Published online Oct 16, 2024. doi: 10.12998/wjcc.v12.i29.6320
Revised: June 29, 2024
Accepted: July 15, 2024
Published online: October 16, 2024
Processing time: 111 Days and 0.2 Hours
Postoperative complications like remnant hepatic vein (HV) outflow block and liver torsion can occur after right hepatectomy. Hepatic falciform ligament fixation is typically used to prevent liver torsion. We report a novel procedure to manage outflow block.
An 80-year-old man developed HV outflow block after remnant right hepatec
This is the first report providing a novel surgical procedure when the falciform ligament is insufficient for remnant liver fixation.
Core Tip: This report presents a case of an 80-year-old man who developed hepatic vein outflow block post right he
- Citation: Higashi H, Abe Y, Abe K, Nakano Y, Tanaka M, Hori S, Hasegawa Y, Yagi H, Kitago M, Kitagawa Y. Novel procedure for hepatic venous outflow block after liver resection: A case report. World J Clin Cases 2024; 12(29): 6320-6326
- URL: https://www.wjgnet.com/2307-8960/full/v12/i29/6320.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i29.6320
In radical hepatectomy for malignant hepatic disease, it is important to balance between safety and logically negative margin (R0) resection to prevent impaired hepatic reserve. Recently, various methods have been developed to expand safety margins of hepatectomy, such as percutaneous transhepatic portal embolization (PTPE)[1], associating liver partition, and portal vein (PV) embolization for staged hepatectomy[2]. These procedures make it possible for an effective radical hepatectomy with caudate lobe resection. Remnant hepatic vein (HV) outflow block after right hepatectomy has been reported as one of the most rare postoperative complications, and various managements and precautionary procedures have been reported[3–6]. Not only HV outflow block but also hepatic venous outflow obstruction of the inferior vena cava (IVC) or the PV were reported as complications related to remnant liver torsion[7–9]. Based on these reports, fixation of the hepatic falciform ligament to the anterior abdominal wall after right hepatectomy is regarded as the gold standard for remnant liver torsion. However, fixation may become insufficient in patients who have had the caudate lobe removed, particularly those with the paracaval portion and Spiegel lobe, or who have experienced multiple postoperative complications including intra-abdominal adhesions. Here we report a case of managing outflow block with a novel procedure using not only the pedicled omental flap but also the mobilized right side colon with ileocecal region.
Cholangiocellular carcinoma of mixed hepatocellular carcinoma.
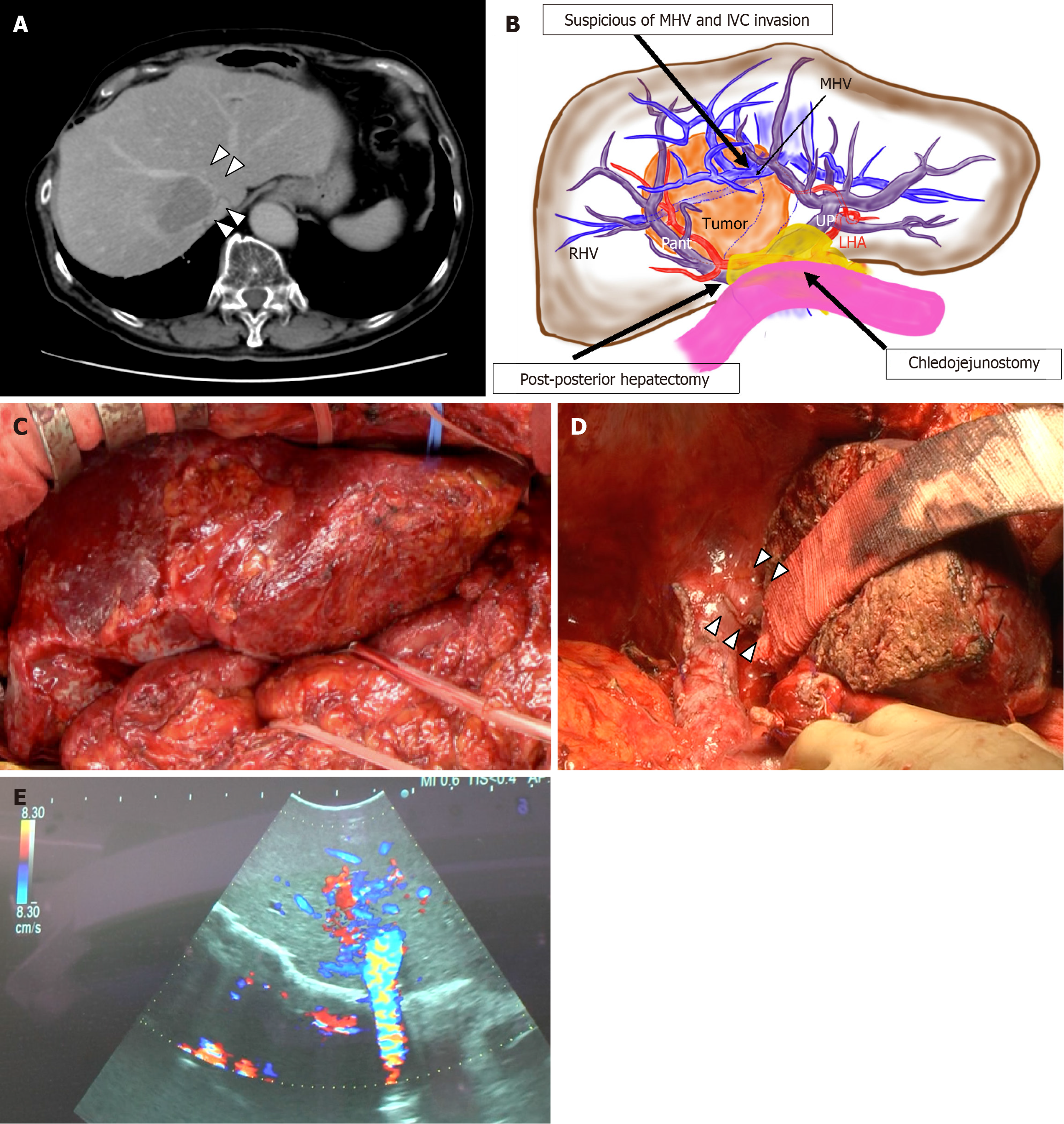
An 80-year-old man with a large cholangiocellular carcinoma of mixed hepatocellular carcinoma of 6 cm × 10 cm at segments VIII and I was referred to our institute. The carcinoma was suspected of invading the middle HV (MHV), right HV (RHV), and IVC (Figure 1A and B). Eighteen years ago, due to cholangiocellular carcinoma, he has undergone posterior segmentectomy without RHV resection, extrahepatic bile duct resection, and choledochojejunostomy.
Four weeks after PTPE of the right PV, a remnant right hepatectomy with caudate lobe resection was performed. Intra-abdominal adhesions were severe, and adhesiolysis around the liver was performed carefully (Figure 1C and D). The anterior branch of the Glisson and all branches of the caudate lobe were ligated and resected. Thereafter, choledochojejunostomy was left untouched and preserved. After complete transection of the liver, the MHV was transected. Since IVC was invaded by the carcinoma, excision of the partial IVC combined with RHV was performed. However, IVC was closed with sutures. The remnant liver was connected to the IVC only by the left HV (LHV) due to the removal of the Spiegel lobe, and the LHV had an unstable neck of about 15 mm. The scarring falciform ligament and the liver parenchyma were fixed to the left side of anterior abdominal wall as much as possible to prevent the LHV kinking and outflow block. There was no sign of HV stenosis or outflow block on ultrasonography (US) before the abdominal wall closure (Figure 1E). The total operation time was 657 minutes and the amount of blood loss was 530 mL without transfusion.
After going back to the intensive care unit, US revealed a regurgitation of umbilical portion (UP), disappearance of triphasic wave and poor blood flow of the LHV.
Eighteen years ago, due to cholangiocellular carcinoma, he has undergone posterior segmentectomy without RHV resection, extrahepatic bile duct resection, and choledochojejunostomy.
None.
No particular abnormalities were observed in the physical examination.
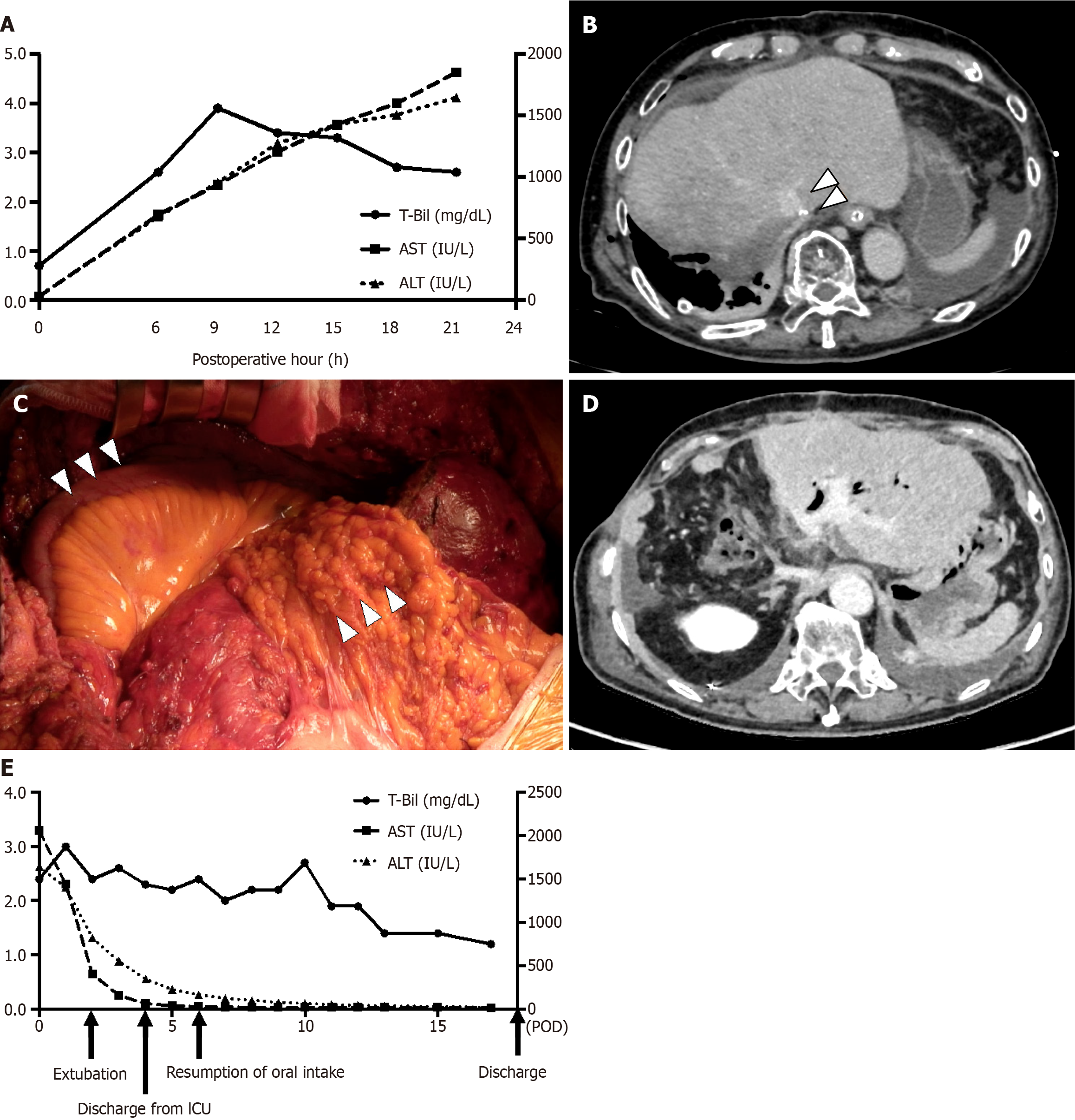
Sustainable increase of aspartate aminotransferase and alanine aminotransferase were observed every 3 hours in blood tests after operation (Figure 2A).
Eighteen hours after the first operation, a contrasted computed tomography (CT) scan revealed remnant liver congestion, and LHV outflow block was suspected (Figure 2B).
Remnant liver congestion, and LHV outflow block.
An emergency operation was performed. When the abdominal wall was re-opened, the remnant liver was located into the right subphrenic space due to the combination of remnant liver hypertrophy by PTPE and the compression of the abdominal wall. Although the fixation of the falciform ligament was remained, it did not keep the remnant liver to the appropriate position. After lifting up the remnant liver and shift it to the left side of the abdominal cavity, the LHV flow was immediately improved. The doppler-US also detected the triphasic wave of the LHV. However, once the remnant liver located in spontaneous position, the root of LHV deformed like S-shaped, and LHV outflow block was replicable. As falciform ligament fixation was insufficient for keeping the remnant liver in its anatomical position, a pedicled omental flap was used to fill in the caudate lobe space, and ileocecal region was also mobilized to fill in the right subphrenic space (Figure 2C). The total operation time was 152 minutes and the amount of blood loss was 513 mL. Remnant liver was kept its anatomical position, and parenchymal contrast enhancement improvement was identified by CT scan at 11 days after the second operation (Figure 2D).
Postoperative course was uneventful, and he discharged at 17 days after the second operation (Figure 2E).
HV outflow block is recognized as one of the most critical complications after living donor liver transplantation and it sometimes leads to graft loss[10,11]. The incidence rate of this complication is reported 5%-13%[12], whereas HV outflow block following major hepatectomy occurred in 1 of 1078 patients (0.09%). Six cases of HV outflow block excluding IVC obstruction have been reported, and mortality reaches up to 33%[3–6]. All cases occurred after right lobe hepatectomy (Table 1), and emergency treatment within 24 hours of liver resection results in good prognosis. Fixation of the remnant liver to its anatomical position in an emergency surgery or placement of expandable metallic stent in angiography has been reported as a treatment of this complication. In addition, LHV outflow is decreased after right hepatectomy even though with the left triangular ligament or MHV is preserved, and the LHV flow is significantly improved when the remnant liver is fixed to its anatomical position[13]. It means that all right hepatectomy has a risk for remnant liver torsion without the left triangular ligament resection or extrahepatic length of the common trunk of the MHV and LHV. Based on this, the remnant liver fixation to its anatomical position is regarded as the gold standard for HV outflow block after right hepatectomy. However, this case is the first to prove that this gold standard was insufficient to prevent the remnant liver torsion.
| Ref. | Age/Sex | Primary disease | Operative procedure | Middle hepatic vein resection | Time to intervension | Treatment procedure | Outcome |
| Pitre et al[3] | 75/M | HCC | RH | Yes | 7 days | Fixation of renmant liver | Dead |
| Pitre et al[3] | 55/M | Colorectal liver metastasis | RH with partial resection of left lobe | Uncertain | Urgent | Fixation of renmant liver | Alive |
| Poon et al[4] | 37/M | HCC | Right trisegmentectomy | Yes | Intraoperative | Fixation of renmant liver | Alive |
| Benesch et al[5] | 15/M | HCC | Right trisegmentectomy with total sement I resection | Yes | 13 days | Expandable metalic stent placement into left hepatic vein | Alive |
| Sato et al[6] | 67/M | HCC | RH | No | 24 hours | Fixation of renmant liver | Alive |
| Ogata et al[13] | Uncertain | Uncertain | RH | Uncertain | Uncertain | Uncertain | Dead |
| This case | 80/M | HCC | RH with total segment I resection | Yes | 21 hours | Filling in the right suphrenic space with omental flap and mobilized ileocecal region | Alive |
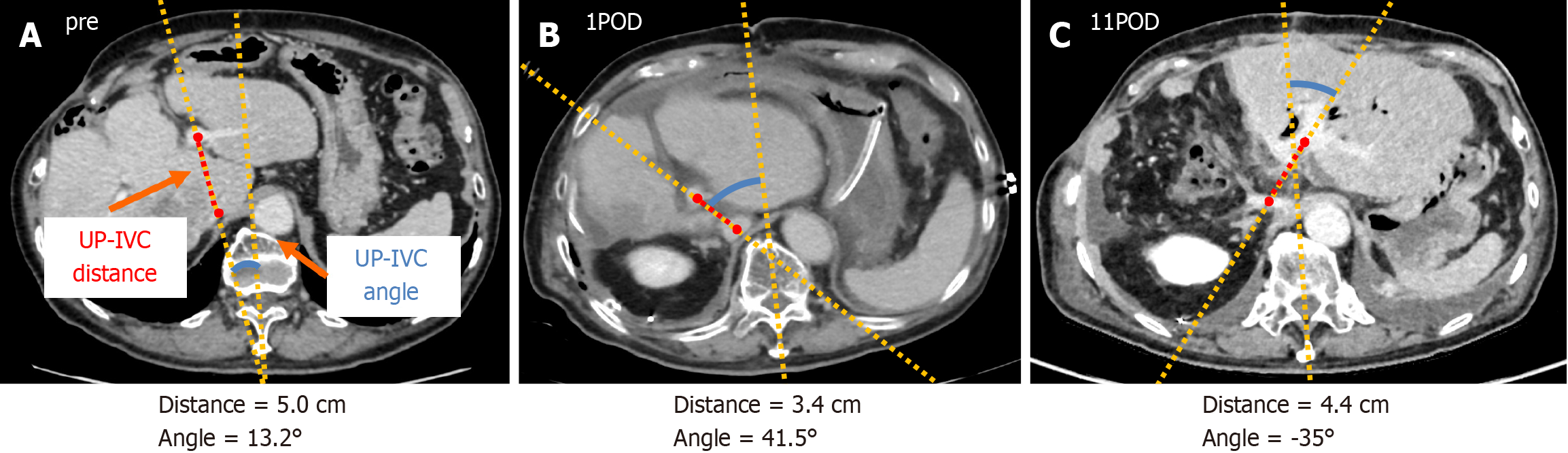
To assess the risk of outflow block after right hepatectomy, two objective indexes are defined; UP-IVC distance, and UP-IVC angle. UP-IVC distance is the distance between the origin of UP and IVC, and UP-IVC angle is the angle between the vertical line and UP-IVC line which is extended UP-IVC distance. Our hypothesis is that UP-IVC distance reflects the absence of segment I, and UP-IVC angle reflects the rotation of the remnant liver (Figure 3A, B and C). Evaluating these indexes by intraoperative US examination may predict the risk of outflow block, however, further analysis is needed for it.
In this case, the falciform ligament had adequate strength to fix the remnant liver to its anatomical position even though the previous posterior segmentectomy. However, mobility of the hepatic flexure, transverse colon, and other organs may be insufficient to fill in the right subphrenic space due to intra-abdominal adhesions of post-choledochojejunostomy. Furthermore, the intra-abdominal adhesions of organs in upper left region may push the remnant liver into the right subphrenic space. Omental flap is reported as a good candidate to fill in the caudate lobe space[9], and this case also suggested that mobilization of the right lower abdominal organ may be a precaution to fill in the right subphrenic space. In addition, a reduction in the length of extrahepatic LHV and a preservation as much of the liver parenchyma sur
In conclusion, the remnant liver fixation with falciform ligament may be insufficient for post-major hepatectomy patients to maintain the remnant liver in its anatomical position and not only intraoperative but also bed-sided Doppler-US is crucial for an early detection of the HV outflow block. In case the HV outflow block is suspected, immediate treatment is vital for good prognosis, and if the right subphrenic space is not filled by the omental flap, small intestine, or transverse colon, mobilized ileocecal region may be a good candidate for filling it.
| 1. | Kinoshita H, Sakai K, Hirohashi K, Igawa S, Yamasaki O, Kubo S. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg. 1986;10:803-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 311] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 2. | Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R, Kroemer A, Loss M, Rümmele P, Scherer MN, Padberg W, Königsrainer A, Lang H, Obed A, Schlitt HJ. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 957] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 3. | Pitre J, Panis Y, Belghiti J. Left hepatic vein kinking after right hepatectomy: a rare cause of acute Budd-Chiari syndrome. Br J Surg. 1992;79:798-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Poon RT, Chan J, Fan ST. Left hepatic vein kinking after right trisegmentectomy: a potential cause of postoperative liver failure. Hepatogastroenterology. 1998;45:508-509. [PubMed] |
| 5. | Benesch M, Urban C, Deutschmann H, Hausegger KA, Höllwarth M. Management of Budd-Chiari syndrome by hepatic vein stenting after extended right hepatectomy. J Pediatr Surg. 2002;37:1640-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Sato N, Kenjo A, Tsuchiya T, Anazawa T, Haga J, Sato T, Muto M, Kimura T, Gotoh M. Remnant left lobe torsion causing hepatic venous outflow obstruction after hepatic right lobectomy for giant hepatocellular carcinoma: report of a case. Fukushima J Med Sci. 2014;60:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Sequeira FW, Weber TR, Smith WL, Careskey JM, Cairo MS. Budd-Chiari syndrome caused by hepatic torsion. AJR Am J Roentgenol. 1981;137:393-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Wang JK, Truty MJ, Donohue JH. Remnant torsion causing Budd-Chiari syndrome after right hepatectomy. J Gastrointest Surg. 2010;14:910-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Narita M, Matsusue R, Hata H, Yamaguchi T, Otani T, Ikai I. Precaution against postoperative venous complications after major hepatectomy using the pedicled omental transposition flap: Report of two cases. Int J Surg Case Rep. 2014;5:646-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Liu XL, Li FQ, Li X, Li B, Yan LN, Wei YG. Treatment of hepatic venous outflow stenosis after living donor liver transplantation by insertion of an expandable metallic stent. Hepatobiliary Pancreat Dis Int. 2009;8:424-427. [PubMed] |
| 11. | Sakamoto S, Egawa H, Kanazawa H, Shibata T, Miyagawa-Hayashino A, Haga H, Ogura Y, Kasahara M, Tanaka K, Uemoto S. Hepatic venous outflow obstruction in pediatric living donor liver transplantation using left-sided lobe grafts: Kyoto University experience. Liver Transpl. 2010;16:1207-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Umehara M, Narumi S, Sugai M, Toyoki Y, Ishido K, Kudo D, Kimura N, Kobayashi T, Hakamada K. Hepatic venous outflow obstruction in living donor liver transplantation: balloon angioplasty or stent placement? Transplant Proc. 2012;44:769-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Ogata S, Kianmanesh R, Belghiti J. Doppler assessment after right hepatectomy confirms the need to fix the remnant left liver in the anatomical position. Br J Surg. 2005;92:592-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/