Published online Mar 6, 2024. doi: 10.12998/wjcc.v12.i7.1235
Peer-review started: November 6, 2023
First decision: January 9, 2024
Revised: January 20, 2024
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: March 6, 2024
Processing time: 115 Days and 13.1 Hours
Intensive care unit-acquired weakness (ICU-AW) is a common complication that significantly impacts the patient's recovery process, even leading to adverse outcomes. Currently, there is a lack of effective preventive measures.
To identify significant risk factors for ICU-AW through iterative machine learning techniques and offer recommendations for its prevention and treatment.
Patients were categorized into ICU-AW and non-ICU-AW groups on the 14th day post-ICU admission. Relevant data from the initial 14 d of ICU stay, such as age, comorbidities, sedative dosage, vasopressor dosage, duration of mechanical ventilation, length of ICU stay, and rehabilitation therapy, were gathered. The relationships between these variables and ICU-AW were examined. Utilizing iterative machine learning techniques, a multilayer perceptron neural network model was developed, and its predictive performance for ICU-AW was assessed using the receiver operating characteristic curve.
Within the ICU-AW group, age, duration of mechanical ventilation, lorazepam dosage, adrenaline dosage, and length of ICU stay were significantly higher than in the non-ICU-AW group. Additionally, sepsis, multiple organ dysfunction syndrome, hypoalbuminemia, acute heart failure, respiratory failure, acute kidney injury, anemia, stress-related gastrointestinal bleeding, shock, hypertension, coronary artery disease, malignant tumors, and rehabilitation therapy ratios were significantly higher in the ICU-AW group, demonstrating statistical significance. The most influential factors contributing to ICU-AW were identified as the length of ICU stay (100.0%) and the duration of mechanical ventilation (54.9%). The neural network model predicted ICU-AW with an area under the curve of 0.941, sensitivity of 92.2%, and specificity of 82.7%.
The main factors influencing ICU-AW are the length of ICU stay and the duration of mechanical ventilation. A primary preventive strategy, when feasible, involves minimizing both ICU stay and mechanical ventilation duration.
Core Tip: The study, utilizing machine learning, identified key risk factors for intensive care unit-acquired weakness (ICU-AW). Findings emphasized the significant impact of length of ICU stay and the duration of mechanical ventilation. Other factors, including age, medication dosage, and specific disease states, were also implicated. The study employed a multilayer perceptron neural network model with an impressive area under receiver operating characteristic curve of 0.941, sensitivity of 92.2%, and specificity of 82.7%. The results underscore the importance of decreasing length of ICU stay and the duration of mechanical ventilation as a primary strategy in preventing ICU-AW, when feasible.
- Citation: Wang L, Long DY. Significant risk factors for intensive care unit-acquired weakness: A processing strategy based on repeated machine learning. World J Clin Cases 2024; 12(7): 1235-1242
- URL: https://www.wjgnet.com/2307-8960/full/v12/i7/1235.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i7.1235
Intensive care unit-acquired weakness (ICU-AW) is a prevalent complication in critically ill patients, marked by secondary neurologic and/or muscular impairments[1,2]. It manifests as symmetrical muscle weakness, profoundly affecting patient survival and quality of life[3,4]. Timely prediction and risk assessment in ICU patients are crucial to implementing interventions, reducing incidence, and enhancing patient outcomes.
The factors influencing the emergence of ICU-AW in critically ill patients are highly intricate. Recent advancements in artificial intelligence and machine learning, particularly neural network models, have shown exceptional capabilities in predicting and diagnosing medical conditions[5,6]. This study aimed to utilize iterative machine learning techniques to pinpoint significant risk factors for ICU-AW, build a predictive model using a multilayer perceptron neural network, and assess its performance, offering guidance for ICU-AW prevention and treatment.
The current study focused on adult patients (age ≥ 18 years) admitted to the People’s Hospital of Qiandongnan Miao and Dong Autonomous Prefecture ICU between January 1, 2022, and August 1, 2023. To ensure the accuracy of ICU-AW diagnosis, individuals with pre-existing conditions impacting it (e.g., severe central nervous system diseases, spinal and limb fractures, multiple myositis, and myasthenia gravis) were excluded. Furthermore, patients choosing treatment discontinuation were also excluded from the study.
Upon ICU admission, the hospital employs aggressive therapeutic measures to address underlying conditions and administers essential organ support therapies for vital sign stability. In this study, mechanical ventilation and sedation therapy were administered when deemed necessary. In cases of hypotension, vasopressor agents were used to maintain a mean arterial pressure of at least 65 mmHg after adequate fluid resuscitation. Infected patients received effective intravenous antibiotic therapy. Furthermore, with patient or family consent, the hospital provided appropriate rehabilitation therapy, such as limb exercises or joint relaxation. In the early stages, the hospital also emphasized nutritional therapy, particularly for patients with serum albumin levels below 30 mg/L, with supplementation of human serum albumin.
The medical research council scale was used to grade muscle strength in upper and lower limb muscle groups for diagnosis. This scale has a score range of 0-5, with a maximum total score of 60. If a patient’s comprehensive score falls below 48 points, he (she) was diagnosed with ICU-AW[1].
Data collection and grouping: Limb muscle strength assessments were conducted daily post-ICU admission. Patients were categorized into the ICU-AW or non-ICU-AW group based on the development of ICU-AW within the initial 14 d of ICU admission. On the 14th day, patient data included gender, age, duration of mechanical ventilation, total sedative dosage (Midazolam), total vasopressor dosage (Norepinephrine), and ICU stay length were collected. Additionally, comorbidities such as hypertension, diabetes, coronary atherosclerotic heart disease, and malignant tumors were noted. On the 14th day, data were collected regarding the occurrence of sepsis, multiple organ dysfunction syndrome, hypoalbuminemia, acute heart failure, respiratory failure, acute kidney injury, anemia, disseminated intravascular coagulation, stress-related gastrointestinal bleeding, shock, and whether the patients underwent rehabilitative treatment during their ICU stay.
Establishment of the multilayer perceptron neural network model: The study utilized a neural network model featuring a single hidden layer, with the number of nodes in the hidden layer determined automatically and the hyperbolic tangent chosen as the activation function. All dependent variables underwent standardization. The dataset was split into a 70% training set and a 30% test set. The input layer incorporated indicators demonstrating statistical significance in correlation analysis, while the output layer determined ICU-AW occurrence (encoded: ICU-AW = 1, non-ICU-AW = 0). The model employed batch processing for training, with the conjugate gradient method and line search chosen as the optimization algorithm. Factors were ordered by importance, and the predictive probability model for ICU-AW was saved. The specificity and sensitivity of the multilayer perceptron neural network model were calculated using the receiver operating characteristic (ROC) curve for analysis convenience.
This study employed SPSS 26.0 statistical software for data processing and analysis. The normality test for continuous variables was conducted using the Kolmogorov-Smirnov method. Normally distributed data were presented as mean ± SD, and inter-group comparisons were made using the independent samples t-test. Non-normally distributed data were expressed as median (interquartile range) [M (QL, QU)], and the Mann-Whitney U-test was used for inter-group comparisons. Categorical data were analyzed using the Chi-squared (χ2) test. The multilayer perceptron neural network model’s predictive capability for ICU-AW was assessed through ROC curve analysis. Statistical significance was set at P < 0.05.
The study compiled data from 1063 cases, spanning ages 18 to 94 years, with an average age of 60.91 ± 19.00 years. Among these, 645 were male, and 418 were female. A total of 370 cases were diagnosed with ICU-AW, while 693 cases did not develop ICU-AW, resulting in an ICU-AW incidence rate of 34.81%.
No significant differences were observed between the two groups in terms of gender, rates of disseminated intravascular coagulation, diabetes, and malignant tumors (U = 1.913, 0.077, 1.564, 0.179, P > 0.05). However, the ICU-AW group exhibited significantly longer ICU stays, prolonged duration of mechanical ventilation, higher total dosages of Midazolam, and increased total dosages of Norepinephrine compared to the non-ICU-AW group (Z = 278.696, 29.905, 127.872, 81.127, P < 0.05). Additionally, the ICU-AW group had higher rates of specific comorbidities compared to the non-ICU-AW group as shown in Table 1.
| Parameters | ICU-AW group (n = 370) | Non-ICU-AW group (n = 693) | χ2/t/Z | P value |
| Sex | 1.913 | 0.167 | ||
| Male, n (%) | 235 (63.51) | 410 (59.16) | ||
| Female, n (%) | 135 (36.49) | 283 (40.84) | ||
| Age in yr, mean ± SD | 65.29 ± 18.72 | 58.58 ± 18.75 | 31.013 | 0.000 |
| Sepsis, n (%) | 31 (8.38) | 31 (4.47) | 6.727 | 0.010 |
| Multiple organ dysfunction syndrome, n (%) | 96 (25.95) | 68 (9.81) | 50.307 | 0.000 |
| Hypoalbuminemia, n (%) | 275 (74.32) | 379 (54.69) | 67.286 | 0.000 |
| Acute cardiogenic failure, n (%) | 157 (42.43) | 205 (29.58) | 18.005 | 0.000 |
| Respiratory failure, n (%) | 231 (62.43) | 277 (39.97) | 83.798 | 0.000 |
| Acute kidney injury, n (%) | 63 (17.03) | 47 (6.78) | 27.956 | 0.000 |
| Anemia, n (%) | 191 (51.62) | 229 (33.04) | 35.942 | 0.000 |
| Diffuse intravascular coagulation, n (%) | 5 (1.35) | 8 (1.15) | 0.077 | 0.781 |
| Stress-related gastrointestinal bleeding, n (%) | 99 (26.76) | 74 (10.68) | 47.733 | 0.000 |
| Shock, n (%) | 224 (60.54) | 276 (39.83) | 43.152 | 0.000 |
| Hypertension, n (%) | 193 (52.16) | 263 (37.95) | 20.661 | 0.000 |
| Diabetes, n (%) | 83 (22.43) | 133 (19.19) | 1.564 | 0.211 |
| Coronary atherosclerotic heart disease, n (%) | 123 (33.24) | 132 (19.05) | 27.292 | 0.000 |
| Malignant tumor, n (%) | 81 (21.89) | 144 (20.78) | 0.179 | 0.673 |
| Rehabilitation therapy, n (%) | 20 (5.40) | 172 (24.82) | 93.687 | 0.000 |
| Mechanical ventilation duration in h, M (QL, QU) | 114 (9.75, 250) | 0 (0, 21) | 29.905 | 0.000 |
| Midazolam in mg, M (QL, QU) | 50 (0, 370) | 0 (0,20) | 127.872 | 0.000 |
| Norepinephrine in mg, M (QL, QU) | 20 (0, 120) | 0 (0, 16) | 81.127 | 0.000 |
| Length of intensive care unit stay in d, M (QL, QU) | 10 (6, 19) | 3 (1, 4) | 278.696 | 0.000 |
Using the presence of ICU-AW as the dependent variable (assigned values: Yes = 1, No = 0) and factors associated with ICU-AW as independent variables, all variables in the input layer were normalized (assigned values: Yes = 1, No = 0). Constructing the ICU-AW neural network model involved creating a multilayer perceptron neural network with one hidden layer and three neurons. The model achieved a prediction accuracy of 86.2% on the training set and 85.5% on the test set as shown in Table 2.
| Samples | Measured results | Number of cases | Predicted results | ||
| Non-ICU-AW | ICU-AW | Correct percentage (%) | |||
| Training set | Non-ICU-AW | 503 | 443 | 60 | 88.1 |
| ICU-AW | 257 | 45 | 212 | 82.5 | |
| Total | 760 | 448 | 272 | 86.2 | |
| Test set | Non-ICU-AW | 190 | 169 | 21 | 88.9 |
| ICU-AW | 113 | 23 | 90 | 79.6 | |
| Total | 303 | 192 | 111 | 85.5 | |
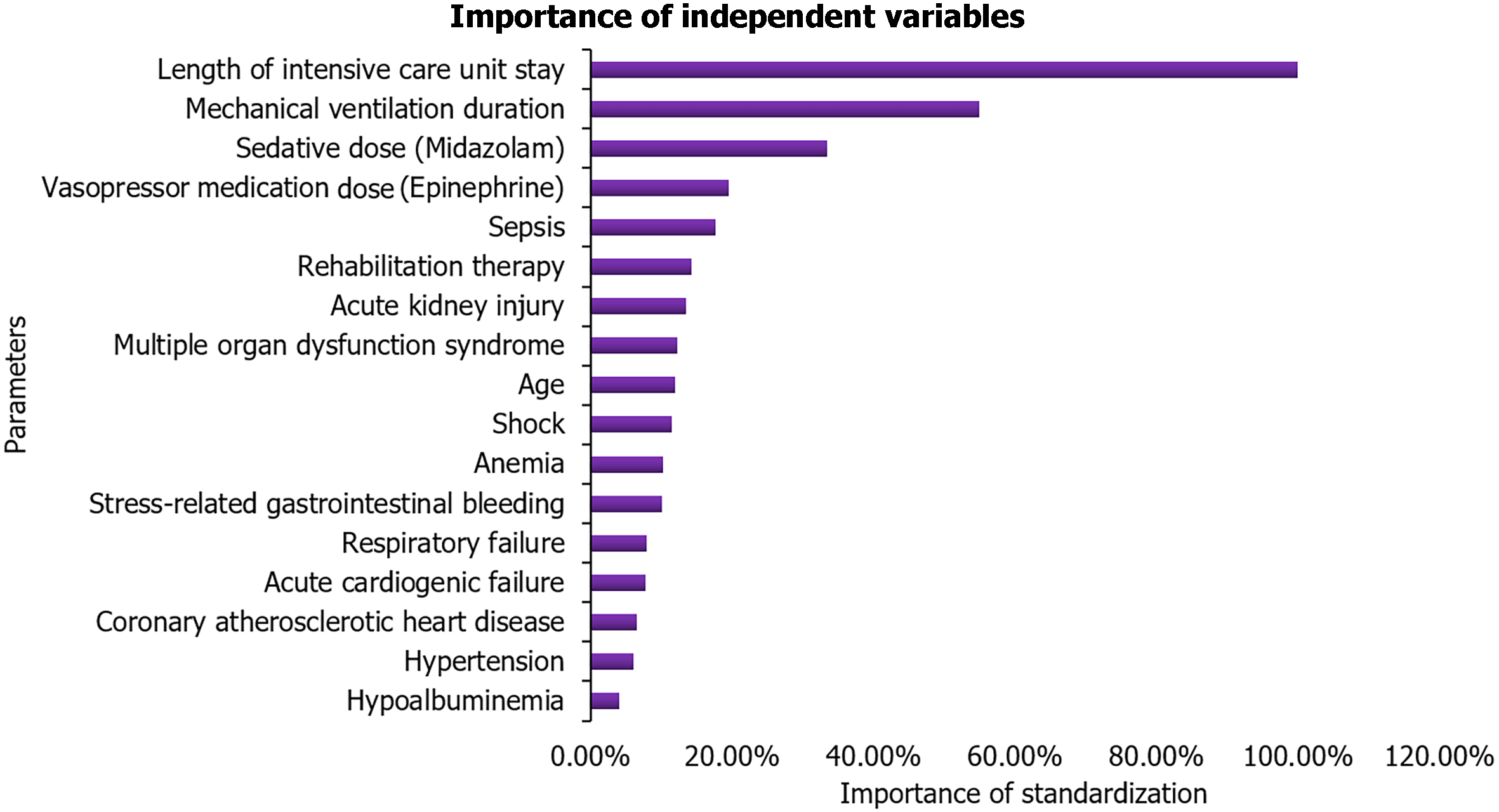
The most significant factors affecting the occurrence of ICU-AW were the length of ICU stay (100.0%) and the duration of mechanical ventilation (54.9%), followed by the dosage of sedatives (33.4%) and the dosage of vasopressor medications (19.5%). For a comprehensive list of influential factors and their respective proportions, please refer to Table 3 and Figure 1.
| Parameters | Importance | The importance of standardization (%) |
| Age | 0.035 | 11.9 |
| Sepsis | 0.052 | 17.6 |
| Multiple organ dysfunction syndrome | 0.036 | 12.2 |
| Hypoalbuminemia | 0.012 | 4.0 |
| Acute cardiogenic failure | 0.023 | 7.8 |
| Respiratory failure | 0.023 | 7.9 |
| Acute kidney injury | 0.039 | 13.4 |
| Anemia | 0.030 | 10.2 |
| Stress-related gastrointestinal bleeding | 0.029 | 10.0 |
| Shock | 0.034 | 11.4 |
| Hypertension | 0.018 | 6.0 |
| Coronary atherosclerotic heart disease | 0.019 | 6.5 |
| Rehabilitation therapy | 0.042 | 14.2 |
| Mechanical ventilation duration | 0.161 | 54.9 |
| Sedative dose (Midazolam) | 0.098 | 33.4 |
| Vasopressor medication dose (Epinephrine) | 0.057 | 19.5 |
| Length of intensive care unit stay | 0.293 | 100.0 |
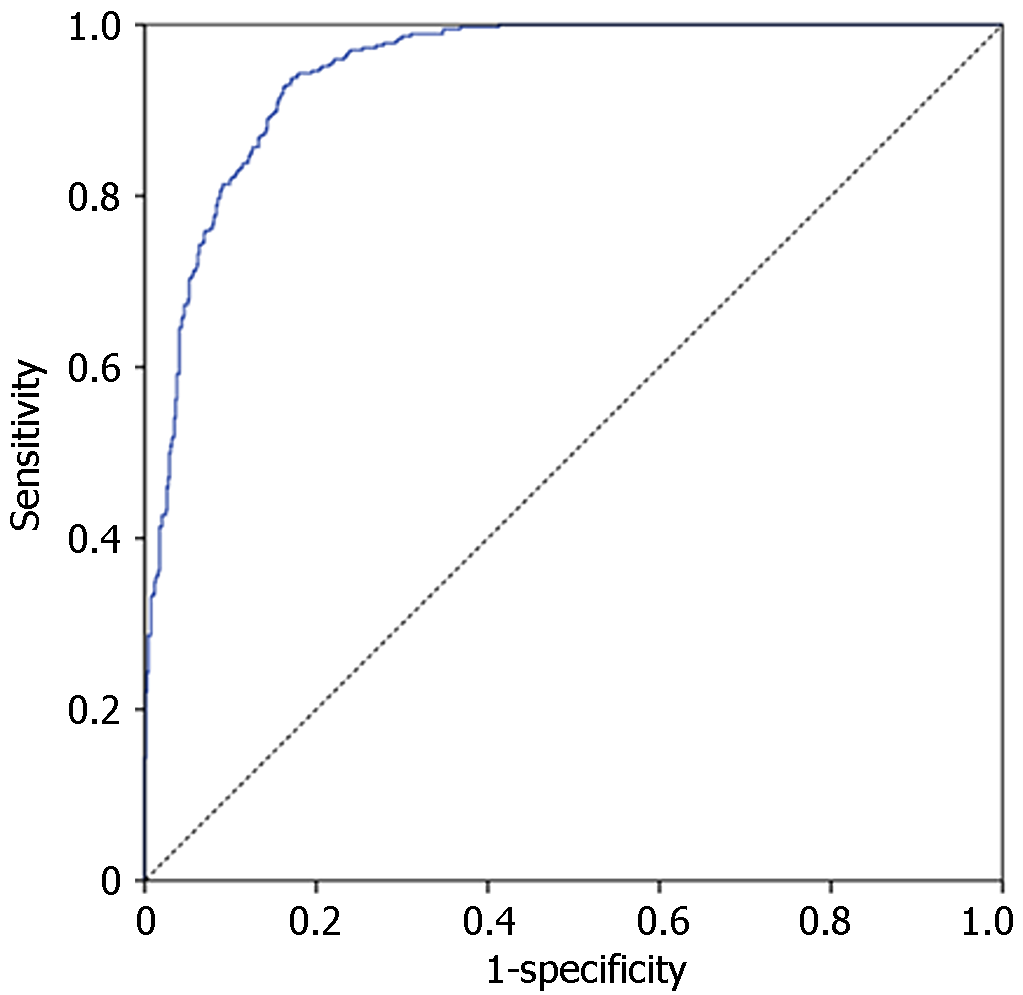
The neural network model predicted ICU-AW with an AUC value of 0.941 (95%CI: 0.928-0.954). Setting the cut-off value at 0.241 yielded a sensitivity of 92.2% and a specificity of 82.7%. Refer to Figure 2 for a visual representation.
The recognition of the increasing impact of ICU-AW on critically ill patients underscores the importance of identifying and addressing its numerous contributing factors. Current treatment and prevention strategies primarily focus on identifying high-risk factors and implementing corresponding measures[7]. Neural networks, through their ability to comprehend intricate data relationships and discern the significance of independent variables[8], play a crucial role in addressing these high-risk factors. This study utilized a multilayer perceptron neural network to assess the risk of ICU-AW among patients, revealing a model with an AUC of 0.941, a sensitivity of 92.2%, and a specificity of 82.7%, showca
The findings of the current study affirmed that the length of ICU stay and the duration of mechanical ventilation as the most pivotal factors for ICU-AW, with sensitivities of 100% and 54.9%, respectively. Previous studies have consistently noted the significant correlation between prolonged ICU stay and the occurrence of ICU-AW[9,10]. Similarly, an extended duration of mechanical ventilation has been linked to a higher incidence of ICU-AW[11,12], with even a few hours of mechanical ventilation potentially triggering ICU-AW[13]. It is inferred that, where clinically viable, reducing both the length of ICU stay and the duration of mechanical ventilation stands as a primary strategy for preventing ICU-AW.
The findings of the current study also indicated that the total dosage of sedatives, total dosage of vasopressor drugs, and sepsis were associated with ICU-AW, aligning with findings in other studies[14-16]. Further exploration of influencing factors in ICU-AW revealed that sedation and analgesia treatments could impact the neuro-musculoskeletal system functions, significantly elevating the risk of ICU-AW[17-19]. Additionally, the duration and dosage of norepinephrine use were notably linked to ICU-AW, with the utilization of vasopressor drugs increasing the risk of occurrence by more than threefold (OR = 3.2, 95%CI: 1.29-7.95)[20]. Patients with sepsis might trigger ICU-AW due to the imbalance in protein synthesis and breakdown caused by cytokines during systemic inflammatory response and inflammatory injury [16]. Studies have also emphasized that malnutrition can impair neuromuscular function, recommending early nutritional intervention[21,22]. The association of muscle relaxants with ICU-AW was widely acknowledged[23]. These results suggest that the factors influencing ICU-AW are complex.
The mechanisms linking ICU stay duration and mechanical ventilation to ICU-AW remain unclear. In the current study, researchers posited that patients with extended ICU stays and mechanical ventilation often experience restricted movement, and heightened use of sedatives, possibly coupled with muscle relaxants, sepsis, hypoxia, and malnutrition. These factors collectively may contribute to neuro-muscular damage.
The current study faced certain limitations. Firstly, its generalizability was constrained as data originated from a single center. Secondly, the potential impacts of disease severity and treatment efficacy on ICU-AW were not thoroughly investigated. Future research endeavors should aim to broaden their scope by encompassing multiple centers and larger sample sizes, while also incorporating a more extensive array of biomarkers and other influential factors for a comprehensive understanding.
This study emphasized the significant influence of the length of ICU stay and the duration of mechanical ventilation on ICU-AW. The primary strategy for preventing ICU-AW involves reducing these durations, where feasible under clinical conditions. However, it is crucial to acknowledge that numerous other factors contribute to the occurrence of ICU-AW. Therefore, minimizing these factors collectively can effectively mitigate the risk of ICU-AW. These insights provide clinicians with valuable information for making informed decisions in the prevention and treatment of ICU-AW.
Intensive care unit-acquired weakness (ICU-AW) is a common complication that significantly impacts the patient's recovery process, even leading to adverse outcomes. Currently, there is a lack of effective preventive measures.
Provide meaningful insights for the prevention of ICU-AW.
Identify the main risk factors for ICU-AW.
Utilizing iterative machine learning techniques, a multilayer perceptron neural network model was developed, and its predictive performance for ICU-AW was assessed using the receiver operating characteristic curve, and analyzed the importance of independent variables in models.
The most influential factors contributing to ICU-AW were identified as the length of ICU stay (100.0%) and the duration of mechanical ventilation (54.9%). The neural network model predicted ICU-AW with an area under the curve of 0.941, sensitivity of 92.2%, and specificity of 82.7%.
The main factors influencing ICU-AW are the length of ICU stay and the duration of mechanical ventilation.
A primary preventive strategy, when feasible, involves minimizing both ICU stay and mechanical ventilation duration. Future research needs to clarify the mechanism of ICU-AW occurrence and refine prevention strategies.
| 1. | Vanhorebeek I, Latronico N, Van den Berghe G. ICU-acquired weakness. Intensive Care Med. 2020;46:637-653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 417] [Article Influence: 69.5] [Reference Citation Analysis (1)] |
| 2. | Li Z, Zhang Q, Zhang P, Sun R, Jiang H, Wan J, Wu F, Wang X, Tao X. Prevalence and risk factors for intensive care unit acquired weakness: A protocol for a systematic review and meta-analysis. Medicine (Baltimore). 2020;99:e22013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Vanpee G, Hermans G, Segers J, Gosselink R. Assessment of limb muscle strength in critically ill patients: a systematic review. Crit Care Med. 2014;42:701-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | Dres M, Jung B, Molinari N, Manna F, Dubé BP, Chanques G, Similowski T, Jaber S, Demoule A. Respective contribution of intensive care unit-acquired limb muscle and severe diaphragm weakness on weaning outcome and mortality: a post hoc analysis of two cohorts. Crit Care. 2019;23:370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | MacEachern SJ, Forkert ND. Machine learning for precision medicine. Genome. 2021;64:416-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 225] [Article Influence: 37.5] [Reference Citation Analysis (80)] |
| 6. | Haug CJ, Drazen JM. Artificial Intelligence and Machine Learning in Clinical Medicine, 2023. N Engl J Med. 2023;388:1201-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 641] [Article Influence: 213.7] [Reference Citation Analysis (1)] |
| 7. | Li XJ, Wu D, Ding XM. Research progress on risk prevention and prediction model of intensive care unit acquired weakness. Zhonghua Xiandai Huli Za Zhi. 2022;28:269-275. [DOI] [Full Text] |
| 8. | Liu Y, Liu S, Wang Y, Lombardi F, Han J. A Survey of Stochastic Computing Neural Networks for Machine Learning Applications. IEEE Trans Neural Netw Learn Syst. 2021;32:2809-2824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Zhu LW, Zhang HF. Analysis of high-risk factors for acquired weakness in ICU and exploration of related nursing interventions. Hangkong Hangtian Yixue Za Zhi. 2021;32:985-987. [DOI] [Full Text] |
| 10. | Yang Z, Wang X, Chang G, Cao Q, Wang F, Peng Z, Fan Y. Development and validation of an intensive care unit acquired weakness prediction model: A cohort study. Front Med (Lausanne). 2023;10:1122936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 11. | van Wagenberg L, Witteveen E, Wieske L, Horn J. Causes of Mortality in ICU-Acquired Weakness. J Intensive Care Med. 2020;35:293-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Needham DM, Dinglas VD, Morris PE, Jackson JC, Hough CL, Mendez-Tellez PA, Wozniak AW, Colantuoni E, Ely EW, Rice TW, Hopkins RO; NIH NHLBI ARDS Network. Physical and cognitive performance of patients with acute lung injury 1 year after initial trophic vs full enteral feeding. EDEN trial follow-up. Am J Respir Crit Care Med. 2013;188:567-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 13. | Friedrich O, Reid MB, Van den Berghe G, Vanhorebeek I, Hermans G, Rich MM, Larsson L. The Sick and the Weak: Neuropathies/Myopathies in the Critically Ill. Physiol Rev. 2015;95:1025-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 264] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 14. | Liu Y, Luo J, Xie L, Liu M, Zhou XT, Ding YH. Systematic evaluation of ICU acquired weakness risk prediction model. Zhonghua Xiandai Huli Za Zhi. 2020;26:4769-4774. [DOI] [Full Text] |
| 15. | Zhang W, Tang Y, Liu H, Yuan LP, Wang CC, Chen SF, Huang J, Xiao XY. Risk prediction models for intensive care unit-acquired weakness in intensive care unit patients: A systematic review. PLoS One. 2021;16:e0257768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Yang T, Li Z, Jiang L, Wang Y, Xi X. Risk factors for intensive care unit-acquired weakness: A systematic review and meta-analysis. Acta Neurol Scand. 2018;138:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 17. | Wang W, Xu C, Ma X, Zhang X, Xie P. Intensive Care Unit-Acquired Weakness: A Review of Recent Progress With a Look Toward the Future. Front Med (Lausanne). 2020;7:559789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 18. | Montejo González JC, Sánchez-Bayton Griffith M, Orejón García L. [Muscle in critically ill patients]. Nutr Hosp. 2019;36:12-17. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Raurell-Torredà M, Arias-Rivera S, Martí JD, Frade-Mera MJ, Zaragoza-García I, Gallart E, Velasco-Sanz TR, San José-Arribas A, Blazquez-Martínez E; MOviPre group. Care and treatments related to intensive care unit-acquired muscle weakness: A cohort study. Aust Crit Care. 2021;34:435-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Wolfe KS, Patel BK, MacKenzie EL, Giovanni SP, Pohlman AS, Churpek MM, Hall JB, Kress JP. Impact of Vasoactive Medications on ICU-Acquired Weakness in Mechanically Ventilated Patients. Chest. 2018;154:781-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Ai MH, Lin SC, Yang CB. Risk Factors Analysis and Prediction Model Establishment of ICU-acquired Weakness. Zhongguo Weisheng Biaozhun Guanli. 2022;13: 38-41. [DOI] [Full Text] |
| 22. | Nakahara S, Takasaki M, Abe S, Kakitani C, Nishioka S, Wakabayashi H, Maeda K. Aggressive nutrition therapy in malnutrition and sarcopenia. Nutrition. 2021;84:111109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | Bellaver P, Schaeffer AF, Leitao CB, Rech TH, Nedel WL. Association between neuromuscular blocking agents and the development of intensive care unit-acquired weakness (ICU-AW): A systematic review with meta-analysis and trial sequential analysis. Anaesth Crit Care Pain Med. 2023;42:101202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ewers A, Austria S-Editor: Zhang H L-Editor: A P-Editor: Li X