Published online Oct 6, 2024. doi: 10.12998/wjcc.v12.i28.6230
Revised: July 9, 2024
Accepted: July 17, 2024
Published online: October 6, 2024
Processing time: 69 Days and 2.3 Hours
Sarcomatoid renal cell carcinoma (SRCC) is a rare variant of renal cell carcinoma associated with an unfavorable prognosis. The efficacy of conventional chemo
A 77-year-old female patient was referred to our hospital following the incidental detection of a right kidney tumor without specific symptoms. The tumor was successfully resected, and subsequent pathological examination confirmed SRCC. She experienced both local recurrence and distant metastasis eight months after the initial laparoscopic resection. Following six cycles of toripalimab combined with pirarubicin chemotherapy, the patient achieved a partial response. Subse
Combination therapy with programmed death 1 antibodies and cytotoxic agents may be a recommended first-line treatment approach for SRCC.
Core Tip: Sarcomatoid renal cell carcinoma (SRCC) is a highly aggressive and uncommon malignant tumor of the kidney, which is associated with a rapid clinical course and unfavorable prognosis compared to conventional RCC. Traditional chemotherapy and targeted therapies have limited efficacy against SRCC. We present a rare case of recurrent and metastatic SRCC that achieved excellent outcomes with the combination of immunotherapy and chemotherapy. This report sheds new light on diagnostic and therapeutic approaches for SRCC.
- Citation: Gao MZ, Wang NF, Wang JY, Ma L, Yang YC. Toripalimab in combination with chemotherapy effectively suppresses local recurrence and metastatic sarcomatoid renal cell carcinoma: A case report. World J Clin Cases 2024; 12(28): 6230-6236
- URL: https://www.wjgnet.com/2307-8960/full/v12/i28/6230.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i28.6230
Sarcomatoid carcinoma is a highly aggressive malignancy characterized by its lack of differentiation and is composed of both epithelial and mesenchymal cells, commonly affecting the female genital system, upper aerodigestive tract, and gastrointestinal tract[1]. It rarely occurs in the urinary system. Sarcomatoid renal cell carcinoma (SRCC) is a distinct type of renal malignant tumor, accounting for approximately 1-8% of renal cell carcinoma[2]. Compared to conventional RCC, SRCC exhibits a higher degree of malignancy and a poorer prognosis, with an increased propensity for infiltration and metastasis[2,3]. Due to its infrequent occurrence in clinical practice, the understanding of this variant is restricted. We here present a case of SRCC managed at our institution in June 2022. To broaden our understanding of this complex disease, we conducted a meticulous review of pertinent domestic and international literature, specifically focusing on the clinical features associated with SRCC.
The patient was diagnosed with SRCC over 8 months ago and had experienced abdominal pain for more than 1 month.
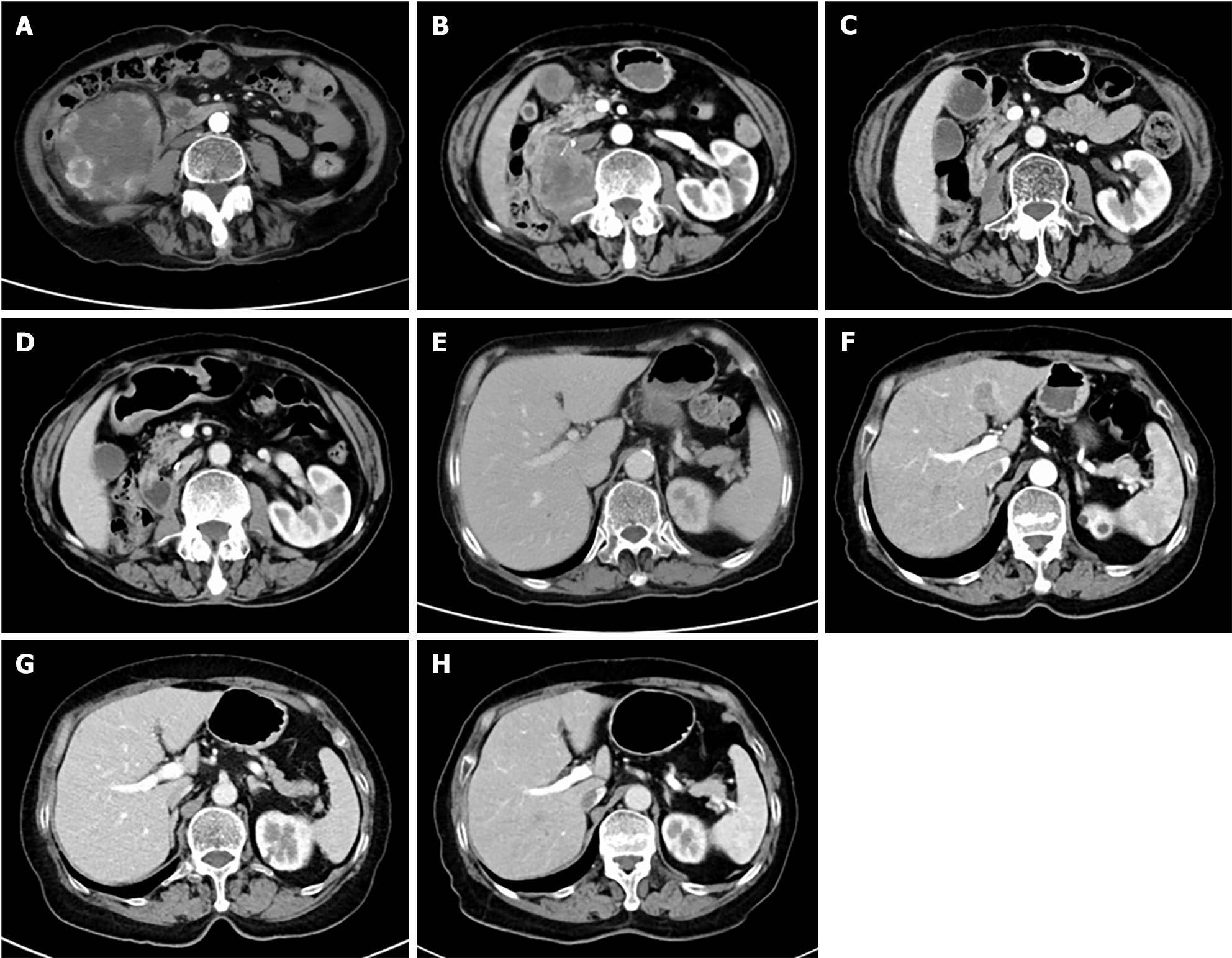
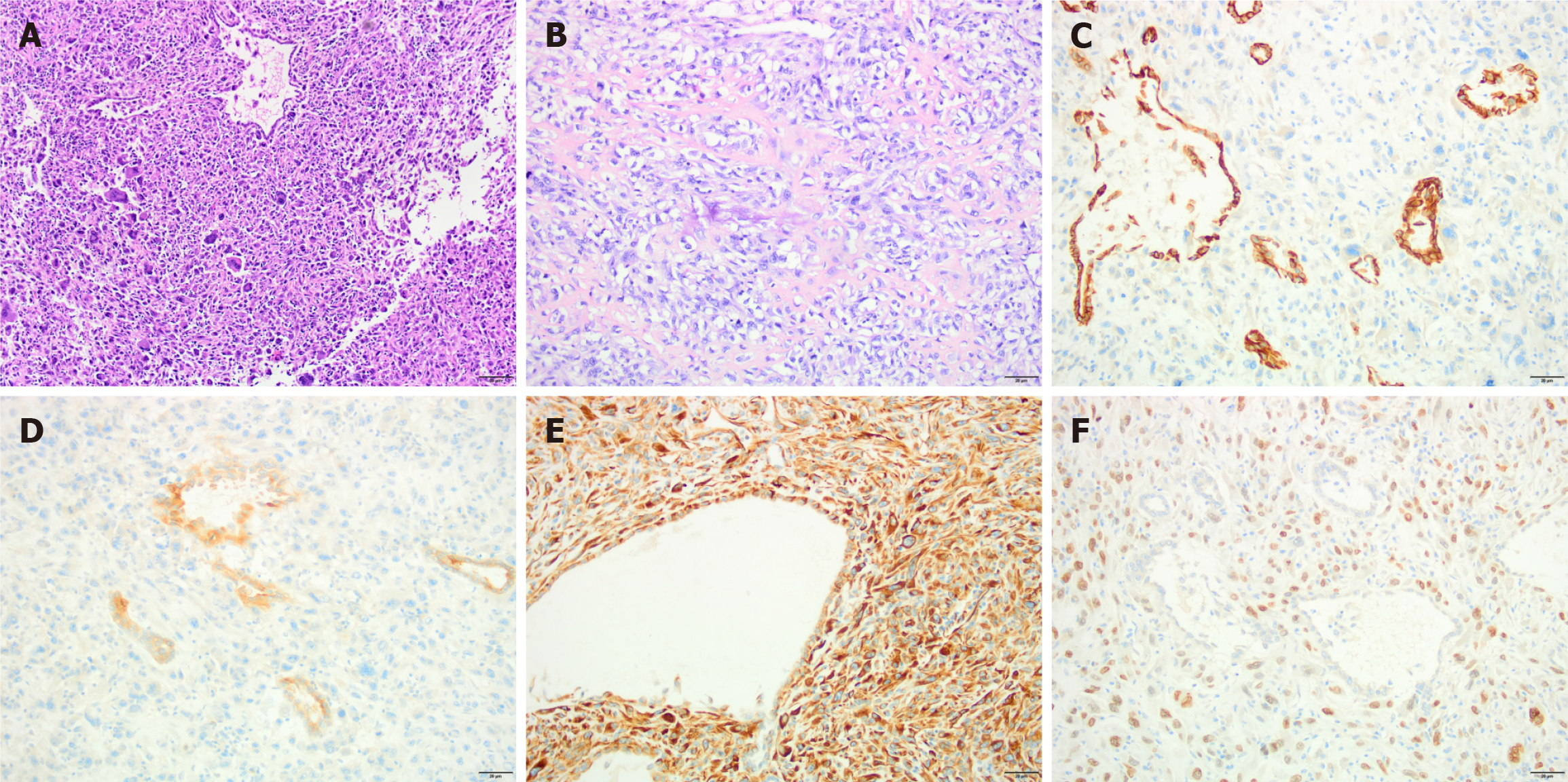
A 77-year-old female patient was found to have a tumor in her right kidney during a medical examination in June 2022, which raised concerns about malignancy (Figure 1A). She was subsequently admitted to the urology department of our hospital. After ruling out distant metastases and surgical contraindications, she underwent a laparoscopic right radical nephrectomy under general anesthesia on June 16, 2022. The pathological examination revealed sarcomatoid dedifferentiation, characterized by a mixture of large, abnormal spindle cells with numerous nuclei resembling a tumor. Further examination identified regions mimicking osteosarcoma and areas harboring tubular structures suggestive of sarcomatoid carcinoma. The sarcomatoid component comprised the predominant element, exceeding 80% of the tumor volume. Notably, the tumor remained confined beneath the renal capsule, with no discernible infiltration into the capsule itself, renal pelvis, ureteral margins, or vasculature. The three perihilar lymph nodes exhibited no signs of metastatic spread. Immunohistochemical analysis depicted in Figure 2 revealed the epithelium to be positive for cytokeratin-pan and Pax-8, indicating epithelial lineage. Conversely, CD34 and S-100 demonstrated a lack of immunoreactivity, further refining the diagnosis. Smooth muscle differentiation was focally evident with smooth muscle actin positivity, while desmin expression highlighted scattered myogenic elements. MyoD1 negativity suggested an immature myogenic phenotype. The proliferative index, as assessed by Ki-67, was approximately 50%, with prominent CD68 expression indicative of macrophages. Ecological momentary assessment (EMA) positivity confirmed the epithelial nature, while Vimentin and SATB2 positivity was also observed. Programmed Cell Death Ligand 1 (PD-L1) expression, evaluated using the 22C3 clone, yielded a combined positive score (CPS) of 1. Due to the COVID-19 outbreak, the patient was unable to attend scheduled post-operative follow-up appointments and did not receive additional therapy. She presented to our hospital in February 2023, complaining of abdominal pain for over one month.
The patient's medical history included long-standing hypertension, which had been managed with nifedipine gastro-resistant tablets (30 mg once daily) to maintain her blood pressure at approximately 140 mmHg/90 mmHg.
The patient's medical and familial histories were unremarkable.
The abdominal examination revealed light pressure pain in the epigastrium. No other physical abnormalities were observed, and the patient’s Eastern Cooperative Oncology Group performance status was 1.
The results of patient’s routine laboratory tests, including whole blood count, liver and kidney function tests, urine analysis, electrolytes, and thyroid function, were all within normal limits.
The computed tomography (CT) scan findings revealed the presence of abnormal tissue in the right renal fossa and psoas muscle, which was indicative of a recurrent SRCC. Additionally, a low-density lesion was observed in the left hepatic lobe, which is suspicious for metastasis disease (Figures 1B and F).
Contemplating the aforementioned clinical data and the lack of a history of SRCC, the patient was diagnosed with postoperative SRCC with local recurrence and hepatic metastases.
From February 2023 to the present, the patient received medical treatment in the Department of Oncology. The treatment involved administering toripalimab at a dose of 240 mg on the first day and pirarubicin at a dose of 50 mg on the first day every 21 days, for a total of six cycles. The treatment was well tolerated without any notable adverse reactions. A follow-up CT scan conducted on June 12, 2023 revealed a significant decrease in the size of the lesions in the right retroperitoneum and liver, indicating a partial response (Figure 1C and G). Subsequently, the patient proceeded with toripalimab 240 mg on day 1 every 21 days for an additional six cycles as part of her maintenance medication. After two treatment cycles, the patient exhibited low FT3 and FT4 levels and high thyrotropin levels, accompanied by fatigue and anorexia. Hypophysitis and adrenal dysfunction were excluded. In the wake of immune checkpoint inhibitor (ICI) therapy, the patient developed iatrogenic hypothyroidism, necessitating levothyroxine replacement therapy. The dosage was titrated based on serial thyroid function assessments.
The serial imaging of CT obtained on December 19, 2023 (Figures 1D and H) demonstrated a dramatic response to treatment, with near complete resolution of the initial renal mass and hepatic lesions, suggestive of a near complete remission. Additionally, thyroid function tests revealed normalization of thyrotropin, free T3, and free T4 levels, with concomitant resolution of clinical symptoms. Notably, the treatment course was well-tolerated, with no significant adverse events documented. The patient remains on active treatment with close clinical monitoring.
SRCC is an exceedingly rare malignancy. The dedifferentiated form is represented rather than being considered a distinct histological subgroup of RCC. Sarcomatoid dedifferentiation can be observed in most varieties of RCC[3-5]. The clear cell and chromophobe subtypes are the ones most frequently affected[6,7]. The etiology of the disease remains elusive. Research suggests that the sarcomatoid component and epithelial component in RCC may share a common cellular origin[8]. The epithelial-mesenchymal transition of the tumor cells is believed to be associated with the sarcomatoid transformation[9].
The clinical manifestation of SRCC is characterized by non-specific symptoms such as flank discomfort, abdominal pain, and hematuria, which vary depending on the stage of the disease at the time of diagnosis. Distant metastases commonly occur in the lung, bone, lymph nodes, and liver[2,10]. Currently, there are no reliable imaging techniques that can distinguish between SRCC and non- SRCC. The diagnosis relies on the evaluation of tissues, particularly through the use of immunohistochemistry staining, which is a crucial component. Commonly employed markers encompass cytokeratin-pan, EMA, PAX-8, vimentin, and others.
The management of SRCC closely resembles that of RCC. Surgery is the primary therapeutic option for localized diseases. Earlier studies have indicated that a majority of SRCC patients who underwent nephrectomy experienced disease recurrence within five to 26 months following the surgical procedure[11]. The patient experienced a recurrence of the disease eight months after the operation, which is consistent with the aggressive nature of the disease. For advanced SRCC, the most common treatments are systemic therapies. However, the role and timing of cytoreductive surgery remain controversial. Prior research has shown that anthracycline-based chemotherapy or antiangiogenic agents have limited efficacy, resulting in a 5-year overall survival rate of approximately 23.5%–33%, regardless of the treatment approach[10]. Nevertheless, those with a more prominent sarcomatoid component may derive greater benefits from cytotoxic chemotherapy[12]. Due to the presence of a sarcomatoid component exceeding 80%, we have decided to consider cytotoxic treatment for our patient. Recently, the discovery of ICIs, specifically programmed death 1 (PD-1) /PD-L1 antibodies, has transformed the treatment landscape of RCC and other cancer types. Researchers have ascertained that SRCC tumor cells exhibit augmented levels of PD-L1 and heightened concentrations of PD-1 on tumor-infiltrating lymphocytes compared to non-sarcomatoid RCC[13]. A study encompassing 118 instances of SRCC further elucidated that a mere 8% of the epithelial components within the sarcomatoid regions concomitantly both PD-L1 and PD-1, while 41% of the sarcomatoid areas did[14]. The control group comprised non-sarcomatoid RCC. The findings intimate that immune checkpoint blockade, which has garnered corroborating evidence from an escalating corpus of clinical data, may prove advantageous for individuals afflicted with SRCC. Patients with metastatic RCC devoid of prior treatment were evaluated in the KEYNOTE-426 trial[15]. It appraised the efficacy of pembrolizumab, an anti-PD-1 antibody, in conjunction with axitinib, a VEGFR TKI, and sunitinib in their management. Within the cohort of 105 patients exhibiting sarcomatoid attributes, the objective response rate (ORR) was 58.8% for those who received pembrolizumab alongside axitinib, contrasted with 31.5% for those who received sunitinib. Furthermore, subsequent to the CheckMate 214 trial, additional studies[16,17] have evinced that the combination of ipilimumab and nivolumab facilitated durable benefits for patients with metastatic clear cell RCC and sarcomatoid features. The median progression-free survival spanned 26 and a half months in the combination group, whereas it was merely 5.1 months in the sunitinib group. Burgeoning evidence indicates that ICIs can potentiate outcomes in SRCC.
Toripalimab, a Chinese-developed humanized anti-PD-1 immunoglobulin G4 monoclonal antibody, by impeding the binding of PD-1 to its ligands PD-L1 and PD-L2, potentiates the immune system's capacity to combat cancer[18]. China has sanctioned its utilization for the management of six neoplastic entities, encompassing melanoma[19], urothelial carcinoma[20], esophageal carcinoma[21], NSCLC[22], and others. The United States Food and Drug Administration approved the employment of toripalimab in combination with chemotherapy as the inaugural treatment for advanced or metastatic nasopharyngeal cancer in October 2023[23]. It was evinced at European Society for Medical Oncology 2023 that the combination of toripalimab and axitinib exhibited a markedly superior overall response rate (ORR 56.7% vs sunitinib) and progression-free survival (18 months) over sunitinib when utilized as the primary treatment for advanced RCC[24]. Congruent with these findings, the 2023 edition of the Chinese Society of Clinical Oncology Guidelines advocates toripalimab as the preferred initial treatment for metastatic RCC.
Based on the patient's age, performance status, pathological subtype, and drug availability, we administered a combination of anthracycline-based chemotherapy (pirarubicin) and toripalimab immunotherapy. Upon completing six cycles, she attained a near-complete response as determined by imaging evaluation. She had not experienced disease progression for 15 months, and her overall survival has reached 24 months thus far. PD-L1 expression was not elevated in this case (CPS 1), and the exceptional outcomes are challenging to elucidate. One reason is that test results exhibit heterogeneity, as PD-L1 is unevenly distributed within tumors. Another reason is that PD-L1 expressions do not confer a significant prognostic impact on SRCC. The post hoc analysis of the CheckMate 214 trial revealed that efficacy was superior in patients treated with nivolumab and ipilimumab versus sunitinib, irrespective of tumor PD-L1 expression level[16]. Until now, there is no standard biomarker that can accurately predict the efficacy of immunotherapy. The specific mechanism by which immunotherapy is effective for sarcomatoid cancer warrants further investigation. Previous studies have reported that skin and endocrine adverse events, such as hypothyroidism, hyperthyroidism, hypophysitis, and diabetes, are the principal toxicities of PD-1/PD-L1 antibodies[25]. Among immune-related adverse events, thyroid dysfunction is the most prevalent. However, thyroid dysfunction is generally reported as moderate or asymptomatic, graded one or two on clinical trials; fewer than 1% of cases are graded three or four[26,27]. It is also not essential for patients to discontinue ICIs therapy, as hormone replacement is generally able to restore functional status promptly[28]. The patient experienced grade-2 hypothyroidism, which was successfully managed with thyroid hormone replacement therapy without treatment interruptions.
The integration of both cytotoxic agents and PD-1 antibodies warrants exploration as a potential first-line therapeutic strategy for SRCC. However, robust prospective trials are imperative to definitively establish the efficacy of this treatment paradigm.
| 1. | Völker HU, Zettl A, Schön G, Heller V, Heinrich E, Rosenwald A, Handwerker M, Müller-Hermelink HK, Marx A, Ströbel P. Molecular genetic findings in two cases of sarcomatoid carcinoma of the ureter: evidence for evolution from a common pluripotent progenitor cell? Virchows Arch. 2008;452:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Blum KA, Gupta S, Tickoo SK, Chan TA, Russo P, Motzer RJ, Karam JA, Hakimi AA. Sarcomatoid renal cell carcinoma: biology, natural history and management. Nat Rev Urol. 2020;17:659-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 3. | Störkel S, Eble JN, Adlakha K, Amin M, Blute ML, Bostwick DG, Darson M, Delahunt B, Iczkowski K. Classification of renal cell carcinoma: Workgroup No. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer. 1997;80:987-989. [PubMed] [DOI] [Full Text] |
| 4. | Delahunt B, Cheville JC, Martignoni G, Humphrey PA, Magi-Galluzzi C, McKenney J, Egevad L, Algaba F, Moch H, Grignon DJ, Montironi R, Srigley JR; Members of the ISUP Renal Tumor Panel. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol. 2013;37:1490-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 615] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 5. | Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol. 2016;70:93-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 2160] [Article Influence: 216.0] [Reference Citation Analysis (2)] |
| 6. | Cheville JC, Lohse CM, Zincke H, Weaver AL, Leibovich BC, Frank I, Blute ML. Sarcomatoid renal cell carcinoma: an examination of underlying histologic subtype and an analysis of associations with patient outcome. Am J Surg Pathol. 2004;28:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 308] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 7. | Shuch B, Bratslavsky G, Linehan WM, Srinivasan R. Sarcomatoid renal cell carcinoma: a comprehensive review of the biology and current treatment strategies. Oncologist. 2012;17:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 8. | Bi M, Zhao S, Said JW, Merino MJ, Adeniran AJ, Xie Z, Nawaf CB, Choi J, Belldegrun AS, Pantuck AJ, Kluger HM, Bilgüvar K, Lifton RP, Shuch B. Genomic characterization of sarcomatoid transformation in clear cell renal cell carcinoma. Proc Natl Acad Sci U S A. 2016;113:2170-2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | Manley BJ, Hsieh JJ. Sarcomatoid renal cell carcinoma: genomic insights from sequencing of matched sarcomatous and carcinomatous components. Transl Cancer Res. 2016;5:S160-S165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Mouallem NE, Smith SC, Paul AK. Sarcomatoid renal cell carcinoma: Biology and treatment advances. Urol Oncol. 2018;36:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Merrill MM, Wood CG, Tannir NM, Slack RS, Babaian KN, Jonasch E, Pagliaro LC, Compton Z, Tamboli P, Sircar K, Pisters LL, Matin SF, Karam JA. Clinically nonmetastatic renal cell carcinoma with sarcomatoid dedifferentiation: Natural history and outcomes after surgical resection with curative intent. Urol Oncol. 2015;33:166.e21-166.e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Haas NB, Lin X, Manola J, Pins M, Liu G, McDermott D, Nanus D, Heath E, Wilding G, Dutcher J. A phase II trial of doxorubicin and gemcitabine in renal cell carcinoma with sarcomatoid features: ECOG 8802. Med Oncol. 2012;29:761-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Joseph RW, Millis SZ, Carballido EM, Bryant D, Gatalica Z, Reddy S, Bryce AH, Vogelzang NJ, Stanton ML, Castle EP, Ho TH. PD-1 and PD-L1 Expression in Renal Cell Carcinoma with Sarcomatoid Differentiation. Cancer Immunol Res. 2015;3:1303-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 14. | Kawakami F, Sircar K, Rodriguez-Canales J, Fellman BM, Urbauer DL, Tamboli P, Tannir NM, Jonasch E, Wistuba II, Wood CG, Karam JA. Programmed cell death ligand 1 and tumor-infiltrating lymphocyte status in patients with renal cell carcinoma and sarcomatoid dedifferentiation. Cancer. 2017;123:4823-4831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Powles T, Plimack ER, Soulières D, Waddell T, Stus V, Gafanov R, Nosov D, Pouliot F, Melichar B, Vynnychenko I, Azevedo SJ, Borchiellini D, McDermott RS, Bedke J, Tamada S, Yin L, Chen M, Molife LR, Atkins MB, Rini BI. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21:1563-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 557] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 16. | Tannir NM, Signoretti S, Choueiri TK, McDermott DF, Motzer RJ, Flaifel A, Pignon JC, Ficial M, Frontera OA, George S, Powles T, Donskov F, Harrison MR, Barthélémy P, Tykodi SS, Kocsis J, Ravaud A, Rodriguez-Cid JR, Pal SK, Murad AM, Ishii Y, Saggi SS, McHenry MB, Rini BI. Efficacy and Safety of Nivolumab Plus Ipilimumab versus Sunitinib in First-line Treatment of Patients with Advanced Sarcomatoid Renal Cell Carcinoma. Clin Cancer Res. 2021;27:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 184] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 17. | Cavillon A, Pouessel D, Houédé N, Mathevet F, Dauxois JY, Chevreau C, Culine S, Delord JP, Porcher R, Filleron T. Assessing Long-term Treatment Benefits Using Complementary Statistical Approaches: An In Silico Analysis of the Phase III Keynote-045 and Checkmate-214 Immune Checkpoint Inhibitor Trials. Eur Urol. 2024;85:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 18. | Zhang L, Hao B, Geng Z, Geng Q. Toripalimab: the First Domestic Anti-Tumor PD-1 Antibody in China. Front Immunol. 2021;12:730666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 19. | Tang B, Chi Z, Chen Y, Liu X, Wu D, Chen J, Song X, Wang W, Dong L, Song H, Wu H, Feng H, Yao S, Qin S, Zhang X, Guo J. Safety, Efficacy, and Biomarker Analysis of Toripalimab in Previously Treated Advanced Melanoma: Results of the POLARIS-01 Multicenter Phase II Trial. Clin Cancer Res. 2020;26:4250-4259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 20. | Sheng X, Chen H, Hu B, Yao X, Liu Z, Yao X, Guo H, Hu Y, Ji Z, Luo H, Shi B, Liu J, Wu J, Zhou F, He Z, Fan J, Wang W, Feng H, Yao S, Keegan P, Huang Y, Guo J. Safety, Efficacy, and Biomarker Analysis of Toripalimab in Patients with Previously Treated Advanced Urothelial Carcinoma: Results from a Multicenter Phase II Trial POLARIS-03. Clin Cancer Res. 2022;28:489-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 21. | Wu HX, Pan YQ, He Y, Wang ZX, Guan WL, Chen YX, Yao YC, Shao NY, Xu RH, Wang F. Clinical Benefit of First-Line Programmed Death-1 Antibody Plus Chemotherapy in Low Programmed Cell Death Ligand 1-Expressing Esophageal Squamous Cell Carcinoma: A Post Hoc Analysis of JUPITER-06 and Meta-Analysis. J Clin Oncol. 2023;41:1735-1746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 22. | Wang Z, Wu L, Li B, Cheng Y, Li X, Wang X, Han L, Wu X, Fan Y, Yu Y, Lv D, Shi J, Huang J, Zhou S, Han B, Sun G, Guo Q, Ji Y, Zhu X, Hu S, Zhang W, Wang Q, Jia Y, Wang Z, Song Y, Wu J, Shi M, Li X, Han Z, Liu Y, Yu Z, Liu AW, Wang X, Zhou C, Zhong D, Miao L, Zhang Z, Zhao H, Yang J, Wang D, Wang Y, Li Q, Zhang X, Ji M, Yang Z, Cui J, Gao B, Wang B, Liu H, Nie L, He M, Jin S, Gu W, Shu Y, Zhou T, Feng J, Yang X, Huang C, Zhu B, Yao Y, Tang X, Yu J, Maher E, Feng H, Yao S, Keegan P, Wang J. Toripalimab Plus Chemotherapy for Patients With Treatment-Naive Advanced Non-Small-Cell Lung Cancer: A Multicenter Randomized Phase III Trial (CHOICE-01). J Clin Oncol. 2023;41:651-663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 134] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 23. | Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen J, Li J, Shi YR, Jin F, Xu R, Pan J, Qu S, Li P, Hu C, Liu YC, Jiang Y, He X, Wang HM, Lim WT, Liao W, He X, Chen X, Liu Z, Yuan X, Li Q, Lin X, Jing S, Chen Y, Lu Y, Hsieh CY, Yang MH, Yen CJ, Samol J, Feng H, Yao S, Keegan P, Xu RH. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat Med. 2021;27:1536-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 295] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 24. | Yan XQ, Ye MJ, Zou Q, Chen P, He ZS, Wu B, He DL, He CH, Xue XY, Ji ZG, Chen H, Zhang S, Liu YP, Zhang XD, Fu C, Xu DF, Qiu MX, Lv JJ, Huang J, Ren XB, Cheng Y, Qin WJ, Zhang X, Zhou FJ, Ma LL, Guo JM, Ding DG, Wei SZ, He Y, Guo HQ, Shi BK, Liu L, Liu F, Hu ZQ, Jin XM, Yang L, Zhu SX, Liu JH, Huang YH, Xu T, Liu B, Sun T, Wang ZJ, Jiang HW, Yu DX, Zhou AP, Jiang J, Luan GD, Jin CL, Xu J, Hu JX, Huang YR, Guo J, Zhai W, Sheng XN. Toripalimab plus axitinib versus sunitinib as first-line treatment for advanced renal cell carcinoma: RENOTORCH, a randomized, open-label, phase III study. Ann Oncol. 2024;35:190-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 48] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 25. | Wang DY, Johnson DB, Davis EJ. Toxicities Associated With PD-1/PD-L1 Blockade. Cancer J. 2018;24:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 26. | Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, Tolaney SM. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 806] [Article Influence: 115.1] [Reference Citation Analysis (1)] |
| 27. | Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V, Marquez-Rodas I, Grob JJ, Butler MO, Middleton MR, Maio M, Atkinson V, Queirolo P, Gonzalez R, Kudchadkar RR, Smylie M, Meyer N, Mortier L, Atkins MB, Long GV, Bhatia S, Lebbé C, Rutkowski P, Yokota K, Yamazaki N, Kim TM, de Pril V, Sabater J, Qureshi A, Larkin J, Ascierto PA; CheckMate 238 Collaborators. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med. 2017;377:1824-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1590] [Cited by in RCA: 1748] [Article Influence: 194.2] [Reference Citation Analysis (0)] |
| 28. | Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, Atkins MB, Brassil KJ, Caterino JM, Chau I, Davies MJ, Ernstoff MS, Fecher L, Ghosh M, Jaiyesimi I, Mammen JS, Naing A, Nastoupil LJ, Phillips T, Porter LD, Reichner CA, Seigel C, Song JM, Spira A, Suarez-Almazor M, Swami U, Thompson JA, Vikas P, Wang Y, Weber JS, Funchain P, Bollin K. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J Clin Oncol. 2021;39:4073-4126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 1315] [Article Influence: 263.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/