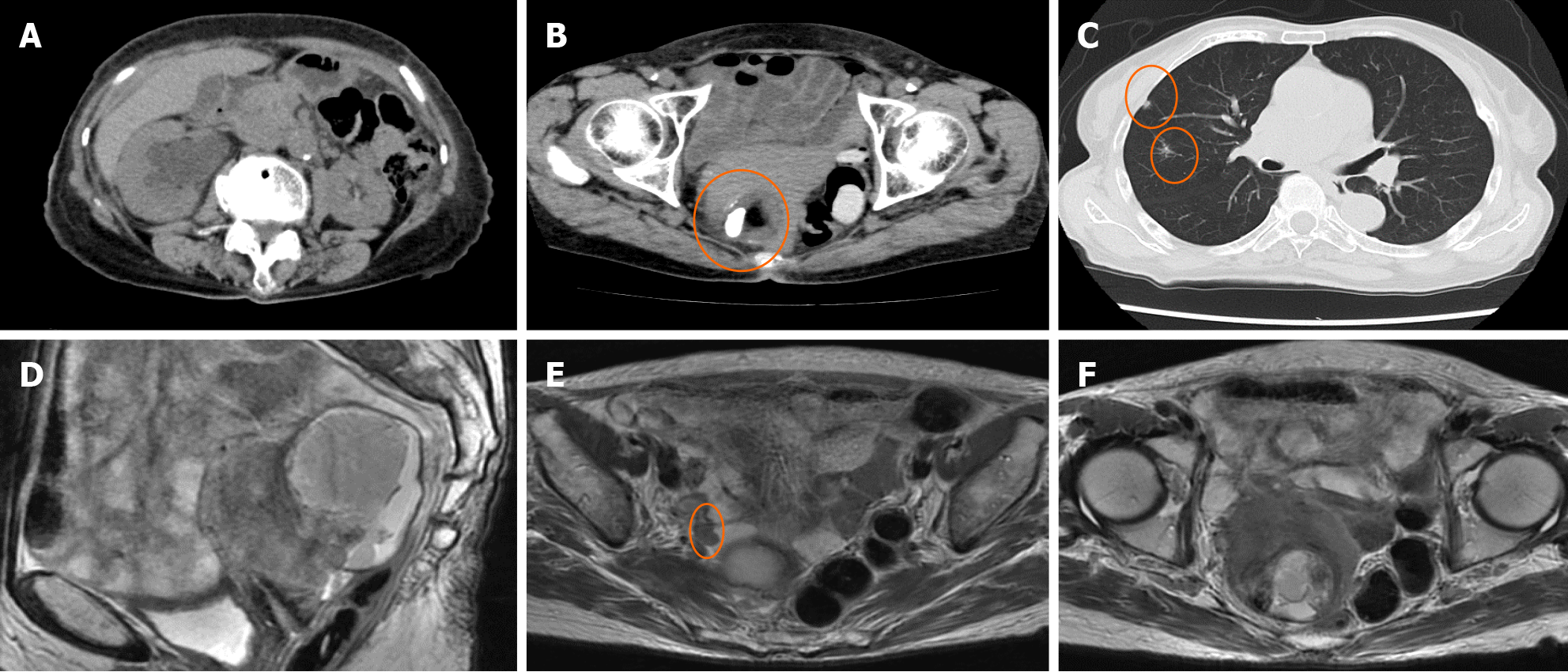
Ultrasonography revealed an irregular solid pattern and a small amount of ascitic fluid. Computed tomography (CT) revealed right hydronephrosis and a 68 cm × 64 cm × 45 mm solid cystic mass with fat and calcification densities in the right pelvic cavity. Chest CT showed multiple grain-sized nodules in both lungs. Magnetic resonance imaging (MRI) showed that the tumor was adhered to both the uterus and rectum, with a 1.5 cm peritoneal dissemination at the right pelvic wall near the ureter (Figure 1).
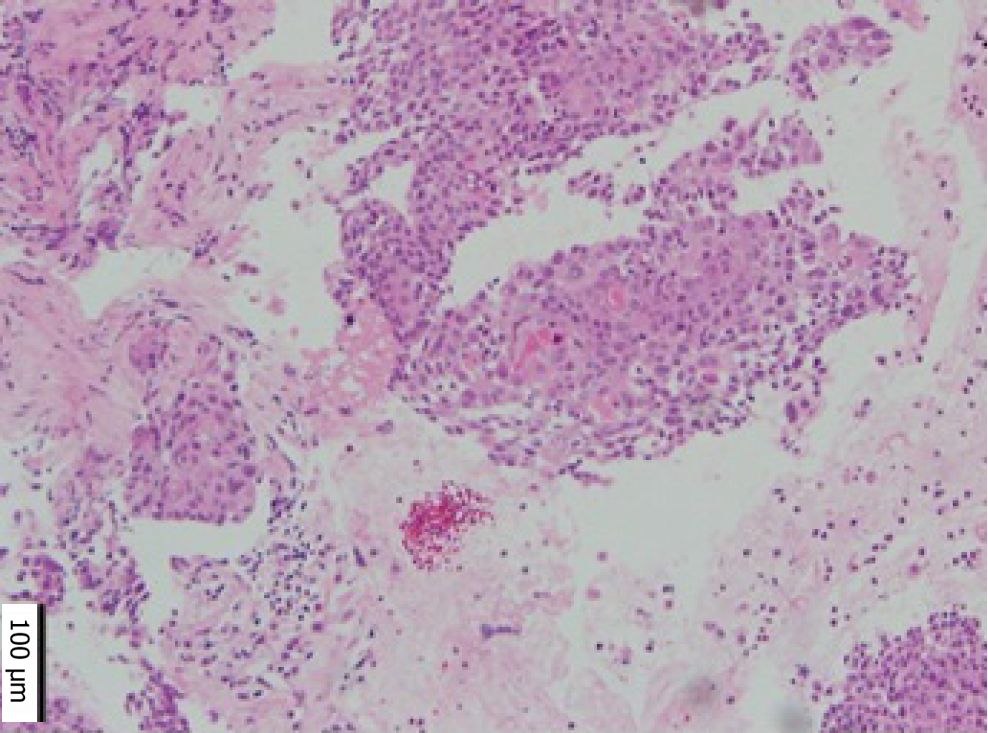
Cervical cytology was negative for intraepithelial lesions or malignancy, and there were no findings suggestive of human papillomavirus infection. We suspected MT of the MCT from CT and MRI findings. Paracentesis or laparoscopic biopsy are usually considered for diagnosis; however, as there was some ascites, and the tumor was located deep in the pelvis and invaded surrounding organs, we concluded that both were difficult. Therefore, we performed EUS-FNB based on the location of the tumor and its adhesion to the rectum. Pathological examination revealed squamous cell carcinoma (Figure 2).
The patient was diagnosed with stage IV MT of the MCT.
We were hesitant to perform debulking surgery because her Eastern Cooperative Oncology Group performance status (PS) score was 2. In addition, multidrug chemotherapy has side effects that could exacerbate renal dysfunction. Since CCRT is performed in hospital, it is possible even if the PS is 2 or higher, and we considered that side effects can be observed and dealt with in detail. In addition, the amount of cisplatin used in combination can be reduced according to renal function, and the treatment results are better than those with radiation alone. Therefore, we selected CCRT. The patient received 50 Gy of radiotherapy in 25 fractions to the lower pelvis with 30 mg/m2 cisplatin for 4 weeks. The side effects included loss of appetite and weight, fever, and diarrhea, all grade 1 according to the Common Terminology Criteria for Adverse Events v5.0. Medication was given for fever and diarrhea, and the patient quickly recovered. After CCRT, the tumor size was reduced by 7.3%, mobility improved, and the PS score improved from 2 to 1. Total abdominal hysterectomy, right salpingo-oophorectomy, and removal of the dissemination were performed. The ascites cytology was positive. No tumors remained in the abdomen postoperatively. The patient was asymptomatic but requested follow-up.
Five months postoperatively, edema was observed in the right lower leg. Positron emission tomography showed peritoneal dissemination in the right pelvic wall, multiple lymph node metastases, and increased lung metastases. The patient requested palliative chemotherapy to reduce the edema. We administered doxorubicin hydrochloride liposome 40 mg/m2 on day 1 but had to stop treatment due to a decrease in blood pressure and generalized rashes that could indicate an allergic reaction. We then administered three courses of gemcitabine 1000 mg/day, on days 1, 8, and 15, but discontinued due to disease progression.
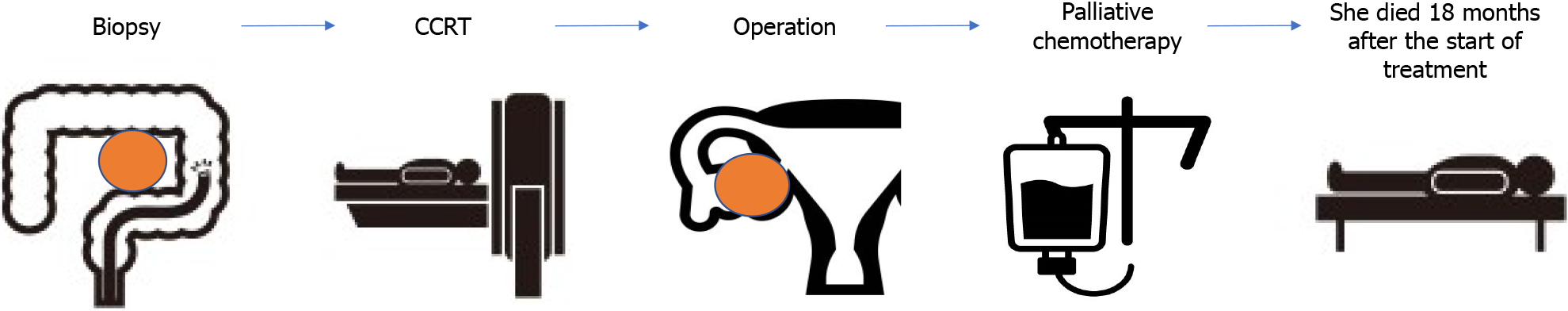
The patient died 18 months after treatment initiation due to multiple organ failure. Information from this case report organized into timeline (Figure 3).
The patient and her family were satisfied with the treatment and its progress.
This case demonstrates the following two points: CCRT had a preoperative reduction effect in MT of MCT, and EUS-FNB is useful for diagnosing MT of MCT.
CCRT reduced the MT of MCT. Table 1 shows previous cases in which CCRT was administered for MT of MCT[3-8]. The advanced cases survived with no evidence of disease. However, all of these cases had CCRTs administered postoperatively. To our knowledge, this is the first case report of preoperative CCRT. Similar to the postoperative cases, CCRT showed a reduction in the MT of MCT, and the side effects were tolerable. According to the literature, cases of long-term survival even in advanced stages have been completely removed by surgery. However, in cases such as this case, where the general condition is poor or the patient is elderly or has complications, treatment that prolongs the prognosis without causing complications is necessary, and CCRT can be said to be a treatment that satisfies such needs. Of course, CCRT also has side effects such as intestinal perforation, radiation enteritis, and cystitis, which may be necessary to treat, but these can be avoided to some extent by narrowing the irradiation field with intensity-modulated radiation therapy. Another long-term disadvantage is that it is difficult to use angiogenesis inhibitors such as bevacizumab because of the increased possibility of intestinal perforation. Although preoperative CCRT for ovarian cancer is currently uncommon, preoperative radiotherapy is often used for colorectal cancer. Recent reports have shown that patients with recurrent MT of MCT have been treated with a combination of surgery, chemotherapy, and CCRT to achieve long-term survival[9,10]. More definitive conclusions can be drawn as more cases accumulate in the future.
| Ref. | Age | Stage | Surgery | Adjuvant therapy | Follow-up (months) | Outcome |
| Rose et al[3], 1993 | 42 | III C | RSO, retroperitoneal dissection, PAN | CCRT (cisplatin) | 11 | DOD |
| Do et al[4], 2001 | 44 | II B | LSO | CCRT (5-FU, leucovorin) | 36 | NED |
| Park et al[5], 2008 | 43 | III C | PLN, PAN, total omentectomy, Appe | CCRT, TP | 13 | NED |
| Yoshida et al[6], 2016 | 37 | II B | TAH, BSO | CCRT (cisplatin, 5-FU) | 27 | NED |
| Tokunaga et al[7], 2016 | 49 | III A | TAH, BSO, pOM, PLN, PAN | CCRT | ? | NED |
| Tokunaga et al[7], 2016 | 40 | III C | TAH, BSO, pOM, PLN, PAN | CCRT | ? | NED |
| Bacalbasa et al[8], 2020 | 47 | I A | TAH, LSO | CCRT (cisplatin) | 24 | NED |
EUS-FNB is useful for diagnosis. Paracentesis or endoscopic biopsy are the usual methods for ovarian cancer biopsy; however, in the present case, there were minimal ascites, the tumor was adhered to the rectum and pelvic wall and had poor mobility, and dissemination was deep in the pelvis. EUS-FNB is a minimally invasive rapid diagnostic technique. At our hospital, EUS-FNB is used for lymph node biopsies and histological examination of intestinal submucosal tumors. Other cases of MT of MCT that were incidentally diagnosed on colonic biopsy have been reported[11]. Advanced MT of MCT may directly invade adjacent organs; however, investigation of further cases is required.
The effects of immune checkpoint inhibitors for recurrence or chemo-resistant cases have been reported in recent years[12-14]. We hope to collect data from additional cases and develop effective treatments.
EUS-FNB is a useful method of diagnosing MT of MCT. Although advanced-stage patients have poor prognosis, CCRT has the potential to suppress the disease temporarily and improve the patient’s quality of life with few adverse effects.
| 1. | Hackethal A, Brueggmann D, Bohlmann MK, Franke FE, Tinneberg HR, Münstedt K. Squamous-cell carcinoma in mature cystic teratoma of the ovary: systematic review and analysis of published data. Lancet Oncol. 2008;9:1173-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 211] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 2. | Tseng CJ, Chou HH, Huang KG, Chang TC, Liang CC, Lai CH, Soong YK, Hsueh S, Pao CC. Squamous cell carcinoma arising in mature cystic teratoma of the ovary. Gynecol Oncol. 1996;63:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Rose PG, Tak WK, Reale FR. Squamous cell carcinoma arising in a mature cystic teratoma with metastasis to the paraaortic nodes. Gynecol Oncol. 1993;50:131-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Do VT, Thomas GM, Bjarnason GA. Postoperative concurrent chronomodulated 5-fluorouracil/leucovorin infusion and pelvic radiotherapy for squamous cell carcinoma of the ovary arising from mature cystic teratoma. Int J Gynecol Cancer. 2001;11:418-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Malignant transformation of mature cystic teratoma of the ovary: experience at a single institution. Eur J Obstet Gynecol Reprod Biol. 2008;141:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Yoshida K, Kajiyama H, Utsumi F, Mitsui H, Shibata K, Kikkawa F. Radiotherapy for persistent malignant transformation from mature cystic teratoma of the ovary. J Obstet Gynaecol Res. 2016;42:584-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Tokunaga H, Watanabe Y, Kaiho M, Yokoyama Y, Futagami M, Watanabe T, Soeda S, Takatori E, Shoji T, Niikura H, Nishigori H, Takahashi F, Sugiyama T, Yaegashi N. Advanced squamous cell carcinomas arising from mature cystic teratoma of the ovary: a retrospective case series at the Tohoku Gynecologic Cancer Unit. Int Cancer Conf J. 2016;5:146-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Bacalbasa N, Cretoiu D, Halmaciu I, Diaconu C, Iliescu L, Dima S, Neacsu A, Balalau C, Bratu OG, Balescu I. Squamous Cell Carcinoma from Abscessed, Mature Cystic Ovarian Teratoma - A Case Report and Literature Review. In Vivo. 2020;34:2141-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Ji X, Zhai P, Yang H, Wang H, Wang X. Recurrent squamous cell carcinoma arising in ovary mature cystic teratoma: A case report. Medicine (Baltimore). 2023;102:e34734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Okuda T, Uda Y, Sakai S, Harada T. Malignant Transformation of Unknown Duration of an Ovarian Mature Cystic Teratoma Presenting as a Trocar Recurrence in a Young Patient: A Case Report and Literature Review. Case Rep Obstet Gynecol. 2023;2023:8875092. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Rojas CP, Ganjei-Azar P, Garcia-Buitrago MT. Unexpected Malignant Diagnosis in Colonic Biopsies: Malignant Transformation of Ovarian Mature Teratomas-Two Case Reports and Review of the Literature. Case Rep Pathol. 2015;2015:905462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Li X, Tang X, Zhuo W. Malignant transformation of ovarian teratoma responded well to immunotherapy after failed chemotherapy: a case report. Ann Palliat Med. 2021;10:8499-8505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 13. | Yoshimura K, Yamanoi K, Kanai M, Okunomiya A, Sagae Y, Sunada M, Taki M, Ukita M, Chigusa Y, Horie A, Yamaguchi K, Hamanishi J, Minamiguchi S, Yamamoto N, Muto M, Mandai M. Nivolumab for malignant transformation of ovarian mature cystic teratoma. Gynecol Oncol Rep. 2022;44:101115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Song XC, Wang YX, Yu M, Cao DY, Yang JX. Case Report: Management of Recurrent Ovarian Squamous Cell Carcinoma With PD-1 Inhibitor. Front Oncol. 2022;12:789228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/