Published online Mar 6, 2024. doi: 10.12998/wjcc.v12.i7.1200
Peer-review started: October 18, 2023
First decision: January 2, 2024
Revised: January 10, 2024
Accepted: February 5, 2024
Article in press: February 5, 2024
Published online: March 6, 2024
Processing time: 133 Days and 18.1 Hours
Hyperparathyroidism (HPT) is a condition in which one or more parathyroid glands produce increased levels of parathyroid hormone (PTH), causing distur
Core Tip: Clinicians should consider a brown tumor in hyperparathyroidism as a differential diagnosis of lytic bone lesions, after excluding more common causes such as metastatic carcinoma or multiple myeloma. A wide number of specialties should be aware of signs and symptoms of a brown tumor in hyperparathyroidism, including internal medicine specialists, orthopaedists, and radiologists, while dentists and oral surgeons should be aware of oral manifestations of systemic diseases.
- Citation: Majic Tengg A, Cigrovski Berkovic M, Zajc I, Salaric I, Müller D, Markota I. Expect the unexpected: Brown tumor of the mandible as the first manifestation of primary hyperparathyroidism. World J Clin Cases 2024; 12(7): 1200-1204
- URL: https://www.wjgnet.com/2307-8960/full/v12/i7/1200.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i7.1200
Hyperparathyroidism (HPT) is a condition in which one or more parathyroid glands produce increased levels of parathyroid hormone (PTH), causing disturbances in calcium homeostasis. HPT can be characterized as primary, secondary, or tertiary. Primary HPT (PHPT) is caused by one or more overactive parathyroid glands due to parathyroid adenoma or hyperplasia, and less commonly, parathyroid carcinoma. Secondary HPT occurs as a response of a parathyroid gland to the reduced calcium level, for example in patients with renal insufficiency or vitamin D deficiency. Eventually, this can lead to autonomous hyperfunction of parathyroid glands, independent of the underlying disease, causing tertiary HPT[1].
The signs and symptoms commonly associated with PHPT are a result of both elevated PTH levels and hypercalcemia. The most frequent clinical manifestation of PHPT is asymptomatic hypercalcemia, which is typically detected through routine biochemical screening. In most cases, asymptomatic patients have a serum calcium concentration slightly above the upper limit of the normal range, typically less than 0.25 mmol/L (1.0 mg/dL). However, atypical presentations are also possible, encompassing a range of disruptions in calcium homeostasis that arise from the combined effects of increased PTH secretion and hypercalcemia[1].
Osteitis fibrosa cystica is a manifestation of PHPT bone disease due to PTH-related activation of osteoclasts and bone resorption. One of the manifestations of osteitis fibrosa cystica is a brown tumor of the bone caused by bone marrow being replaced with osteoclast-like giant cells and fibrous tissue[2]. Osteitis fibrosa cystica is rare and occurs more often in patients with uncontrolled disease.
The brown tumor is a rare, benign, tumor-like lesion of bone, first described by Henry Jaffe in 1942[3]. Its presence is an uncommon complication of uncontrolled HPT, representing the terminal stage of a bone remodeling process and is usually the sign of a poorly controlled disease pathway. The incidence reported in PHPT is 1%-3%, being more common in developing countries. When faced with insufficient access to medical care and screening programs, the incidence can reach up to 15%[3]. Due to improved screening techniques for PHPT, most cases of PHPT in developed countries are detected before a brown tumor appears, consequently, it is rare to diagnose brown tumor as the initial manifestation in PHPT, before the onset of systemic manifestations.
Brown tumors can occur as solitary or multifocal lesions and are most commonly found in the ribs, clavicles, pelvic girdle, extremities, and facial bones such as the maxilla, mandible, and hard palate[4]. A brown tumor can present as an asymptomatic swelling or a painful exophytic mass. Furthermore, it can cause a pathological fracture or skeletal pain and be radiologically described as a lytic bone lesion[5]. Consequently, the brown tumor can be mistaken for a metastatic carcinoma or multiple myeloma.
The diagnosis of a brown tumor in HPT is typically confirmed by assessing the levels of serum calcium, phosphorus, and PTH. There are no pathognomonic histological features for the brown tumor, consequently, histology is often insufficient for confirming the diagnosis. The brown color, seen in a pathohistological analysis, is caused by an excess osteoclast activity, due to a massive secretion of PTH, intralesional haemorrhage and hemosiderin deposition. A histological diagnosis of a giant cell tumor is often made; however, it may be a case of a brown tumor, thereby delaying the correct diagnosis and appropriate treatment[5].
Treatment of a brown tumor is often directed to the management of the underlying HPT, including partial or complete parathyroidectomy, which frequently results in spontaneous regression and resolution of these lesions without surgical intervention. However, when necessary, surgical treatment is a therapeutic choice in refractory cases, including extensive cortical involvement or in large symptomatic lesions, often causing pathologic fractures. A few case reports were published, reporting brown tumors failing to resolve after a parathyroidectomy, thus requiring additional surgical treatment[6-15].
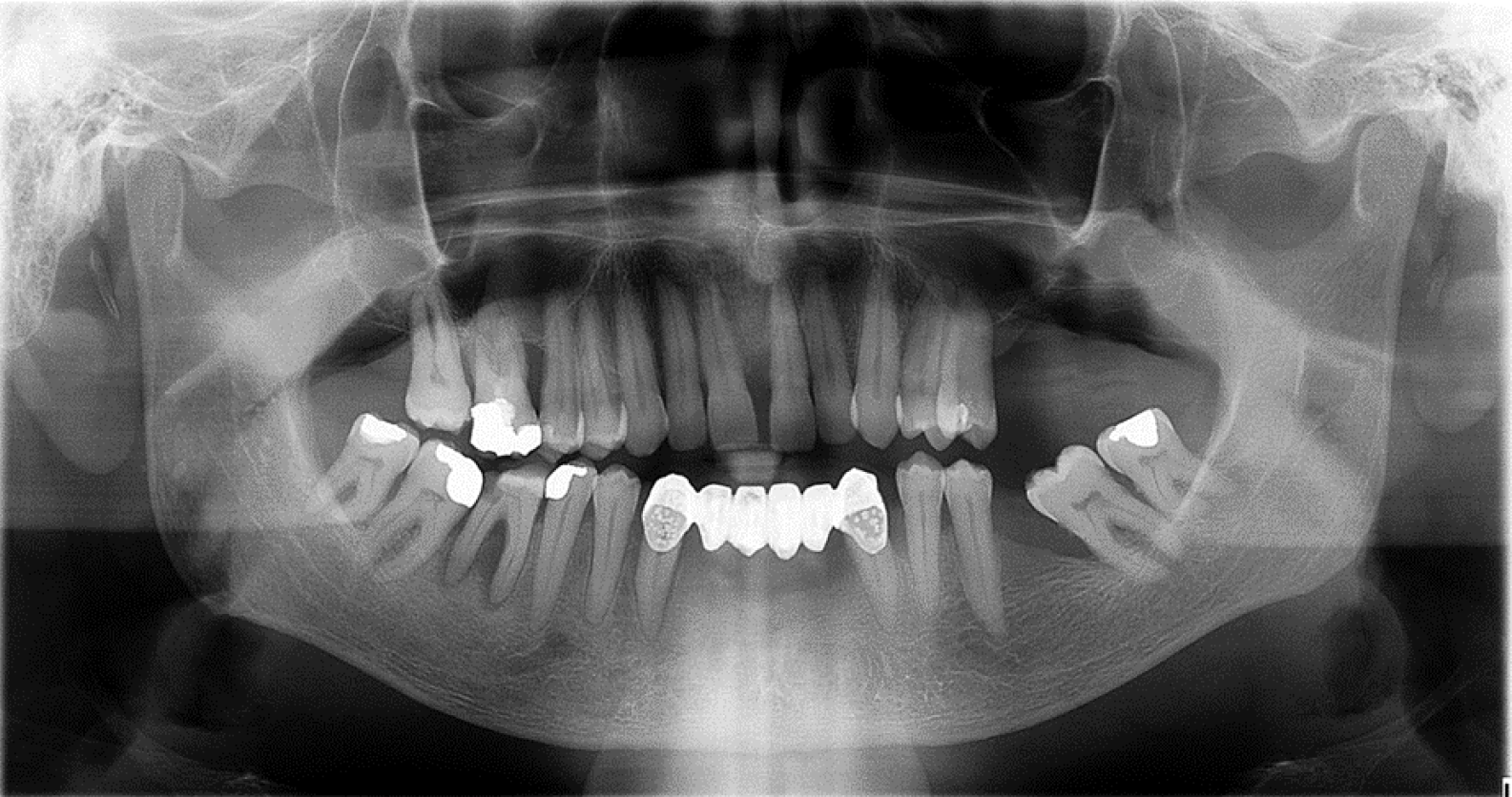
A 67-year-old male patient was initially referred to an oral surgeon due to a mass and persistent bleeding in the right mandible, which had been present for about one year (Figure 1). The patient's medical history included an occurrence of recurring nephrolithiasis.
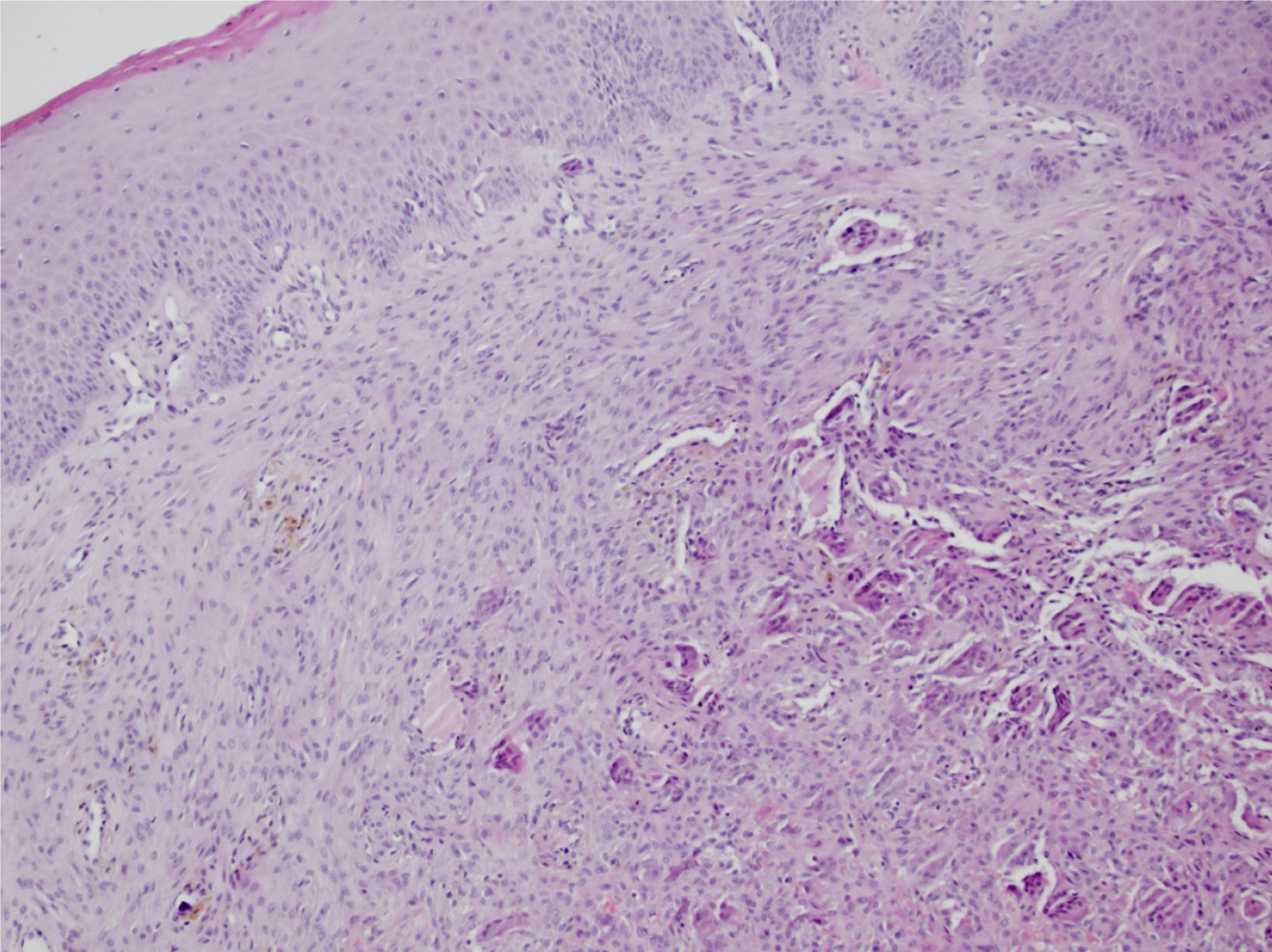
A surgical procedure was conducted to obtain an incisional biopsy of the mass. Histologically, there was a regular stratified squamous epithelium seen on the surface. In the underlying connective tissue stroma, clusters of spindle-shaped and oval cells with numerous giant cells resembling osteoclasts, as well as extravasated erythrocytes with hemosiderotic pigment, were described. No elements of bone trabeculae were detected in the examined sections. The histologic findings were consistent with a diagnosis of a peripheral giant cell tumor/epulis (Figure 2).
Although the histologic diagnosis was made, the skilled oral surgeon suspected a brown tumor in HPT. As a result, the patient was referred to an endocrinologist for further evaluation. Laboratory results are shown in the Table 1.
| Patient’s laboratory values | Normal range |
| Total calcium level = 2.94 mmol/L | 2.14-2.53 mmol/L |
| Ionised calcium level = 1.61 mmol/L | 1.18-1.32 mmol/L |
| Phosphorus level = 0.66 mmol/L | 0.79-1.42 mmol/L |
| Daily urine calcium level = 11.69 mmol/dU | < 7.9 mmol/dU |
| PTH level = 44.02 pmol/L | 1.59-7.24 pmol/L |
| 25 OH vitamin D level = 27.97 nmol/L | |
| Creatinine level = 66 μmol/L | 64-104 μmol/L |
| Estimated glomerular filtration rate (CKD-EPI formula) = 102.8 mL/min/1.73 m2 |
The patient's renal function was normal, with a creatinine level of 66 µmol/L (normal range: 64-104) and an estimated glomerular filtration rate [using Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula] of 102.8 mL/min/1.73 m2 when diagnosed. A kidney ultrasound revealed mild bilateral nephrocalcinosis without nephrolithiasis. Additionally, a bone density test indicated the presence of osteoporosis. The patient had no complaints of bone pain, headache, abdominal pain, nausea, vomiting, or any changes in intestinal transit.
A neck ultrasound revealed a solitary lesion in the left inferior parathyroid gland. Further imaging, using the neck and thorax scintigraphy with 99mTc-MIBI, showed abnormal uptake of radiopharmaceuticals behind the left thyroid lobe. The finding was indicative of a parathyroid adenoma.
The patient underwent a left inferior parathyroidectomy, and the pathological examination confirmed the diagnosis of a parathyroid adenoma. After one year of follow-up, the patient's laboratory results of interest returned to normal. Additionally, the patient had an exophytic mandible mass that required surgical intervention by an oral surgeon due to slow bone healing.
In conclusion, the brown tumor can be the initial manifestation of uncontrolled HPT and is frequently histologically misdiagnosed as a giant cell tumor. When evaluating patients with osteolytic bone lesions, it is important to consider brown tumor as a potential differential diagnosis, after excluding more common diagnoses such as multiple myeloma or metastatic carcinoma. Establishing the diagnosis of brown tumor requires a high level of suspicion, and measuring serum calcium, phosphorus, and parathyroid hormone levels can be valuable and widely accessible diagnostic tools. A wide number of specialties should be aware of signs and symptoms of brown tumor in HPT, including internal medicine specialists, orthopaedists, radiologists, and oral surgeons.
| 1. | Melmed S, Polonsky KS, Reed Larsen P, Kronenberg HM. Williams Textbook of Endocrinology. 13th ed. Canada: Elsevier; 2016. |
| 2. | Naji Rad S, Anastasopoulou C, Deluxe L. Osteitis Fibrosa Cystica. StatPearls [Internet] 2023 Jan [accessed: 25-Sep-2023]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559097/. |
| 3. | Jaffe HL, Lichtenstein L. Benign Chondroblastoma of Bone: A Reinterpretation of the So-Called Calcifying or Chondromatous Giant Cell Tumor. Am J Pathol. 1942;18:969-991. [PubMed] |
| 4. | Ngo QX, Ngo DQ, Tran TD, Le DT, Hoang GN, Le QV. Multiple brown tumors with primary hyperparathyroidism mimicking bone metastases. Int J Surg Case Rep. 2021;79:375-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Brabyn P, Capote A, Belloti M, Zylberberg I. Hyperparathyroidism Diagnosed Due to Brown Tumors of the Jaw: A Case Report and Literature Review. J Oral Maxillofac Surg. 2017;75:2162-2169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Qaisi M, Loeb M, Montague L, Caloss R. Mandibular Brown Tumor of Secondary Hyperparathyroidism Requiring Extensive Resection: A Forgotten Entity in the Developed World? Case Rep Med. 2015;2015:567543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Azria A, Beaudreuil J, Juquel JP, Quillard A, Bardin T. Brown tumor of the spine revealing secondary hyperparathyroidism. Report of a case. Joint Bone Spine. 2000;67:230-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Panagopoulos A, Tatani I, Kourea HP, Kokkalis ZT, Panagopoulos K, Megas P. Osteolytic lesions (brown tumors) of primary hyperparathyroidism misdiagnosed as multifocal giant cell tumor of the distal ulna and radius: a case report. J Med Case Rep. 2018;12:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Bilezikian JP, Silverberg SJ, Shane E, Parisien M, Dempster DW. Characterization and evaluation of asymptomatic primary hyperparathyroidism. J Bone Miner Res. 1991;6 Suppl 2:S85-9; discussion S121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Tarello F, Ottone S, De Gioanni PP, Berrone S. [Brown tumor of the jaws]. Minerva Stomatol. 1996;45:465-470. [PubMed] |
| 11. | Xu W, Qu Y, Shi W, Ma B, Jiang H, Wang Y, Qu N, Zhu Y. Multiple bone brown tumor secondary to primary hyperparathyroidism: a case report and literature review. Gland Surg. 2019;8:810-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Triantafillidou K, Zouloumis L, Karakinaris G, Kalimeras E, Iordanidis F. Brown tumors of the jaws associated with primary or secondary hyperparathyroidism. A clinical study and review of the literature. Am J Otolaryngol. 2006;27:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Can Ö, Boynueğri B, Gökçe AM, Özdemir E, Ferhatoğlu F, Canbakan M, Şahin GM, Titiz Mİ, Apaydın S. Brown Tumors: A Case Report and Review of the Literature. Case Rep Nephrol Dial. 2016;6:46-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Shetty AD, Namitha J, James L. Brown tumor of mandible in association with primary hyperparathyroidism: a case report. J Int Oral Health. 2015;7:50-52. [PubMed] |
| 15. | Xu B, Yu J, Lu Y, Han B. Primary hyperparathyroidism presenting as a brown tumor in the mandible: a case report. BMC Endocr Disord. 2020;20:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed by the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer-reviewed.
Peer-review model: Single-blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jin S, China S-Editor: Liu JH L-Editor: A P-Editor: Zhao S