Published online Feb 26, 2024. doi: 10.12998/wjcc.v12.i6.1063
Peer-review started: October 26, 2023
First decision: December 15, 2023
Revised: December 21, 2023
Accepted: January 29, 2024
Article in press: January 29, 2024
Published online: February 26, 2024
Processing time: 117 Days and 0.5 Hours
Alzheimer’s disease (AD) is a serious disease causing human dementia and social problems. The quality of life and prognosis of AD patients have attracted much attention. The role of chronic immune inflammation in the pathogenesis of AD is becoming more and more important.
To study the relationship among cognitive dysfunction, abnormal cellular im
A retrospective analysis of 62 hospitalized patients clinical diagnosed with AD who were admitted to our hospital from November 2015 to November 2020. Co
Univariate analysis showed that abnormal cellular immune function, extrapyramidal symptoms, obvious dis
The decrease in the proportion of T lymphocytes may have predictive value for the poor prognosis of AD. It is recommended that the proportion of T lymphocytes < 55% is used as the cut-off threshold for predicting the poor prog
Core Tip: Abnormal cellular immune function, extrapyramidal symptoms, abnormal electroencephalogram, increased neutrophils and lymphocyte ratio, abnormal magnetic spectroscopy, and complicated pneumonia were related to the poor prognosis of Alzheimer’s disease (AD) patients. The decrease in the proportion of T lymphocytes in the blood after abnormal cellular immune function was an independent risk factor for predicting the poor prognosis of AD. The number of days of donepezil treatment to improve cognitive function was negatively correlated with modified Rankin scale score. The decrease in the proportion of T lymphocytes may have predictive value for the poor prognosis of AD.
- Citation: Bai H, Zeng HM, Zhang QF, Hu YZ, Deng FF. Correlative factors of poor prognosis and abnormal cellular immune function in patients with Alzheimer’s disease. World J Clin Cases 2024; 12(6): 1063-1075
- URL: https://www.wjgnet.com/2307-8960/full/v12/i6/1063.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i6.1063
Alzheimer’s disease (AD) is a neurodegenerative disease with severe cognitive dysfunction. The prominent clinical manifestations are memory loss, confusion of thinking and logic, and abnormal mental behavior. It accounts for about 40%-60% of dementia patients[1,2]. At present, it is also inclined to think that AD is a chronic inflammatory disease mediated by abnormal autoimmune function. Mononuclear RNA sequencing and transcriptomics analysis show that the abnormal changes in microglia in the brain of AD patients induce a series of abnormal immune function. The activation of abnormal inflammasome represented by nucleotide-binding domain leucine-rich repeat and pyrin domain containing receptor protein 3 (NLRP3) inflammasome mediates the secretion of many immune inflammatory factors and subsequent cascades of chronic cascades reactions in immune inflammation[3,4]. The amyloid β (Aβ) peptide produced by abnormal neurons precipitates and aggregates outside the cell. The hyperphosphorylation of tau protein can also easily cause aggregation, leading to neuron and nerve synaptic dysfunction and cell death, especially small glial cells. Reactive proliferation of glial cells often causes secondary cytopathological reactions in diseased brain regions[5]. The activation of NLRP3 inflammasome promotes the aggregation of Aβ protein and the pathological formation of AD. The activation of NLRP3 inflammasome also contributes to the phosphorylation of tau protein and the accelerated development of AD. The interaction between Aβ and tau protein promotes the progression of AD. The onset and development of AD are usually mediated by abnormal immune function[6-8].
At present, the diagnostic criteria of AD mostly depend on the screening of cognitive function scale and the exclusion of similar diseases. Although there are some biochemical markers of dementia in serum or cerebrospinal fluid (CSF), their specificity and sensitivity are not high[9,10]. Combining some biochemical markers in blood or CSF for early diagnosis of AD may be a direction of future efforts, among which some biochemical markers related to immunity have great research prospects. Some scholars have combined the detection results of magnetic resonance spectroscopy (MRS) with blood biochemical markers and achieved good results[11]. On the other hand, the research on the factors affecting the prognosis of AD also has important clinical and social significance. Some AD patients may have a long life, but whether this lon
The role of chronic immune inflammation in the pathogenesis of AD is becoming more and more important. The ratio of neutrophil to lymphocyte (NLR) in blood is an important systemic inflammatory biomarker. NLR is calculated by ab
This retrospective case study was reviewed and approved by the Medical Ethics Committee of the Third Affiliated Hospital of Guizhou Medical University in China. AD patients and their families hospitalized in the Department of Neurology and Psychiatry of the Third Affiliated Hospital of Guizhou Medical University were told to participate in the study and signed an informed consent form in accordance with the Declaration of Helsinki. The researchers checked the electronic medical records of 229 patients initially diagnosed with various types of dementia. These cases were patients who were discharged from the hospital between November 2015 and November 2020. The researchers re-evaluated the basis for the diagnosis of dementia in these cases, first confirmed or ruled out dementia through the Mini Mental State Examination Scale (MMSE) and the Cognitive Function Screening Scale, and then based on the medical history, clinical manifestations, and laboratory test results. In the diagnosis of AD, pay special attention to the use of the Harkinski Ischemic Scale to identify AD. Excluded 14 patients with incomplete data and 7 patients lacking the basis for the diag
The 68 patients in this retrospective study are all clinically diagnosed AD patients. The 68 AD patients who met the needs of this study were selected for follow-up. After the patients are discharged from the hospital, they will be followed up and followed up by family members or guardians by telephone every 3 months. The prognosis will be assessed after detailed inquiries, and semi-quantitative according to the classic scale.
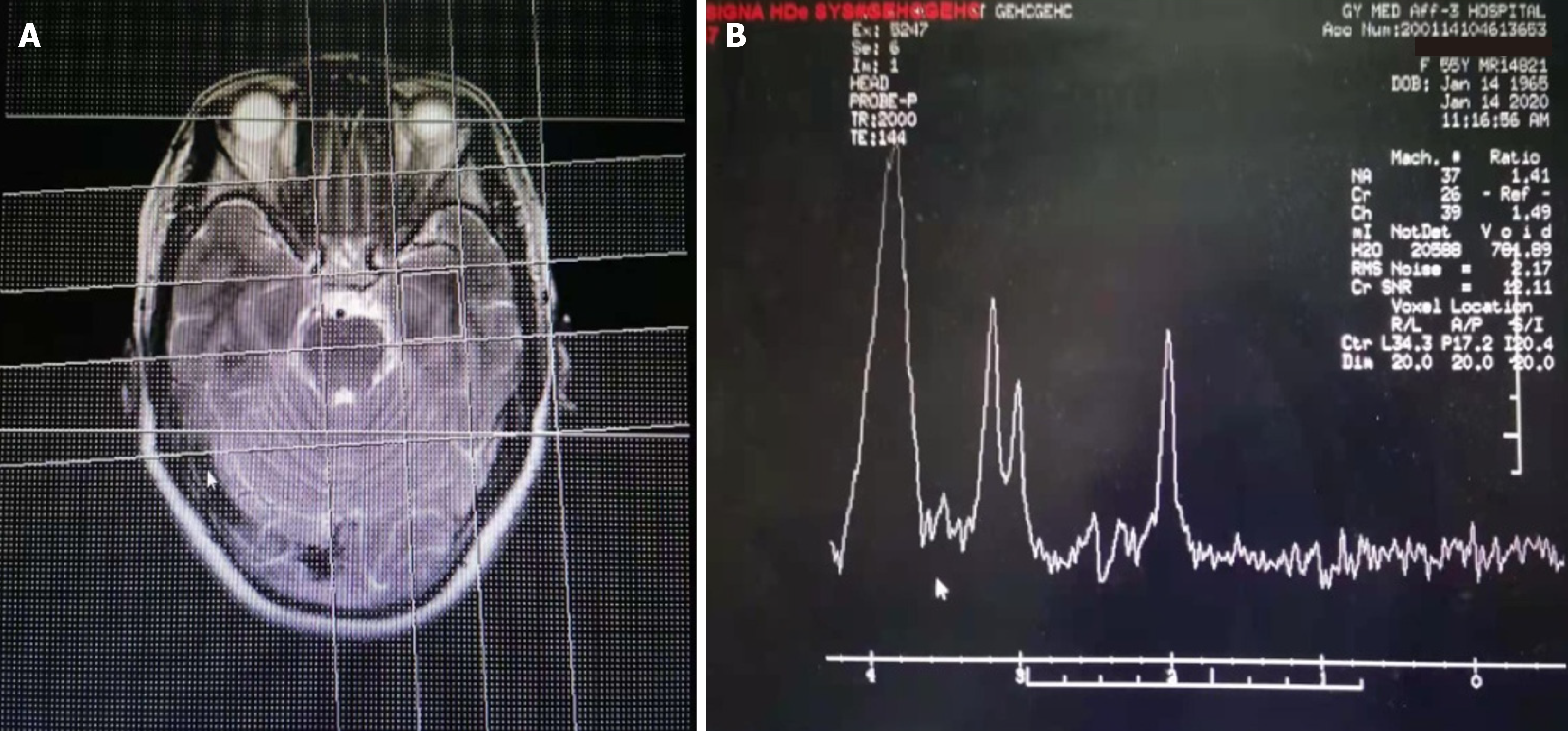
Collect the following medical history and clinical data: Age of onset, gender, chief complaint, duration of disease, first symptoms, other symptoms, main positive signs, cranial magnetic resonance imaging (MRI), cranial MRS, EEG, blood routine, blood immunity Results of cell examination and drug treatment. The main metabolites detected by MRS include N-acetylaspartate (NAA), creatine (Cr), choline (Cho), inositol (MI), etc. NAA/Cr ratio and MI/Cr ratio were collected as key analysis indicators. Regarding EEG data, it is mainly to pay attention to the abnormal β wave and slow wave (θ wave and δ wave), especially the ratio of (θ + δ) to (α + β) in the whole brain [(θ + δ)/(α + β)]. We also pay attention to the ratio of neutrophils to lymphocytes (NLR) in the blood. The percentage values of T lymphocytes, B lymphocytes, and natural killer (NK) cells detected by flow cytometry are also collected. As the value of Aβ protein and tau protein in the blood in the diagnosis of AD is controversial sometimes, this study was not collected. The decrease of Aβ42 protein in the CSF and the increase of phosphorylated tau protein do have certain value in the diagnosis of AD, but there are many lacks of data in this group of cases, and they have not been collected. In addition, we collected MMSE score data and cognitive func
The modified rankin scale (mRS) was used to assess neurological function at admission, discharge, and follow-up. There are 6 grades of mRS score: 0 score is for full recovery; a score of 1 score is defined as having no apparent dysfunction or being able to perform daily life and work tasks despite symptoms; 2 score is mild disability, but basically able to complete daily life and work tasks independently; 3 score is moderate disability, unable to complete all previous activities, difficult to handle own affairs independently; 4 score is severely disabled and needs to be cared for by someone else; 5 score is severe disability who require intensive care by medical staff; and 6 score is defined as death case.
According to the mRS during the follow-up period, all patients were divided into two groups: Those with mRS score of 0-2 scores were defined as “good prognosis”; 3-6 scores was defined as “poor prognosis”.
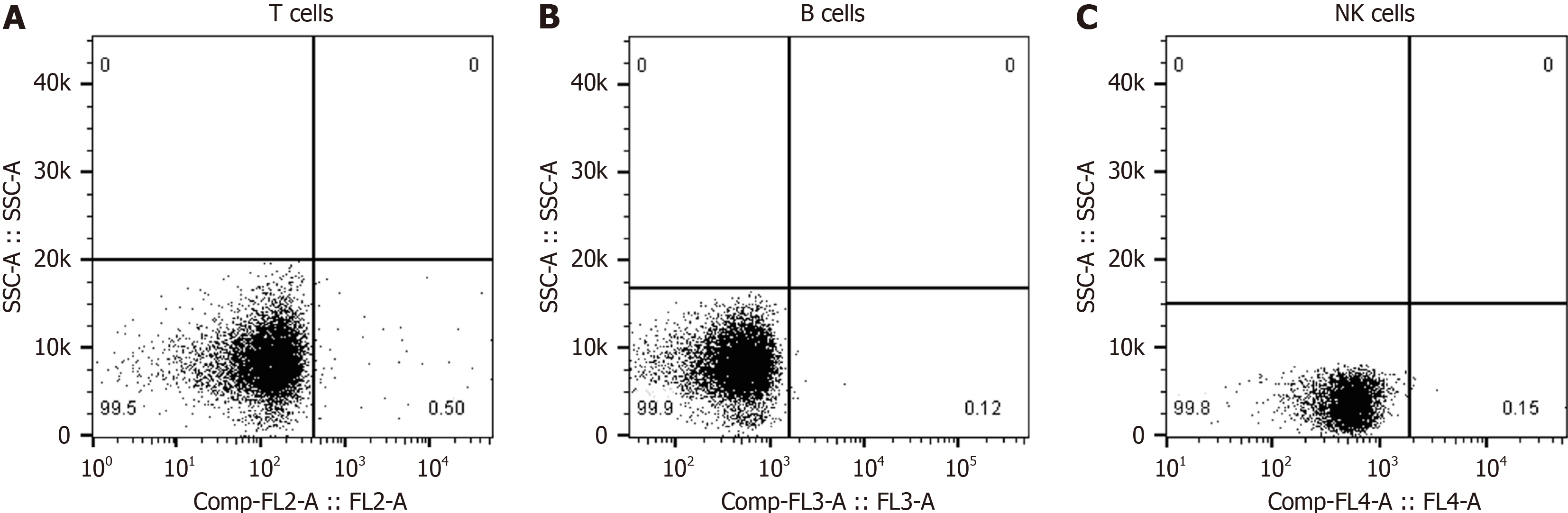
The FC500 automatic flow cytometer was used to perform the detection by direct immunofluorescence. The percentage of quantitative counts of T lymphocytes, B lymphocytes, and NK cells in the blood of patients was measured at one time. FITC-labeled anti-CD mAbs and normal mouse IgG were prepared. Cell wash with 2% BSA, 0.1% NaN and PBS. The fixative was prepared to a volume of 100 mL with 25% glutaraldehyde 3.2 mL, 2 g glucose, and BSA-free cell wash. De
SPSS software was used for statistical analysis (version 17.0). The data collected are expressed as mean ± SD or median (range). Count data is expressed as a ratio or percentage. Univariate correlation analysis was used to compare the di
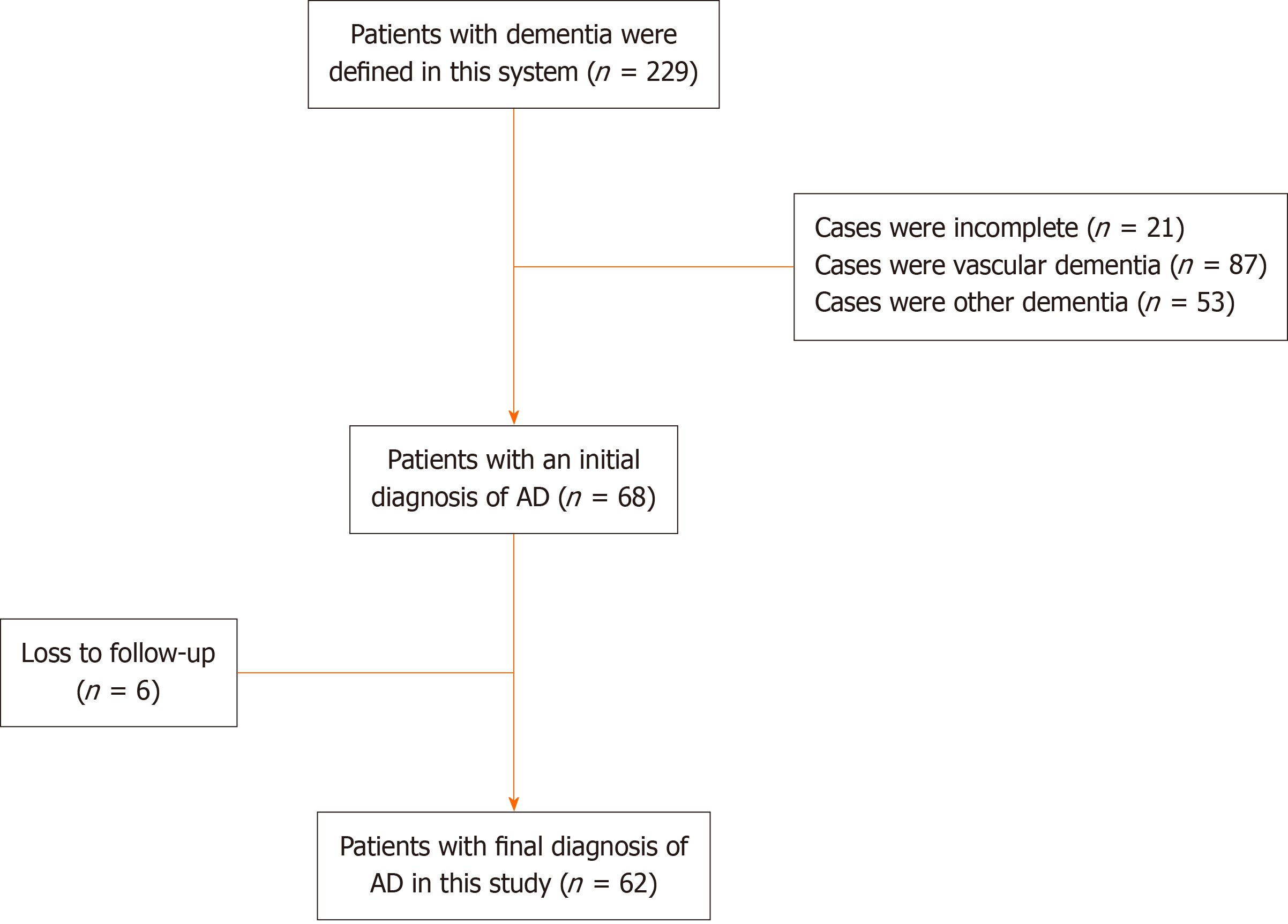
Through the electronic medical records database of the inpatient department and medical record room of the hospital, 229 cases of patients with clinical diagnosis of single or combined dementia were collected, including 87 cases of VD, 68 cases of AD, and 53 cases of other dementia. These patients with other types of dementia included 3 cases of frontotemporal dementia, 2 cases of Lewy body dementia, 5 cases of Parkinson’s disease dementia, 10 cases of chronic alcoholism de
The 62 patients in this follow-up study were 55 to 92 years old (71.0 years old ± 8.7 years old), 24 males and 38 females. All of them had chronic insidious onset and were sent to the hospital for treatment after they were found to have abnormal symptoms by their families. These patients had no history of major mental trauma, no history of head trauma, no history of drug abuse and toxicosis. The first symptoms were as follows: 19 cases (30.6%) of memory loss, 15 cases (24.2%) of personality changes, and 28 cases (45.1%) of abnormal mental behavior. The time from onset to hospital ad
| Characteristics | Patients |
| Sex (male/female) | 24/38 |
| Age, mean, range (yr) | 71 (55-92) |
| Interval between onset and hospitalization | 87, 5-547 |
| Mean, range (d) | |
| Initial symptom | |
| Hypomnesis, n (%) | 19 (30.6) |
| Apparent personality change | 15 (24.2) |
| Abnormal mental behavior | 28 (45.1) |
| Personality abnormality, n (%) | 34 (54.8) |
| Recent memory deficits, n (%) | 54 (87.1) |
| Hallucination, n (%) | 26 (41.9) |
| Delusion of victimization, n (%) | 19 (30.6) |
| Disturbance of consciousness, n (%) | 10 (16.1) |
| Depressed, n (%) | 20 (32.2) |
| Abnormal EEG results, n (%) | 48 (77.4) |
| (θ + δ)/(α + β) more than 1.8, n (%) | 42 (67.7) |
| Abnormal brain MRI results, n (%) | 52 (83.9) |
| Encephalatrophy, n (%) | 43 (69.4) |
| Abnormality of hippocampus, n (%) | 36 (58.1) |
| Ratio of NAA/Cr decreased, n (%) | 59 (95.2) |
| NLR (median interquartile) | 2.25 (1.59-2.97) |
| Proportion of T lymphocytes in blood | 0.55 (0.47-0.63) |
| Proportion of B lymphocytes in blood | 0.13 (0.09-0.18) |
| Proportion of NK cell in blood | 0.11 (0.07-0.17) |
The data of Cranial MRI, MRS, EEG, blood routine, and blood immune cell tests were available for all patients. There were 52 cases with abnormal brain MRI, including 43 (69.4%) with brain atrophy, 22 (35.5%) with demyelinating lesions around the ventricle (white matter osteoporosis), and 36 (25.8%) with abnormal T2 signals in the hippocampus (Figure 2). There were 60 cases with abnormal brain MRS, including 59 cases with decreased NAA/Cr (95.1%) and 56 cases with increased MI/Cr (90.3%). There were 48 cases (77.4%) with abnormal EEG examination, including 18 patients with high-amplitude β waves, 17 patients with more theta waves, 13 patients with δ waves, and the ratio of (θ + δ)/(α + β) is greater than 1.8 in 42 patients. Among the AD patients in this study, only 5 patients had mild abnormalities in routine blood tests, while the NLR value exceeded 4.5 in 20 patients and exceeded 4.0 in 46 patients. Blood immune cell examination found abnormal in 38 cases, mainly the proportion of T lymphocytes or NK cells decreased. The proportion of T lympho
All patients were treated with medications, mainly medications that may improve cognitive function. Among them, 48 patients were treated with Donepezil (5-10 mg/d), 7 patients were treated with nicergoline, 5 patients were treated with Galantamine. Huperzine A was used in 2 cases. In addition, piracetam, oxiracetam, adenosine triphosphate, coenzyme Q10, vitamin E and other drugs were used for treatment. Risperidone, or olanzapine, or clozapine was used at the same time to control mental symptoms. All patients were not treated with transcranial magnetic therapy, acupuncture therapy, music therapy, psychotherapy, and other treatment methods. The average follow-up time is 10 months (6-24 months). At the end of the follow-up, 16 patients (25.8%) had a good prognosis, 19 patients (30.6%) had a moderate prognosis, and 27 patients (43.6%) had a poor prognosis. mRS score: 5 points for 3 cases, 4 points for 7 cases, 3 points for 4 cases, 2 points for 17 cases, 1 point for 23 cases, and 0 points for 8 cases. Among them, 28 cases were treated with risperidone alone to control their psychiatric symptoms, and the follow-up mRS score was 1-5 (3.00 ± 0.72) points. Four patients died during the follow-up period, and 40 patients were hospitalized again during the follow-up period.
Univariate analysis showed that there were significant differences in five indexes in the corresponding auxiliary exa
| Variables | Good prognosis (n = 27) | Poor prognosis (n = 35) | P value |
| Age (yr) | 71.2 ± 15.3 | 70.6 ± 16.7 | 0.829 |
| Sex, n (%) | |||
| Male | 11 (40.7) | 15 (42.9) | 0.867 |
| Female | 16 (59.3) | 20 (57.1) | |
| Duration from onset to admission, n (%) | |||
| < 90 d | 18 (66.7) | 22 (62.9) | 0.755 |
| ≥ 90 d | 9 (33.3) | 13 (37.1) | |
| Personality abnormality | |||
| Yes | 15 | 19 | 0.092 |
| No | 12 | 16 | |
| Recent memory deficits | |||
| Yes | 22 | 32 | 0.247 |
| No | 5 | 3 | |
| Hallucination | |||
| Yes | 7 | 19 | 0.025 |
| No | 20 | 16 | |
| Delusion of victimization | |||
| Yes | 5 | 14 | 0.069 |
| No | 22 | 21 | |
| Disturbance of consciousness | |||
| Yes | 2 | 8 | 0.101 |
| No | 25 | 27 | |
| Depressed | |||
| Yes | 8 | 12 | 0.698 |
| No | 19 | 23 | |
| Abnormal EEG results | |||
| Yes | 16 | 32 | 0.003 |
| No | 11 | 3 | |
| (θ + δ)/(α + β) from EEG | |||
| ≥ 1.8 | 14 | 28 | 0.019 |
| < 1.8 | 13 | 7 | |
| Abnormal brain MRI results | |||
| Yes | 24 | 28 | 0.346 |
| No | 3 | 7 | |
| Encephalatrophy | |||
| Yes | 20 | 23 | 0.479 |
| No | 7 | 12 | |
| Abnormality of hippocampus | |||
| Yes | 8 | 28 | 0.001 |
| No | 19 | 7 | |
| NAA/Cr ratio decreased | |||
| Yes | 25 | 34 | 0.408 |
| No | 2 | 1 | |
| NLR (median IQR) | 2.19 (1.51-2.87) | 2.34 (1.62-3.28) | 0.379 |
| Proportion of T lymphocytes in blood | 0.63 (0.38-0.77) | 0.37 (0.24-0.49) | 0.008 |
| Proportion of B lymphocytes in blood | 0.11 (0.08-0.18) | 0.14 (0.10-0.23) | 0.282 |
| Proportion of NK cell in blood | 0.15 (0.08-0.19) | 0.10 (0.06-0.16) | 0.075 |
| Variables | OR | 95%CI | P value |
| Hallucination | 2.961 | 0.265-18.397 | 0.723 |
| Abnormal EEG results | 1.983 | 0.079-7.531 | 0.682 |
| Abnormal brain MRI results | 12.369 | 0.592-39.127 | 0.849 |
| Abnormality of hippocampus | 5.394 | 0.275-78.364 | 0.231 |
| NAA/Cr ratio decreased | 1.398 | 0.056-135.284 | 0.816 |
| Proportion of T lymphocytes in blood | 3.265 | 1.156-5.681 | 0.038 |
The NLR ratio in blood, the severity of memory impairment and the time of drug treatment had no significant cor
In this project, we retrospectively studied the subsequent prognosis of patients initially diagnosed with AD. We analyzed the clinical features, blood examination results, imaging data, EEG results and flow cytometry results. It also focused on the factors that are closely related to the poor prognosis. This study showed that the median T lymphocyte percentage in the poor prognosis group was significantly lower than that in the good prognosis group. The percentage of T lympho
MRS is an imaging technique that uses the principle of magnetic resonance and chemical shift phenomena to perform imaging and quantitative analysis of specific nuclei and related compounds[26,27]. In the normal human brain, there are 5 resonance spectrum peaks in the MRS examination: NAA peak, Cho peak, Cr peak, inositol peak, and glutamate peak. The decrease in NAA peak can be used as a sign of neuron loss or damage in the brain. The content of Cr in the gray matter of the brain is higher than that of the white matter, and it is a high energy phosphoric acid reserve substance for ATP/ADP conversion[28]. This research found that the NAA/Cr ratio of the AD poor prognosis group was significantly lower than the NAA/Cr ratio of the good prognosis group. The decrease in NAA/Cr ratio indicates that there is more loss of bilateral hippocampal neurons, which can be used as a biomarker for the transition from mild cognitive im
EEG examination is mainly used for differential diagnosis of epilepsy, as well as auxiliary diagnosis of encephalitis and certain encephalopathy[31]. This study also found that the prognosis of AD patients with a ratio of (θ + δ)/(α + β) greater than or equal to 1.8 obtained by EEG was poor, suggesting that careful EEG analysis also has a certain value in judging the prognosis of AD. Engedal et al[32] used statistical pattern recognition quantitative EEG to predict the conversion rate of dementia in patients with subjective cognitive decline (SCD) and MCI, and conducted follow-up. Of the 200 parti
NLR is considered to be an easy to detect and operate systemic inflammatory index, which is related to the abnormal cellular immune function. Based on the above considerations, we analyzed the impact of NLR on the prognosis of AD patients. The results showed that there was no significant correlation between the ratio of NLR in blood and the pro
However, our research had some limitations. First, this study was a retrospective analysis, and it is difficult to control confounding factors. Second, the items related to the detection of cellular immune function were incomplete, and lym
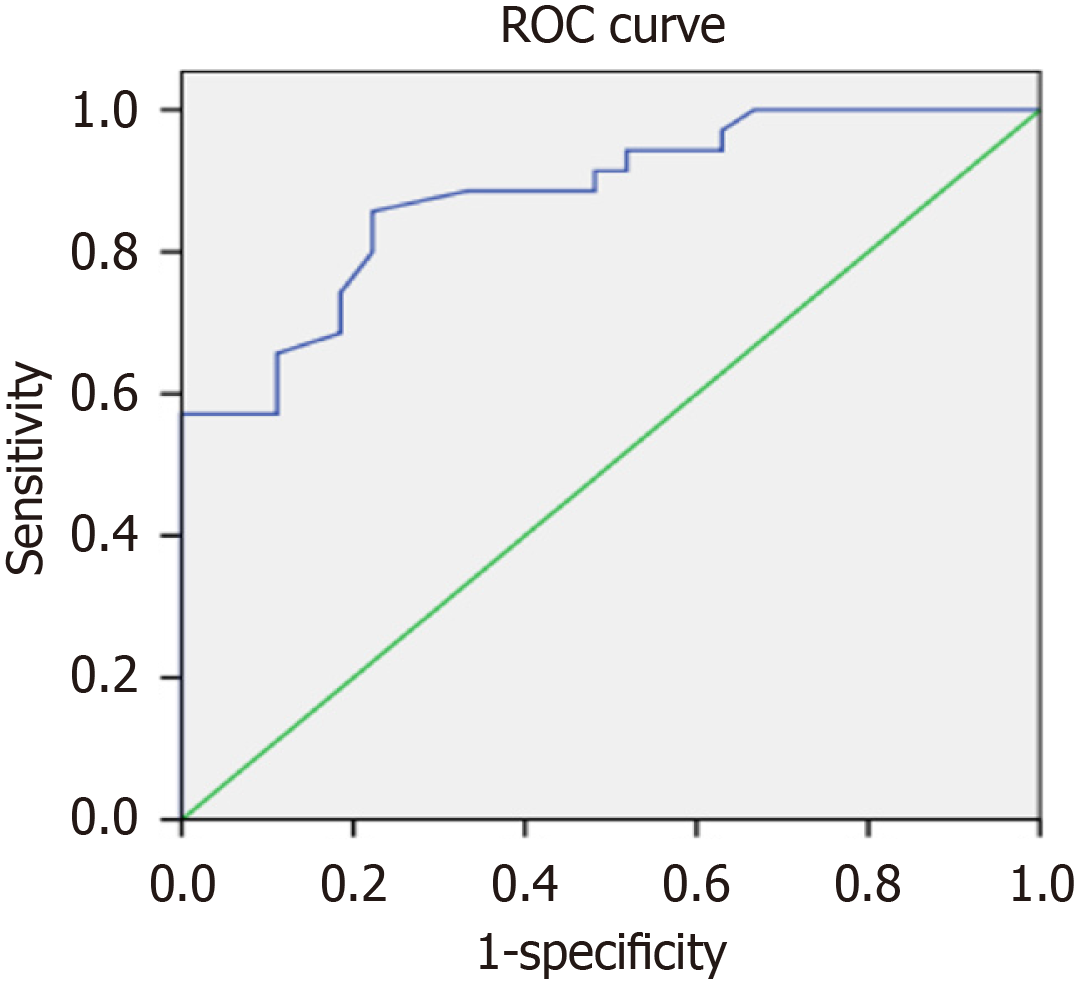
The decrease in the proportion of T lymphocytes may have predictive value for the poor prognosis of AD (Figure 4). It is suggested that the proportion of T lymphocytes less than 55% should be used as the cut-off threshold for predicting the poor prognosis of AD. In addition, MRS combined with EEG detection is also worthy of recognition in predicting the poor prognosis of AD. Yet the early and continuous drug treatment that improve cognitive function is associated with a good prognosis.
Alzheimer’s disease (AD) is a neurodegenerative disease with severe cognitive dysfunction. The prominent clinical ma
The role of chronic immune inflammation in the pathogenesis of AD is becoming more and more important. The ratio of NLR in blood is an important systemic inflammatory biomarker. NLR is calculated by ab
To explore the correlation between abnormal immune function and adverse prognostic factors in AD patients, and hope to find some valuable clues.
A retrospective analysis of 62 hospitalized patients clinical diagnosed with AD who were admitted to our hospital from November 2015 to November 2020. Collect cognitive dysfunction performance characteristics, laboratory test data and neuroimaging data from medical records within 24 h of admission, including MMSE score, drawing clock test, blood T lymphocyte subsets, and NLR, disturbance of consciousness, extrapyramidal symptoms, electroencephalogram (EEG) and head nucleus MRS and other data. Multivariate logistic regression analysis was used to determine independent prognostic factors. the modified Rankin scale (mRS) was used to determine whether the prognosis was good. The cor
Univariate analysis showed that abnormal cellular immune function, extrapyramidal symptoms, obvious disturbance of consciousness, abnormal EEG, increased NLR, abnormal MRS, and complicated pneumonia were related to the poor prognosis of AD patients. Multivariate logistic regression analysis showed that the decrease in the proportion of T lymphocytes in the blood after abnormal cellular immune function (odd ratio: 2.078, 95% confidence interval: 1.156-3.986, P < 0.05) was an independent risk factor for predicting the poor prognosis of AD. The number of days of donepezil treatment to improve cognitive function was negatively correlated with mRS score (r = 0.578, P < 0.05).
The decrease in the proportion of T lymphocytes may have predictive value for the poor prognosis of AD. It is suggested that the proportion of T lymphocytes less than 55% should be used as the cut-off threshold for predicting the poor prognosis of AD. In addition, MRS combined with EEG detection is also worthy of recognition in predicting the poor prognosis of AD. Yet the early and continuous drug treatment that improve cognitive function is associated with a good prognosis.
It is speculated that the decline of cellular immune function is not only closely related to the onset of AD, but also has a greater relationship with the poor prognosis of AD. There is increasing evidence that the occurrence of AD is closely related to slow immune inflammation, and the changes of lymphocytes in the blood may be directly related to this slow immune inflammation. In a word, this research still has some valuable findings in predicting the correlation between abnormal cellular immune function and poor prognosis in AD patients.
| 1. | Jacus JP, Mayelle A, Voltzenlogel V, Cuervo-Lombard CV, Antoine P. Modelling Awareness in Alzheimer's Disease. J Alzheimers Dis. 2020;76:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM. Alzheimer's disease. Lancet. 2016;388:505-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1969] [Cited by in RCA: 2391] [Article Influence: 239.1] [Reference Citation Analysis (0)] |
| 3. | Lee JY, Jin HK, Bae JS. Sphingolipids in neuroinflammation: a potential target for diagnosis and therapy. BMB Rep. 2020;53:28-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Olichney JM, Taylor JR, Gatherwright J, Salmon DP, Bressler AJ, Kutas M, Iragui-Madoz VJ. Patients with MCI and N400 or P600 abnormalities are at very high risk for conversion to dementia. Neurology. 2008;70:1763-1770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Ising C, Venegas C, Zhang S, Scheiblich H, Schmidt SV, Vieira-Saecker A, Schwartz S, Albasset S, McManus RM, Tejera D, Griep A, Santarelli F, Brosseron F, Opitz S, Stunden J, Merten M, Kayed R, Golenbock DT, Blum D, Latz E, Buée L, Heneka MT. NLRP3 inflammasome activation drives tau pathology. Nature. 2019;575:669-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 1047] [Article Influence: 149.6] [Reference Citation Analysis (0)] |
| 6. | Li L, Ismael S, Nasoohi S, Sakata K, Liao FF, McDonald MP, Ishrat T. Thioredoxin-Interacting Protein (TXNIP) Associated NLRP3 Inflammasome Activation in Human Alzheimer's Disease Brain. J Alzheimers Dis. 2019;68:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 7. | Olsen I, Singhrao SK. Inflammasome Involvement in Alzheimer's Disease. J Alzheimers Dis. 2016;54:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Stancu IC, Cremers N, Vanrusselt H, Couturier J, Vanoosthuyse A, Kessels S, Lodder C, Brône B, Huaux F, Octave JN, Terwel D, Dewachter I. Aggregated Tau activates NLRP3-ASC inflammasome exacerbating exogenously seeded and non-exogenously seeded Tau pathology in vivo. Acta Neuropathol. 2019;137:599-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 344] [Article Influence: 49.1] [Reference Citation Analysis (4)] |
| 9. | Cable J, Holtzman DM, Hyman BT, Tansey MG, Colonna M, Kellis M, Brinton RD, Albert M, Wellington CL, Sisodia SS, Tanzi RE. Alternatives to amyloid for Alzheimer's disease therapies-a symposium report. Ann N Y Acad Sci. 2020;1475:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Egan MF, Kost J, Voss T, Mukai Y, Aisen PS, Cummings JL, Tariot PN, Vellas B, van Dyck CH, Boada M, Zhang Y, Li W, Furtek C, Mahoney E, Harper Mozley L, Mo Y, Sur C, Michelson D. Randomized Trial of Verubecestat for Prodromal Alzheimer's Disease. N Engl J Med. 2019;380:1408-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 414] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 11. | Mielke MM, Hagen CE, Xu J, Chai X, Vemuri P, Lowe VJ, Airey DC, Knopman DS, Roberts RO, Machulda MM, Jack CR Jr, Petersen RC, Dage JL. Plasma phospho-tau181 increases with Alzheimer's disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement. 2018;14:989-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 480] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 12. | Reitz C. Genetic diagnosis and prognosis of Alzheimer's disease: challenges and opportunities. Expert Rev Mol Diagn. 2015;15:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Wang M, Peng IF, Li S, Hu X. Dysregulation of antimicrobial peptide expression distinguishes Alzheimer's disease from normal aging. Aging (Albany NY). 2020;12:690-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Hamelin L, Lagarde J, Dorothée G, Potier MC, Corlier F, Kuhnast B, Caillé F, Dubois B, Fillon L, Chupin M, Bottlaender M, Sarazin M. Distinct dynamic profiles of microglial activation are associated with progression of Alzheimer's disease. Brain. 2018;141:1855-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Kim MS, Kim Y, Choi H, Kim W, Park S, Lee D, Kim DK, Kim HJ, Hyun DW, Lee JY, Choi EY, Lee DS, Bae JW, Mook-Jung I. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer's disease animal model. Gut. 2020;69:283-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 445] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 16. | Sayed A, Bahbah EI, Kamel S, Barreto GE, Ashraf GM, Elfil M. The neutrophil-to-lymphocyte ratio in Alzheimer's disease: Current understanding and potential applications. J Neuroimmunol. 2020;349:577398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 17. | Speciale L, Calabrese E, Saresella M, Tinelli C, Mariani C, Sanvito L, Longhi R, Ferrante P. Lymphocyte subset patterns and cytokine production in Alzheimer's disease patients. Neurobiol Aging. 2007;28:1163-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Bregman N, Kavé G, Zeltzer E, Biran I; Alzheimer's Disease Neuroimaging Initiative. Memory impairment and Alzheimer's disease pathology in individuals with MCI who underestimate or overestimate their decline. Int J Geriatr Psychiatry. 2020;35:581-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Fani L, Georgakis MK, Ikram MA, Ikram MK, Malik R, Dichgans M. Circulating biomarkers of immunity and inflammation, risk of Alzheimer's disease, and hippocampal volume: a Mendelian randomization study. Transl Psychiatry. 2021;11:291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Fohner AE, Sitlani CM, Buzkova P, Doyle MF, Liu X, Bis JC, Fitzpatrick A, Heckbert SR, Huber SA, Kuller L, Longstreth WT, Feinstein MJ, Freiberg M, Olson NC, Seshadri S, Lopez O, Odden MC, Tracy RP, Psaty BM, Delaney JA, Floyd JS. Association of Peripheral Lymphocyte Subsets with Cognitive Decline and Dementia: The Cardiovascular Health Study. J Alzheimers Dis. 2022;88:7-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Bonotis K, Krikki E, Holeva V, Aggouridaki C, Costa V, Baloyannis S. Systemic immune aberrations in Alzheimer's disease patients. J Neuroimmunol. 2008;193:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Lodygin D, Hermann M, Schweingruber N, Flügel-Koch C, Watanabe T, Schlosser C, Merlini A, Körner H, Chang HF, Fischer HJ, Reichardt HM, Zagrebelsky M, Mollenhauer B, Kügler S, Fitzner D, Frahm J, Stadelmann C, Haberl M, Odoardi F, Flügel A. β-Synuclein-reactive T cells induce autoimmune CNS grey matter degeneration. Nature. 2019;566:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 23. | Regen F, Hellmann-Regen J, Costantini E, Reale M. Neuroinflammation and Alzheimer's Disease: Implications for Microglial Activation. Curr Alzheimer Res. 2017;14:1140-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 167] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 24. | Hur JY, Frost GR, Wu X, Crump C, Pan SJ, Wong E, Barros M, Li T, Nie P, Zhai Y, Wang JC, Tcw J, Guo L, McKenzie A, Ming C, Zhou X, Wang M, Sagi Y, Renton AE, Esposito BT, Kim Y, Sadleir KR, Trinh I, Rissman RA, Vassar R, Zhang B, Johnson DS, Masliah E, Greengard P, Goate A, Li YM. The innate immunity protein IFITM3 modulates γ-secretase in Alzheimer's disease. Nature. 2020;586:735-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 267] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 25. | Marsh SE, Abud EM, Lakatos A, Karimzadeh A, Yeung ST, Davtyan H, Fote GM, Lau L, Weinger JG, Lane TE, Inlay MA, Poon WW, Blurton-Jones M. The adaptive immune system restrains Alzheimer's disease pathogenesis by modulating microglial function. Proc Natl Acad Sci U S A. 2016;113:E1316-E1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 336] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 26. | Chaney A, Williams SR, Boutin H. In vivo molecular imaging of neuroinflammation in Alzheimer's disease. J Neurochem. 2019;149:438-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 27. | Chandra A, Dervenoulas G, Politis M; Alzheimer’s Disease Neuroimaging Initiative. Magnetic resonance imaging in Alzheimer's disease and mild cognitive impairment. J Neurol. 2019;266:1293-1302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 240] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 28. | Joe E, Medina LD, Ringman JM, O'Neill J. (1)H MRS spectroscopy in preclinical autosomal dominant Alzheimer disease. Brain Imaging Behav. 2019;13:925-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Zhang B, Ferman TJ, Boeve BF, Smith GE, Maroney-Smith M, Spychalla AJ, Knopman DS, Jack CR Jr, Petersen RC, Kantarci K. MRS in mild cognitive impairment: early differentiation of dementia with Lewy bodies and Alzheimer's disease. J Neuroimaging. 2015;25:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Kantarci K, Petersen RC, Boeve BF, Knopman DS, Tang-Wai DF, O'Brien PC, Weigand SD, Edland SD, Smith GE, Ivnik RJ, Ferman TJ, Tangalos EG, Jack CR Jr. 1H MR spectroscopy in common dementias. Neurology. 2004;63:1393-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 31. | Bagattini C, Mutanen TP, Fracassi C, Manenti R, Cotelli M, Ilmoniemi RJ, Miniussi C, Bortoletto M. Predicting Alzheimer's disease severity by means of TMS-EEG coregistration. Neurobiol Aging. 2019;80:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 32. | Engedal K, Barca ML, Høgh P, Bo Andersen B, Winther Dombernowsky N, Naik M, Gudmundsson TE, Øksengaard AR, Wahlund LO, Snaedal J. The Power of EEG to Predict Conversion from Mild Cognitive Impairment and Subjective Cognitive Decline to Dementia. Dement Geriatr Cogn Disord. 2020;49:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 33. | Rembach A, Watt AD, Wilson WJ, Rainey-Smith S, Ellis KA, Rowe CC, Villemagne VL, Macaulay SL, Bush AI, Martins RN, Ames D, Masters CL, Doecke JD; AIBL Research Group. An increased neutrophil-lymphocyte ratio in Alzheimer's disease is a function of age and is weakly correlated with neocortical amyloid accumulation. J Neuroimmunol. 2014;273:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Laurent C, Buée L, Blum D. Tau and neuroinflammation: What impact for Alzheimer's Disease and Tauopathies? Biomed J. 2018;41:21-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 255] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 35. | Lee JY, Han SH, Park MH, Baek B, Song IS, Choi MK, Takuwa Y, Ryu H, Kim SH, He X, Schuchman EH, Bae JS, Jin HK. Neuronal SphK1 acetylates COX2 and contributes to pathogenesis in a model of Alzheimer's Disease. Nat Commun. 2018;9:1479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 36. | Long H, Zhong G, Wang C, Zhang J, Zhang Y, Luo J, Shi S. TREM2 Attenuates Aβ1-42-Mediated Neuroinflammation in BV-2 Cells by Downregulating TLR Signaling. Neurochem Res. 2019;44:1830-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Velázquez-Soto H, Mexico S-Editor: Chen YL L-Editor: A P-Editor: Xu ZH