Published online Sep 6, 2024. doi: 10.12998/wjcc.v12.i25.5814
Revised: June 10, 2024
Accepted: July 2, 2024
Published online: September 6, 2024
Processing time: 81 Days and 13.1 Hours
An ependymoma is a glial tumor that usually occurs in or near the ventricle, close to the ependyma. It rarely occurs exclusively in the brain parenchyma without being associated with the ventricle.
Here, we report a rare case of a cerebellar ependymoma completely located in the brain parenchyma. A previously healthy 32-year-old female with a 1-month his
We conducted a literature review and summarized three theories regarding ependymomas located exclusively in the brain parenchyma, which are key to the diagnosis of intraparenchymal cerebellar ependymomas. Surgery and postope
Core Tip: Ependymomas seldom occur exclusively in the brain parenchyma without being associated with the ventricles. Here, we report a rare case of cerebellar ependymoma completely located in the brain parenchyma. Moreover, we conducted a literature review to summarize three theories of brain parenchymal ependymoma and treatment options for this type of tumor to strengthen clinicians’ capacity to identify and treat cerebellar ependymoma located in the brain parenchyma.
- Citation: Yang CG, Xue RF, Yang LX, Jieda XL, Xiang W, Zhou J. Ventricular system-unrelated cerebellar ependymoma: A case report. World J Clin Cases 2024; 12(25): 5814-5820
- URL: https://www.wjgnet.com/2307-8960/full/v12/i25/5814.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i25.5814
Ependymomas are a series of glial tumors that account for only 2%–6% of intracranial tumors[1,2]. Its pathological basis involves ependymal cells originating from the ventricles and ependymal cell nests in the extraventricular white matter. It usually occurs in the infratentorial area in the pediatric population and in the supratentorial area in adults[3-5]. Approximately 70% of ependymomas are infratentorial and usually originate from the fourth ventricle[4,6]. Ependymomas rarely occur in the brain parenchyma alone, and more commonly, ventricular lesions can locally extend into the adjacent parenchyma through the intima of the ependyma[4,7]. To date, only a few cases of infratentorial ependymoma located entirely within the cerebellar brain parenchyma have been reported. In the present case, dizziness was the first symptom, with no obvious evidence to support the ependymoma diagnosis. However, intraoperative frozen sections indicated an ependymoma. Owing to the rare location of cerebellar ependymomas, their characteristic imaging findings are limited, and their diagnosis is relatively difficult. However, early diagnosis, combined with surgery and radiotherapy, remains the main treatment for this tumor.
A 32-year-old Chinese female presented to our neurosurgery clinic with complaints of dizziness that had persisted for more than 1 month.
The patient’s physical examination more than 1 year prior revealed an intracranial space-occupying lesion with no discomfort; therefore, no treatment was administered. Moreover, more than 1 month prior, the patient had no obvious dizziness symptoms.
The patient had been previously healthy with no history of hypertension, heart disease, diabetes, infectious disease, surgery, trauma, blood transfusion, food allergies, or drug allergies.
She denied any history of exposure to chemicals or radiation, alcohol consumption, or smoking and has no hereditary or similar diseases in her family.
On physical examination, the vital signs were as follows: Body temperature, 36.7 °C; blood pressure, 115/76 mmHg; heart rate, 65 beats per minute; respiratory rate, 16 breaths per minute. Neurological examination demonstrated no obvious positive signs.
There were no obvious abnormalities in blood routine, electrolyte, biochemistry, urine routine and coagulation function.
Magnetic resonance imaging (MRI) of the brain revealed a space-occupying lesion measuring 57 mm × 41 mm × 51 mm in the right cerebellar hemisphere and inferior cerebellar vermis. T1-weighted images showed a low signal intensity, whereas T2-weighted images showed slightly heterogeneous hyperintensities (Figure 1). No obvious signs of cerebral edema were noted around the lesions. No abnormalities in the size or shape of the cerebral ventricles were observed. The differential radiological diagnoses were hemangioblastoma or glioma.
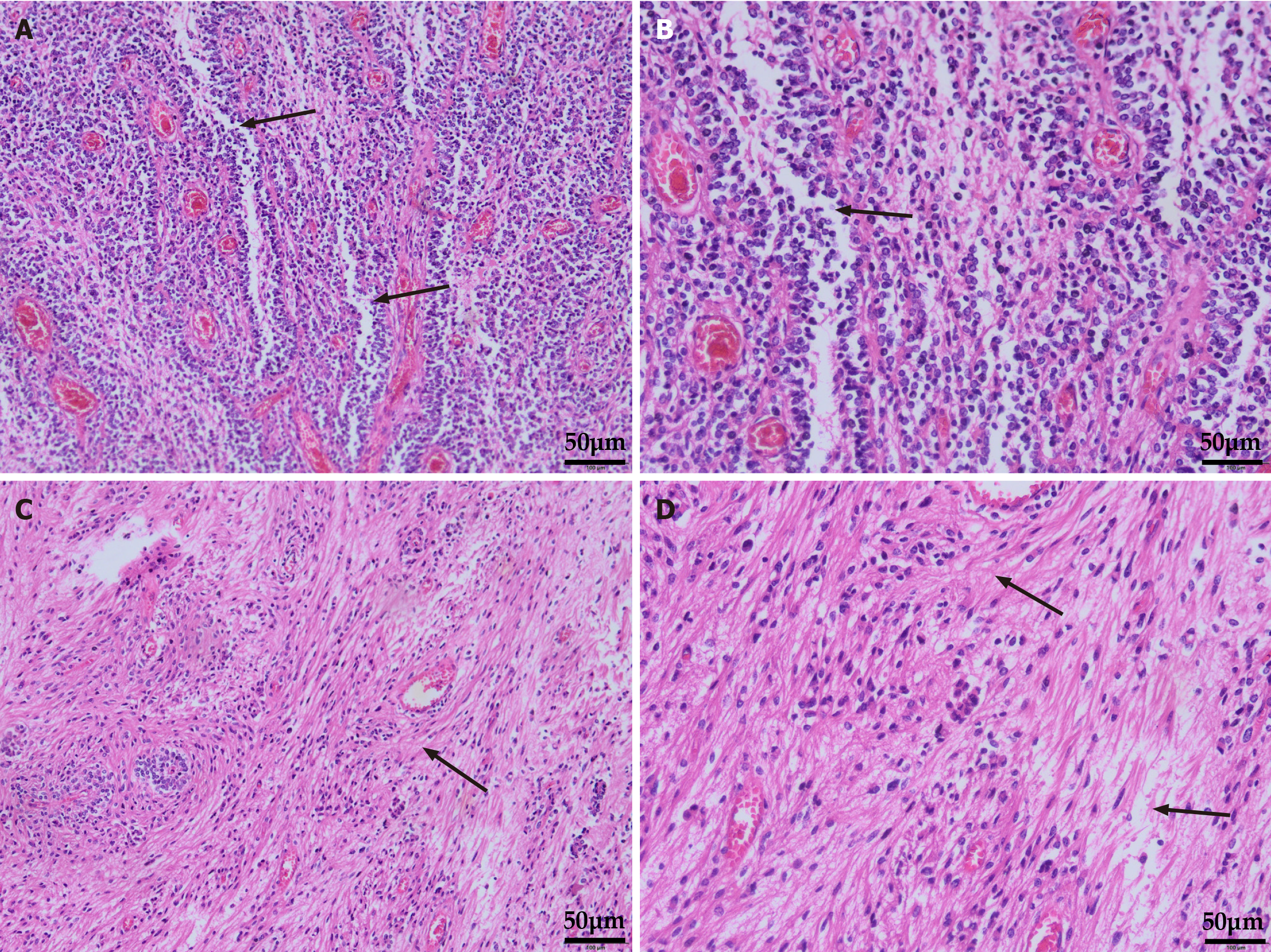
Ependymal fissures were detected by hematoxylin and eosin staining of tumor sections postoperatively. The cells surrounding the blood vessels were blurred, and the cytoplasm gathered around the blood vessels (Figure 2). Immunohistochemical study revealed tumor cells: Glial fibrillary acidic protein (+), S100(+), EMA (punctate positivity), and oligodendrocyte transcription factor 2 (-); according to histomorphology and immunohistochemistry staining, a diagnosis of ependymoma (World Health Organization grade Ⅱ) was made (Figure 3).
The patient underwent surgical resection of the right cerebellar mass, and she recovered well and was discharged 2 weeks postoperatively. One month postoperatively, the patient underwent radiotherapy in the oncology department.
At 1 year follow-up, the patient was doing well and showed no recurrence (Figure 1).
Ependymomas typically occur in or near the ventricle, ependyma, occasionally, in the brain parenchyma. However, as in this case, posterior fossa ventricular system-unrelated ependymomas located exclusively in the cerebellar parenchyma are extremely rare[8,9].
To further explore the characteristics of cerebellar ependymomas, we reviewed recently published English literature. To the best of our knowledge, only 10 cases of cerebellar ependymomas have been reported. The mean age of the seven patients with cerebellar ependymoma with reported age and sex was 48.88 years (28–78 years). Among them, five (62.5%) were female. Maximal surgical resection is the primary treatment option for cerebellar ependymoma. Total resection was achieved in all six patients with reported treatment modalities, three of whom (including this patient) received adjuvant radiotherapy postoperatively. The patients were followed for an average of 11.1 months (8–24 months), and none of the patients died during the follow-up period (Table 1)[4,8,10-15].
| No. | Ref. | year | Age/sex | Location of the tumor | Diagnose | Therapy | Follow-up (months) |
| 1 | Losa et al[20] | 2019 | 44/F | Cerebellomedulllary cistern and surrounding brain tissue | CCE (WHO II) | GTTR | NS |
| 2 | Zhang et al[21] | 2017 | 62/F | Right cerebellum | TE + CCE (WHO II) | GTTR | 9 |
| 3 | Ebrahimi et al[8] | 2020 | 36/F | Left cerebellopontine angle | PE (WHO II) | GTTR + AR | 12 |
| 4 | Lan et al[22] | 2019 | 28/M | Left cerebellopontine angle | NS (WHO II) | GTTR + AR | 24 |
| 5 | Schild et al[23] | 2017 | 78/F | Fourth ventricle and surrounding brain tissue | NS | GTTR | 9 |
| 6 | Gill et al[24] | 2015 | 48/M | Right cerebellopontine angle | NS (WHO II) | GTTR | NS |
| 7 | O'Donnell et al[4] | 2016 | 63/M | Right cerebellum | NS (WHO II) | NS | NS |
| 8-10 | Leng et al[25] | 2016 | NS | Cerebellar hemisphere | AE (NS) | NS | NS (mean, 8) |
The mechanism of posterior fossa ependymoma remains unclear, and evidence for its molecular “driving” alterations and cellular origin is still lacking. In ependymomas occurring only in the posterior fossa without invading the spinal cord, a basically balanced chromosomal profile is shown. Only chromosome 1q gain, 6q loss, and 9q gain were observed. Notably, among the many prognostic correlates, the gain of chromosome 1q has been shown to be an independent prognostic factor that is positively associated with poor prognosis[16,17]. A study by Mack et al[18] showed that central pattern generator methylation was relatively increased in isolated posterior fossa ependymomas and that these CpG hypermethylated regions were concentrated at the target of the polycomb repressor complex 2 (PRC2). However, PRC2 inhibits stem cell differentiation and maintains pluripotency through trimethylation of H3K27, suggesting that this may be a driver of posterior fossa ependymoma[19,20]. In addition, three theories have been postulated regarding ependy
Most patients experience dizziness and headaches. Owing to the nature of brain parenchymal tumors, the typical symptoms of elevated intracranial pressure occur relatively late. Cerebellar parenchyma ependymomas may develop into a large, asymptomatic mass until intracranial hypertension occurs. Depending on the tumor location, various symptoms of nerve compression may occur, suggesting that this type of tumor may not have the typical symptoms[23].
Ependymomas can spread into the cerebrospinal fluid (CSF) through the subarachnoid space. The key factor in the staging, prognosis, and treatment of tumors is the spread of tumor cells into the CSF[24]. MRI or CSF cytology can be used to determine the spread of a lesion into the CSF. In the absence of CSF invasion, maximum surgical resection with safe and localized radiation to the surgical area has been defined as the standard treatment for these tumors[17]. The most important factor affecting overall survival and progression-free survival is total tumor resection, and postoperative radiotherapy is associated with longer progression-free survival for tumors that are not completely resected. To date, no chemotherapeutic regimen has demonstrated a clear overall survival benefit in clinical trials of ependymoma[2,7,17,25]. In addition, ependymomas occurring outside the midline may have a worse prognosis than midline lesions, possibly due to the involvement of key structures such as cranial nerves[6]. In the present case, no abnormalities occurred in the size and morphology of the ventricle and cistern, the midline structure was not compressed, and the occupying effect was not obvious, potentially an important factor in the good prognosis of the patient.
Intraparenchymal cerebellar ependymomas are rare. The location of the lesion highlights the necessity and difficulty of the differential diagnosis of such tumors. Histopathology is key to diagnosing intraparenchymal cerebellar ependy
| 1. | Li JY, Lopez JI, Powell SZ, Coons SW, Fuller GN. Giant cell ependymoma-report of three cases and review of the literature. Int J Clin Exp Pathol. 2012;5:458-462. [PubMed] |
| 2. | Zhao L, Jiang Y, Wang Y, Bai Y, Liu L, Li Y. Case Report: Sellar Ependymomas: A Clinic-Pathological Study and Literature Review. Front Endocrinol (Lausanne). 2021;12:551493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Liu MQ, Liu Y, Chen ZY. [Ependymoma in Sellar Region:Report of One Case]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2019;41:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | O'Donnell K, Tsui A, Drummond K, Gaillard F. Intraparenchymal infratentorial ependymoma. J Clin Neurosci. 2016;24:158-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Martínez León MI, Vidal Denis M, Weil Lara B. [Magnetic resonance imaging of infratentorial anaplastic ependymoma in children]. Radiologia. 2012;54:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Yuh EL, Barkovich AJ, Gupta N. Imaging of ependymomas: MRI and CT. Childs Nerv Syst. 2009;25:1203-1213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Qiu SJ, Zhang XL. [MRI features of 13 cases of intracranial ependymoma]. Di Yi Jun Yi Da Xue Xue Bao. 2003;23:1224-1225. [PubMed] |
| 8. | Ebrahimi H, Jelodar S, Karimi Yarandi K, Eftekhar Javadi A, Alimohamadi M. Adult cerebellopontine angle ependymoma presenting as an isolated cisternal mass: A case report. J Med Imaging Radiat Sci. 2020;51:689-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Yang Y, Tian KB, Hao SY, Wu Z, Li D, Zhang JT. Primary Intracranial Extra-Axial Anaplastic Ependymomas. World Neurosurg. 2016;90:704.e1-704.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Losa M, Carta MC, Frontzek K, Krayenbühl N, Wichmann W, Rushing EJ. An Infratentorial Tumor in a 44-Year-Old Female Patient. Brain Pathol. 2019;29:145-146. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Zhang XP, Liu Y, Zhang D, Zheng Q, Wang C, Wang L, Li QC, Qiu XS, Wang EH. Cerebellar ependymoma with overlapping features of clear-cell and tanycytic variants mimicking hemangioblastoma: a case report and literature review. Diagn Pathol. 2017;12:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Lan Z, Richard SA, Zhang Y. Cerebellopontine angle ependymoma in a young adult: A case report. Medicine (Baltimore). 2019;98:e15019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Schild MH, Doane EP, Friedman AH, Cummings TJ. Mixed hemangioblastoma and ependymoma collision tumor of the cerebellum. Clin Neuropathol. 2017;36:248-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Gill AS, Taheri MR, Hamilton J, Monfared A. Extra-Axial Ependymoma Presenting as a Cerebellopontine Angle Mass. Otol Neurotol. 2015;36:e138-e139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Leng X, Tan X, Zhang C, Lin H, Qiu S. Magnetic resonance imaging findings of extraventricular anaplastic ependymoma: A report of 11 cases. Oncol Lett. 2016;12:2048-2054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Witt H, Mack SC, Ryzhova M, Bender S, Sill M, Isserlin R, Benner A, Hielscher T, Milde T, Remke M, Jones DT, Northcott PA, Garzia L, Bertrand KC, Wittmann A, Yao Y, Roberts SS, Massimi L, Van Meter T, Weiss WA, Gupta N, Grajkowska W, Lach B, Cho YJ, von Deimling A, Kulozik AE, Witt O, Bader GD, Hawkins CE, Tabori U, Guha A, Rutka JT, Lichter P, Korshunov A, Taylor MD, Pfister SM. Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell. 2011;20:143-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 397] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 17. | Pajtler KW, Mack SC, Ramaswamy V, Smith CA, Witt H, Smith A, Hansford JR, von Hoff K, Wright KD, Hwang E, Frappaz D, Kanemura Y, Massimino M, Faure-Conter C, Modena P, Tabori U, Warren KE, Holland EC, Ichimura K, Giangaspero F, Castel D, von Deimling A, Kool M, Dirks PB, Grundy RG, Foreman NK, Gajjar A, Korshunov A, Finlay J, Gilbertson RJ, Ellison DW, Aldape KD, Merchant TE, Bouffet E, Pfister SM, Taylor MD. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol. 2017;133:5-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 250] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 18. | Mack SC, Witt H, Piro RM, Gu L, Zuyderduyn S, Stütz AM, Wang X, Gallo M, Garzia L, Zayne K, Zhang X, Ramaswamy V, Jäger N, Jones DT, Sill M, Pugh TJ, Ryzhova M, Wani KM, Shih DJ, Head R, Remke M, Bailey SD, Zichner T, Faria CC, Barszczyk M, Stark S, Seker-Cin H, Hutter S, Johann P, Bender S, Hovestadt V, Tzaridis T, Dubuc AM, Northcott PA, Peacock J, Bertrand KC, Agnihotri S, Cavalli FM, Clarke I, Nethery-Brokx K, Creasy CL, Verma SK, Koster J, Wu X, Yao Y, Milde T, Sin-Chan P, Zuccaro J, Lau L, Pereira S, Castelo-Branco P, Hirst M, Marra MA, Roberts SS, Fults D, Massimi L, Cho YJ, Van Meter T, Grajkowska W, Lach B, Kulozik AE, von Deimling A, Witt O, Scherer SW, Fan X, Muraszko KM, Kool M, Pomeroy SL, Gupta N, Phillips J, Huang A, Tabori U, Hawkins C, Malkin D, Kongkham PN, Weiss WA, Jabado N, Rutka JT, Bouffet E, Korbel JO, Lupien M, Aldape KD, Bader GD, Eils R, Lichter P, Dirks PB, Pfister SM, Korshunov A, Taylor MD. Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature. 2014;506:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 467] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 19. | Shen X, Liu Y, Hsu YJ, Fujiwara Y, Kim J, Mao X, Yuan GC, Orkin SH. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol Cell. 2008;32:491-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 837] [Cited by in RCA: 797] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 20. | Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, Bell GW, Otte AP, Vidal M, Gifford DK, Young RA, Jaenisch R. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2098] [Cited by in RCA: 1986] [Article Influence: 99.3] [Reference Citation Analysis (0)] |
| 21. | Donich D, Lee JH, Prayson R. Giant extra-axial cerebellopontine angle/cavernous sinus ependymoma: case report. Neurosurgery. 1999;44:195-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Goto T, Ohata K, Tsuyuguchi N, Takami T, Hara M. Extra-axial subarachnoid ependymoma of the cerebral convexity. Acta Neurochir (Wien). 2003;145:913-917; discussion 917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Ma L, Xiao SY, Liu XS, You C, Zhang YK. Intracranial extraaxial ependymoma in children: a rare case report and review of the literature. Neurol Sci. 2012;33:151-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Bertero L, Ricci AA, Tampieri C, Cassoni P, Modena P. Ependymomas. Pathologica. 2022;114:436-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 25. | Mandera M, Makarska J, Sobol G, Musioł K. Infratentorial ependymomas--a study of the centre in Katowice. Childs Nerv Syst. 2015;31:1089-1096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/