Published online Nov 6, 2024. doi: 10.12998/wjcc.v12.i31.6431
Revised: May 14, 2024
Accepted: May 27, 2024
Published online: November 6, 2024
Processing time: 175 Days and 23.7 Hours
Li-Fraumeni syndrome (LFS) is a well-defined autosomal dominant predisposition syndrome due to TP53 germline mutation that causes many cancer malig
Core Tip: In this editorial, we comment on a case report by Huffaker et al. According to the authors of this article, the objective of presenting this case was to bring medical attention to evolve effective strategies to detect and manage cardiac masses in rare clinical conditions like Li-Fraumeni syndrome (LFS). Though it has been reported in many cancers, its presence should not rule out other possible predictions in the diagnosis of unexplored cardiac masses. Hence in this editorial article, we will focus specifically on the significance of alternative modus operandi to scrutinize cardiac masses in LFS.
- Citation: Bharathi SP, Ramaiyan V. Complexity in interpreting cardiac valve-associated thrombus from tumors in Li-Fraumeni syndrome. World J Clin Cases 2024; 12(31): 6431-6435
- URL: https://www.wjgnet.com/2307-8960/full/v12/i31/6431.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i31.6431
Li-Fraumeni syndrome (LFS) is a rare autosomal dominant disorder caused by loss of function of P53, the “guardian of the genome”. It belongs to a group of tumor suppressor genes located on the short arm of chromosome 17, which plays a pivotal role in maintaining genomic integrity, cell cycle regulation, and apoptosis in response to cellular stressors. Some 100 years back, the concept of hereditary cancers was put forward that described a generation of offspring carrying cancer-susceptible genes of a specific tumor. In consecutive years, Frederick Li and Joseph Fraumeni Jr proved the occurrence of multiple tumors in an individual vulnerable to cancer pre-disposition genes at an early stage. This kind of familial genetic predisposition was pioneered in children in 1969; hence, discoverers named it as ‘Li-Fraumeni syndrome’. While it has no sex differences, it is very commonly identified in females[1].
Most cancer vulnerability genes encode tumor suppressors, which are proteins that limit cell development by inhibiting cell cycle progression, promoting cell death, triggering senescence, or stimulating transformation. Tumor suppressors further assist in recognizing genomic damage and facilitate the restoration of DNA[1,2]. Among such genes, P53 is very important because it masters the interplay of cell regulation. Generally, it prevents DNA damage and repair either temporarily (quiescence) or permanently (senescence) by arresting the cell-cycle through phosphorylating two protein kinases and activating GADD45. They trigger apoptosis by BAX proteins due to its increased half-life if not bound to MDM2 in stressful conditions. This syndrome that alters the function of the p53 gene is commonly conferred with different types of mutations causing various primary cancers. Upon chemotherapy or radiotherapy treatment, these tumors are the cause of secondary tumors in LFS. Well-known chemotherapeutics that do not damage genes can be strategically understood by specific mutations to overcome LFS, terminating as severe malignancies[3].
In LFS, early onset tumorigenesis is predominantly observed in the case of a dominant-negative missense mutation of p53 and poorly seen with point or frameshift mutations. Despite being vigilant in LFS cases, new heterozygous mutant variants of TP53 have been identified, which in the future may suggest a suitable strategy like whole genome, whole exome, and next-generation genome sequencing (WGS, WES and NGS) to be best handled on time[4].
Following its finding, the French LFS task force modified the definition standards for LFS to encompass the many clinical implications correlated with germline variations in TP53 and to aid in clinical assessment[4-7]. The Chompret standards consider three clinical scenarios that signify LFS: (1) inherited manifestation [the subject with an LFS tumor (breast cancer, STS, osteosarcoma, CNS tumor, adrenocortical carcinomas (ACC), leukemia, bronchoalveolar lung cancer) under 46 years and one first- or second-degree relative with an LFS tumor under 56 years or with many tumors]; (2) several primary malignancies (a pair of which are related to the narrowed LFS spectrum); and (3) ACC or choroid plexus carcinoma (CPC) irrelevant to family history. This states that LFS, an inherited condition more commonly encountered as soft-tissue tumors, is predominantly seen under 46-years-old or under 56-years-old in the case of first- or-second-degree relative with LFS. Otherwise, known history of multiple primary tumors and those specifically with non-inherited ACC or CPC were broadly prone to LFS. The accuracy and precision associated with these measures are predicted to be 82%-95% and 47%-58%, respectively[4,7-9].
In the case of LFS in a female with cardiac anomaly, as demonstrated by Huffaker et al[10], the visualization of mass around the tricuspid valve using transthoracic echocardiography, transesophageal echocardiography (TEE) followed by magnetic resonance imaging (MRI) suggested it was an intra-cardiac tumor. Whereupon during invasive surgery, they encountered it instead as an enhanced thrombus, which could be dealt with by catheter-based thrombectomy. This mandates how to authenticate a mass as a tumor merely by usual routine screenings.
SEOM guidelines enlisted certain surveillance measures in LFS, such as annual whole-body MRI (WB-MRI) using gadolinium to detect early multiple solid cancers, excluding soft-tissue sarcomas[11]. However, a case report by Ye et al[12] instigated genetic detection of the presence of secondary malignancies, especially of gynecological origin, apart from regular annual MRI. A 9-year-old case of LFS in a study real-time probed the use of chromosomal microarray testing to deeply investigate and detect specific genes of interest (alteration) to medically manage tumors[13].
To ease an understanding of cardiac masses, they are grouped into true and pseudo masses. Pseudo masses are normal cardiac structures but presented as lesions like Taenia sagittalis, Crista terminalis, etc. They were ridges observed in the wall of the right atrium that may be visualized as linear structures in CT/MRI, which can help rule out the lesions of tumor origin. True masses can be neoplastic (subclassified as benign or malignant) or non-neoplastic (thrombus, vegetation, or aneurysms). It is very difficult to distinguish non-neoplastic from neoplastic masses, though both can be presented in echocardiography, CT, or MRI[14-17].
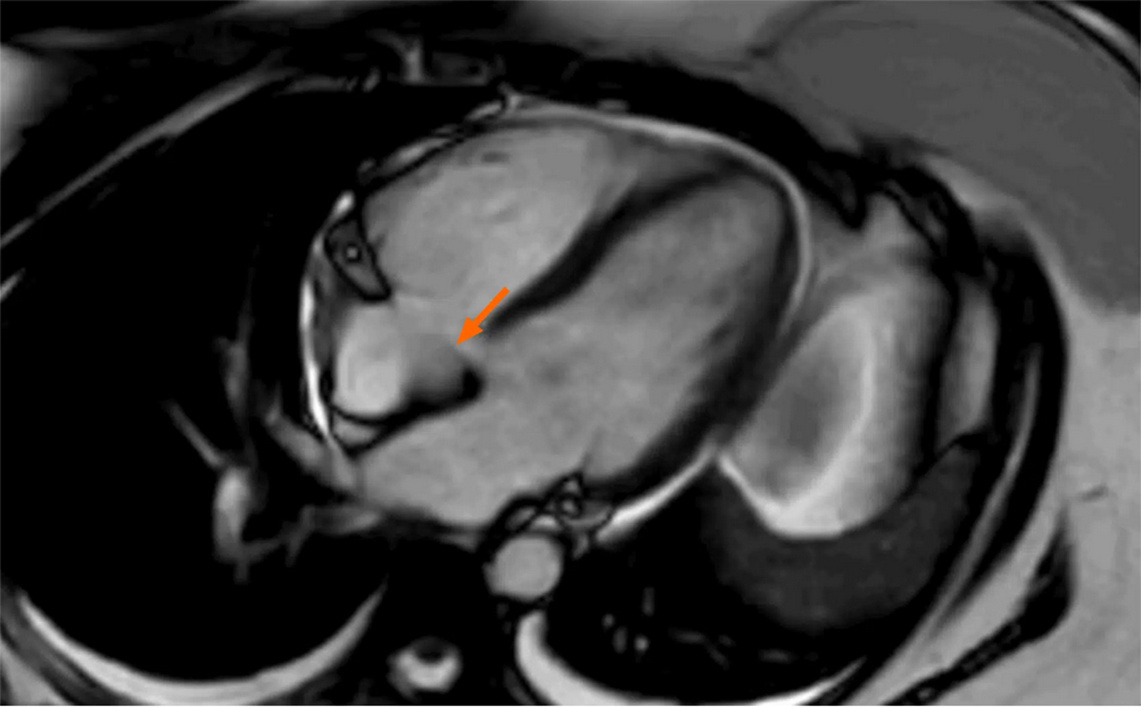
Thrombus is very infrequent in the right atrium (RA), and vice versa in the left atrium (LA) characterized as non-enhancing intracardiac lesions. Neoplastic masses are not often seen, though noted they would be malignant ones like cardiac sarcomas and would be benign forms in LFS. Cardiac myxomas and papillary fibroelastoma were the most common benign tumors. All of these masses can be visualized using ultrasonography, echocardiography, and CT/MRI with specific key characteristics. The MRI on intracardiac thrombus would reveal hyper T1w and T2w with no enhancement in acute cases[14,18]. Rather, in the case of Huffaker et al[10], false enhancement was observed in MR imaging, as shown in Figure 1.
This case clearly explained the complexity of assessing the malignancies associated with cardiac masses in an LFS patient. Above all, the chances of suspecting these masses to be benign are crucial. This perspective necessitates appropriate diagnostic strategies to prioritize a proper plan of treatment. Rather, solely relying on the usual diagnosis using CT or MRI, as proposed by Urbini et al[19], characterizing each secondary tumor at the molecular level may probe the possible undiscovered imprints noted antemortem using immunohistochemistry (IHC), which streamlines valid markers to orchestrate the metastatic tumors. This can also be improvised by scaling diagnostic techniques towards next genome sequencing (NGS), which can picture molecular targets specific for malignant cardiac neoplasms. one drawback of NGS is that its functionality requires the optimal imaging modality to preliminarily eliminate the dubious neoplastic masses[19].
Notable characteristics to rule out these masses using primary screening were well-documented. Hence, the optimal method of diagnosis cannot exempt the routine screenings. Echocardiography is commonly used to evaluate patients with suspected cardiac tumors due to its accessibility, cost-effectiveness, and non-invasive nature. TEE is the primary diagnostic tool for finding cardiac masses, with a 97% sensitivity[20]. It assesses tumor position, dimension, and motion, as well as potential consequences including pericardial drainage or valvular abnormalities[21]. Echocardiography can help in diagnosis and treatment planning[22].
Echocardiography has constraints, including difficulty in precisely defining different tissue types and reliance on operator ability and technique[20,22]. CT scans can improve comprehension of cardiac tumor architecture and identify systemic metastases. It also aids in the visualization of tumor-associated calcifications and facilitates transthoracic biopsies[23]. Cardiac MRI is superior to CT for characterizing soft tissue and discriminating between myocardial anomalies as shown in Figure 1[10]. It can distinguish between thrombi and tumors in the heart cavity[22].
Metastases are common in terminal cancer patients, and malignancies account for only a small percentage of initial cardiac tumors[15]. Transthoracic echocardiography typically detects cardiac lesions that require additional evaluation, while transesophageal testing can confirm the presence of a mass or diagnose a specific pseudomass[24]. Advanced echocardiography, including three-dimensional, transesophageal, and contrast-enhanced ultrasound, can accurately assess a lesion's size, connection to the vicinity, and cardiovascular impact[14].
However, CT or MRI scans are routinely used. MRI is the most effective imaging technique for this condition due to its ability to detect intrinsic contrast between tissue components. It is well-recognized that tissue characterization is an unquestionable advantage of MR imaging. This method is beneficial when the mass is intramural rather than intracavitary, demonstrating whether the lesion is innocuous or deadly. CT pictures can imply an infiltrative growth pattern, which can be independently confirmed by MR imaging[2]. Conte et al[25] first reported a soft tissue spindle cell sarcoma of the aorta related to LFS when a case presented with a clinical manifestation of an embolism. No genetic testing or family pedigree analysis was performed. This rare tumor may have been missed, as the patient reported here was confined to possess a thrombus from prime investigations. However, differential diagnosis like IHC on tissue biopsy confirmed the authenticity of this primary mass as well as LFS[25].
MRI better evaluates the intracardiac masses compared to echocardiography in several aspects, especially based on signal intensity changes observed in morphology as well as extracardiac manifestations. A study by Gulati et al[20] determined the ratio of missed diagnosis of thrombus from that of tumor located in an intracardiac region using echocardiography. Prospective developments in T1 and T2 imaging patterns radiomics, artificial intelligence, and PET/MRI might strengthen MRI accuracy and discriminate between non-neoplastic lesions, benign tumors, and malignant neoplasms. In a few decades, spectral imaging may replace MR imaging for the characterization of cardiac lesions, making CT a more comprehensive imaging modality[14].
This editorial is a plea for action to medical professionals to collaboratively join in tackling the diagnostic challenges associated with managing valvular thrombus in LFS, supporting advances in precision medicine and enhancing prognosis through interdisciplinary methods.
Rare forms of cardiac masses that are scrutinized in LFS could be either a tumor or a thrombus, but its exact diagnosis may not just be purely based on the regular imaging modalities mentioned in clinical guidelines. Differential diagnoses like WGS, NGS, IHC, high-contrast CT/MRI otherwise PET scans or any other novel markers must be experimentalized to determine the obscure masses specifically in rare cancer predisposition syndromes like LFS. Concluding diagnosis means suitable management is well-planned. These medical decisions should not involve risk on the grounds of precarious findings. Hence, adopting alternative diagnostic approaches apart from expert opinions in these unique scenarios may profoundly decide multimodal treatment options and quality of survival.
I would like to thank Saveetha University for providing me with adequate access and my professor who encouraged me to write this article.
| 1. | Mcgee RB, Nichols KE. Introduction to cancer genetic susceptibility syndromes. Available from: http://ashpublications.org/hematology/article-pdf/2016/1/293/1249510/hem088422.pdf. |
| 2. | Sarac S, Krsmanovic Z, Milic R, Radevic T, Lazovic-popovic B, Vasiljevic M, Sarac M. Li-Fraumeni syndrome: A case report. Vojnosanit Pregl. 2023;80:362-367. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Bougeard G, Renaux-Petel M, Flaman JM, Charbonnier C, Fermey P, Belotti M, Gauthier-Villars M, Stoppa-Lyonnet D, Consolino E, Brugières L, Caron O, Benusiglio PR, Bressac-de Paillerets B, Bonadona V, Bonaïti-Pellié C, Tinat J, Baert-Desurmont S, Frebourg T. Revisiting Li-Fraumeni Syndrome From TP53 Mutation Carriers. J Clin Oncol. 2015;33:2345-2352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 566] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 4. | Papadimitriou DT, Stratakis CA, Kattamis A, Glentis S, Dimitrakakis C, Spyridis GP, Christopoulos P, Mastorakos G, Vlahos NF, Iacovidou N. A Novel Variant in the TP53 Gene Causing Li-Fraumeni Syndrome. Children (Basel). 2023;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 5. | Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26738] [Cited by in RCA: 24723] [Article Influence: 2247.5] [Reference Citation Analysis (0)] |
| 6. | Abou Tayoun AN, Pesaran T, DiStefano MT, Oza A, Rehm HL, Biesecker LG, Harrison SM; ClinGen Sequence Variant Interpretation Working Group (ClinGen SVI). Recommendations for interpreting the loss of function PVS1 ACMG/AMP variant criterion. Hum Mutat. 2018;39:1517-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 647] [Article Influence: 80.9] [Reference Citation Analysis (0)] |
| 7. | Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2:a001008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1549] [Article Influence: 96.8] [Reference Citation Analysis (0)] |
| 8. | Gonzalez KD, Noltner KA, Buzin CH, Gu D, Wen-Fong CY, Nguyen VQ, Han JH, Lowstuter K, Longmate J, Sommer SS, Weitzel JN. Beyond Li Fraumeni Syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol. 2009;27:1250-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 413] [Article Influence: 24.3] [Reference Citation Analysis (1)] |
| 9. | Ruijs MW, Verhoef S, Rookus MA, Pruntel R, van der Hout AH, Hogervorst FB, Kluijt I, Sijmons RH, Aalfs CM, Wagner A, Ausems MG, Hoogerbrugge N, van Asperen CJ, Gomez Garcia EB, Meijers-Heijboer H, Ten Kate LP, Menko FH, van 't Veer LJ. TP53 germline mutation testing in 180 families suspected of Li-Fraumeni syndrome: mutation detection rate and relative frequency of cancers in different familial phenotypes. J Med Genet. 2010;47:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 10. | Huffaker T, Pak S, Asif A, Otchere P. Tricuspid mass-curious case of Li-Fraumeni syndrome: A case report. World J Clin Cases. 2024;12:1936-1939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Sánchez-Heras AB, Ramon Y Cajal T, Pineda M, Aguirre E, Graña B, Chirivella I, Balmaña J, Brunet J; SEOM Hereditary Cancer Working Group and AEGH Hereditary Cancer Committee. SEOM clinical guideline on heritable TP53-related cancer syndrome (2022). Clin Transl Oncol. 2023;25:2627-2633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Ye QL, Qi Y, Liu JJ, Hu YX, Lv Y, Lin B. First case of endometrial cancer after yolk sac tumor in a patient with Li-Fraumeni syndrome. BMC Womens Health. 2023;23:329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Coulter ME, Miller DT, Harris DJ, Hawley P, Picker J, Roberts AE, Sobeih MM, Irons M. Chromosomal microarray testing influences medical management. Genet Med. 2011;13:770-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Tagliati C, Fogante M, Palmisano A, Catapano F, Lisi C, Monti L, Lanni G, Cerimele F, Bernardini A, Procaccini L, Argalia G, Esposto Pirani P, Marcucci M, Rebonato A, Cerimele C, Luciano A, Cesarotto M, Belgrano M, Pagnan L, Sarno A, Cova MA, Ventura F, Regnicolo L, Polonara G, Uguccioni L, Quaranta A, Balardi L, Barbarossa A, Stronati G, Guerra F, Chiocchi M, Francone M, Esposito A, Schicchi N. Cardiac Masses and Pseudomasses: An Overview about Diagnostic Imaging and Clinical Background. Medicina (Kaunas). 2023;60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Maleszewski JJ, Basso C, Bois MC, Glass C, Klarich KW, Leduc C, Padera RF, Tavora F. The 2021 WHO Classification of Tumors of the Heart. J Thorac Oncol. 2022;17:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Lakhani DA, Balar AB, Kim C. Prominent crista terminalis mimicking a right atrial mass: A case report and brief review of the literature. Radiol Case Rep. 2022;17:434-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Kucybała I, Ciuk K, Klimek-Piotrowska W. Clinical anatomy of human heart atria and interatrial septum - anatomical basis for interventional cardiologists and electrocardiologists. Part 1: right atrium and interatrial septum. Kardiol Pol. 2018;76:499-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Adeniyi A, Abadir S, Parikh K, Khanna R, Yusuf S, Anais Hichard M. Atypical Intracavitary Cardiac Mass: Tumor or Thrombus? Cureus. 2022;14:e21937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 19. | Urbini M, Astolfi A, Indio V, Nannini M, Pizzi C, Paolisso P, Tarantino G, Pantaleo MA, Saponara M. Genetic aberrations and molecular biology of cardiac sarcoma. Ther Adv Med Oncol. 2020;12:1758835920918492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Gulati G, Sharma S, Kothari SS, Juneja R, Saxena A, Talwar KK. Comparison of echo and MRI in the imaging evaluation of intracardiac masses. Cardiovasc Intervent Radiol. 2004;27:459-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Syed IS, Feng D, Harris SR, Martinez MW, Misselt AJ, Breen JF, Miller DV, Araoz PA. MR imaging of cardiac masses. Magn Reson Imaging Clin N Am. 2008;16:137-164, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Kumari N, Bhandari S, Ishfaq A, Butt SRR, Ekhator C, Karski A, Kadel B, Altayb Ismail MA, Sherpa TN, Al Khalifa A, Khalifah B, Nguyen N, Lazarevic S, Zaman MU, Ullah A, Yadav V. Primary Cardiac Angiosarcoma: A Review. Cureus. 2023;15:e41947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 23. | Riles E, Gupta S, Wang DD, Tobin K. Primary cardiac angiosarcoma: A diagnostic challenge in a young man with recurrent pericardial effusions. Exp Clin Cardiol. 2012;17:39-42. [PubMed] |
| 24. | Parato VM, Nocco S, Alunni G, Becherini F, Conti S, Cucchini U, Di Giannuario G, Di Nora C, Fabiani D, La Carrubba S, Leonetti S, Montericcio V, Tota A, Petrella L. Imaging of Cardiac Masses: An Updated Overview. J Cardiovasc Echogr. 2022;32:65-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 25. | Conte GA, Alidoost M, Devita MS, Harmon JS, Schuler JW, Brea F, Farooq T, Chinnici AA. Diagnostic Enigma: Spindle Cell Sarcoma of the Aorta Presenting as Pulmonary Embolism and Chronic Anaemia. Eur J Case Rep Intern Med. 2020;7:001832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/