Published online Sep 6, 2024. doi: 10.12998/wjcc.v12.i25.5805
Revised: June 22, 2024
Accepted: June 28, 2024
Published online: September 6, 2024
Processing time: 89 Days and 2.1 Hours
Lung cancer is increasing in incidence worldwide, and targeted therapies are developing at a rapid pace. Furthermore, the KRAS specific gene is strongly associated with non-small cell lung cancer (NSCLC). Adult patients with locally advanced or metastatic NSCLC who have tested positive for the KRAS G12C mutation and have progressed after at least one systemic treatment are treated with sotorasib.
In this study, we report on an advanced NSCLC with a KRAS G12C mutation. The histological diagnosis indicates stage IVB left lung adenocarcinoma with pelvic and bone metastases, identified as cT4N2bM1c. Using circulating tumor DNA analysis, it was possible to determine the mutation abundance of the KRAS gene exon 2, c.34G>Tp.G12C, which was 32.3%. The patient was advised to take sotorasib as part of their treatment. The imaging data were compared before and after treatment. Furthermore, clinical reassessments and regular serial blood testing were conducted. We found that the patient’s clinical symptoms significantly improved after receiving sotorasib medication, and there were no notable side effects, such as liver toxicity, during the treatment.
Sotorasib has shown promising clinical efficacy in patients with the KRAS G12c mutation and has no apparent toxic side effects.
Core Tip: In this study, we demonstrate the efficacy of sotorasib in treating advanced non-small cell lung cancer with the KRAS G12C mutation. A patient diagnosed with stage IVB adenocarcinoma, exhibiting pelvic and bone metastases, showed a significant improvement in clinical symptoms post-sotorasib treatment, with no severe side effects observed. This case highlights the potential of targeted therapies in personalized cancer treatment, emphasizing the importance of genomic profiling for therapeutic decision-making.
- Citation: Wang MX, Zhu P, Shi Y, Sun QM, Dong WH. Returning from the afterlife? Sotorasib treatment for advanced KRAS mutant lung cancer: A case report. World J Clin Cases 2024; 12(25): 5805-5813
- URL: https://www.wjgnet.com/2307-8960/full/v12/i25/5805.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i25.5805
Primary bronchogenic carcinoma, also known as lung cancer, primarily consists of two types: small cell lung cancer and non-small cell lung cancer (NSCLC), with the latter accounting for approximately 85% of all lung cancer cases[1,2]. Tobacco usage is the most common cause of lung cancer[3]. The International Agency for Research on Cancer of the World Health Organization has released its latest data on the global cancer burden. In 2020, there were 9.96 million cancer-related deaths worldwide, with 1.8 million of those fatalities attributed to lung cancer, which is significantly higher than any other type of cancer[4]. Currently, chemotherapy, targeted therapy, and immunotherapy are the primary treatments for non-small cell lung cancer worldwide.
As a form of systemic treatment, chemotherapy has significant toxic side effects on the entire body. In recent years, the effectiveness of immunotherapy and targeted therapy has been remarkable. Immunotherapy, which targets tumor cells or immune cells through immune checkpoint inhibitors to produce therapeutic effects, has demonstrated superior efficacy in treating esophageal cancer, lung cancer, and other tumors. This treatment can significantly extend the survival period, but can also increase the occurrence of adverse reactions, such as liver function abnormalities[5-8]. Consequently, targeted therapy is increasingly crucial in lung cancer treatment.
One of the frequently mutated genes in the RAS (rat sarcoma) gene family is KRAS (Kirsten-RAS), which regulates the signaling pathways responsible for cell development, differentiation, proliferation, and survival. The first small molecule chemical that directly suppresses RAS was discovered by Herbert Waldmann[9]. Since then, other RAS inhibitors have been discovered[10]. Twenty-three percent of patients with NSCLC have KRAS mutations, with the most common mutation being G12C. KRAS G12C mutants are associated with cancer due to their ability to maintain an active state[11]. The United States Food and Drug Administration authorized sotorasib, the first medication specifically designed to treat KRAS mutations, on 28 May 2021. This medication is a KRASG12C inhibitor developed by Amgen, which locks KRAS in an inactive conformation, thereby irreversibly inhibiting KRASG12C[12].
We present a case study of a 52-year-old female patient who was diagnosed with adenocarcinoma in her left lung. With cT4N2M1 characteristics, the tumor was classified as stage IV and was accompanied by metastases of the bone and pelvis. A G12C mutation was identified through genetic testing. Following comprehensive systemic targeted therapy and immunotherapy, the patient received sotorasib—a novel targeted medication—that resulted in substantial clinical benefits. We mention this case because the effectiveness of sotorasib, a targeted drug used in patients with G12C mutant NSCLC, has not been widely clinically validated in China. Although sotorasib has been approved in other countries for the treatment of certain types of advanced NSCLC, particularly those carrying the G12C gene mutation, the efficacy and safety data are insufficient in China due to a limited number of clinical trials or inadequate sample size. Therefore, this case provides practical clinical evidence of the positive therapeutic effect that sotorasib may have on Chinese patients. This will not only help promote further research and development of the drug in China but also provide additional reference information for doctors when selecting treatment options. This case may stimulate more clinical research interest and promote future larger-scale clinical trials in China to accurately evaluate the efficacy and safety of sotorasib in Chinese lung cancer patients.
A 52-year-old female patient was admitted to our hospital after experiencing recurrent episodes of unexplained chest tightness.
At the beginning of September 2019, the patient experienced repeated chest distress without obvious inducement. During the course of the disease, there was no fever, hemoptysis, cough, or expectoration. The patient experienced occasional chest distress, shortness of breath, left chest pain, dizziness, headache, skin petechiae, ecchymosis, normal defecation, poor appetite, sleep, and weight loss of approximately 9 kg in the April prior.
The patient was in good health, and denied a history of hypertension, diabetes, coronary heart disease, hepatitis, tuberculosis, other infectious diseases, trauma and blood transfusion, surgery, and food allergies. She reported a history of penicillin allergy, and had no history of vaccination with the Xinguan vaccine.
The patient denied ever smoking, having a family history of cancer, or being exposed to high-risk work environments.
The patient’s skin and mucosa showed no yellow staining or petechiae. The superficial lymph nodes were not enlarged. Respiratory sounds of both lungs included wet rales. Percussion revealed dullness. The heart rate was 90 beats/min, with a regular rhythm and no pathological murmur heard. The abdomen was soft, with the liver and spleen not palpable under the ribs. There was no tenderness or rebound pain, and the ascites sign was negative. Both lower limbs were not swollen, and there was no edema.
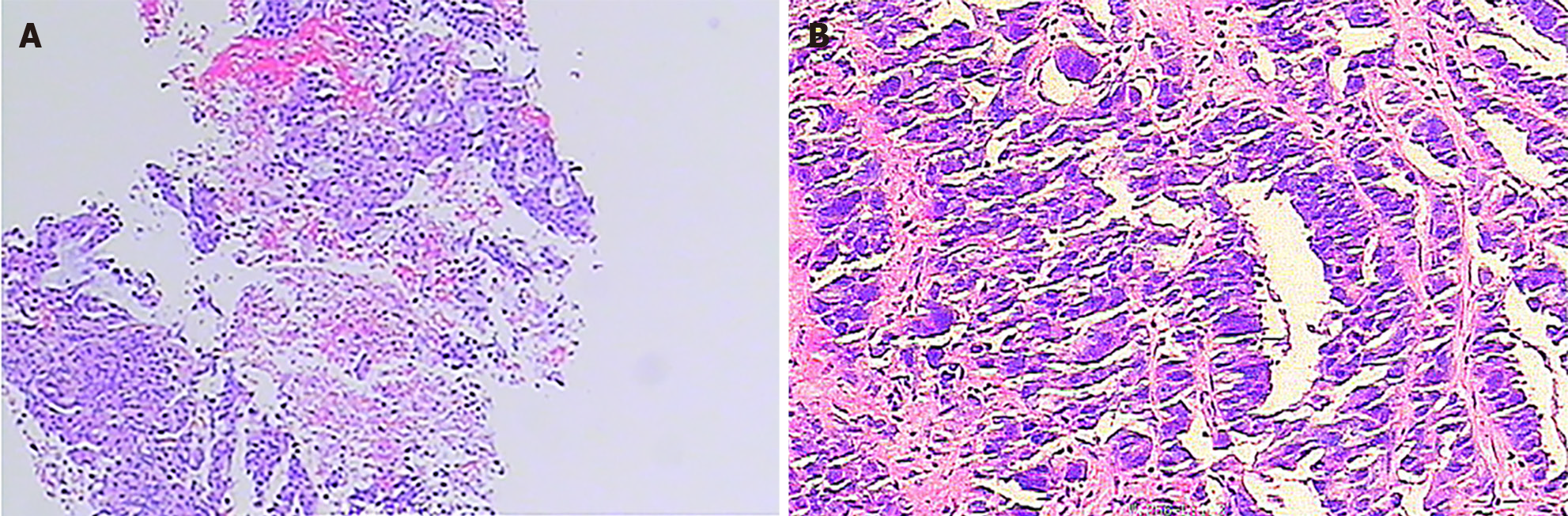
Adenocarcinoma was revealed by a puncture biopsy of the left lung lesion (Figure 1A). Other results included the following IHC results: P40 negativity; CK5/6 negativity; P63 negativity; Ki-67 approximately 30% positivity; CK7 positivity; TTF-1 positivity; and NapsinA partial positivity. Genetic testing revealed a KRAS G12C mutation and wild-type epidermal growth factor receptor (EGFR). The following lung tumor markers were evaluated on 2 November 2019: CYFRA21-1 4.05 ng/mL (elevated); carbohydrate antigen 125 (CA125) 242.60 U/mL (elevated); neuron-specific enolase 17.54 ng/mL (elevated); and carcinoembryonic antigen (CEA) ≤ 1000 ng/mL. On 22 February 2023, an enzyme-linked immunosorbent assay enzyme substrate biopsy of the abdominal and pelvic masses revealed adenocarcinoma, suggesting lung cancer as a potential source (Figure 1B).
On 11 September 2019, thoracic and abdominal enhanced computed tomography (CT) showed the following: (1) Inflammation in the lower left lung, small pleural effusion on the left side, small nodules in the posterior segment of the upper lobe of the left lung, and enlarged hilar lymph nodes on the left lung; and (2) Occupying the uterus, full cervical volume.
A chest CT on 17 October 2019 revealed possible lung cancer in the lower lobe of the left lung, enlarged lymph nodes in the mediastinum and hilum of the lung, and multiple nodules on the left pleura, suggesting metastasis; left pleural effusion. A pelvic MRI conducted on 18 July 2022 revealed the presence of both bone and pelvic metastases. On 12 February 2023, a CT scan revealed multiple metastases in the abdomen and pelvis, along with abdominal pelvic effusion.
After consulting with pulmonary disease experts, the diagnosis was confirmed as adenocarcinoma of the left lung with a KRAS G12c gene mutation.
The patient was finally diagnosed with adenocarcinoma of the left lung, stage cT4N2bM1c.
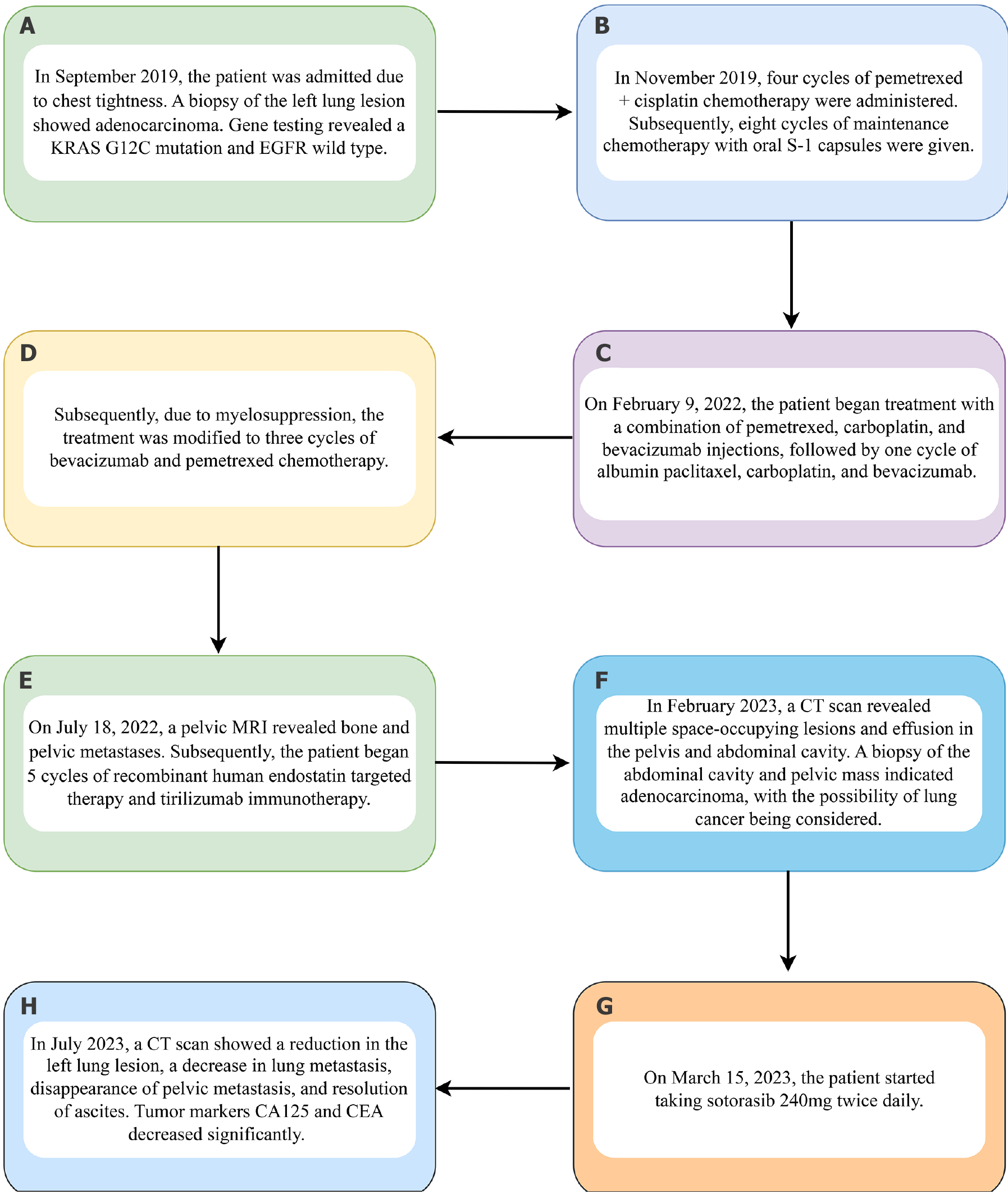
In our department, we delivered four consecutive cycles of chemotherapy using a pemetrexed + cisplatin regimen. Eight sessions of oral maintenance chemotherapy using Tegio capsules as the sole medication followed this. We then treated the patient with a combination of pemetrexed + carboplatin + bevacizumab injections. On 9 February 2022, a single cycle of chemotherapy began with an injection of albumin paclitaxel, carboplatin, and bevacizumab. This was followed by three cycles of chemotherapy, including two cycles of bevacizumab, pemetrexed, and carboplatin, and a final adjustment to bevacizumab and pemetrexed due to bone marrow suppression.
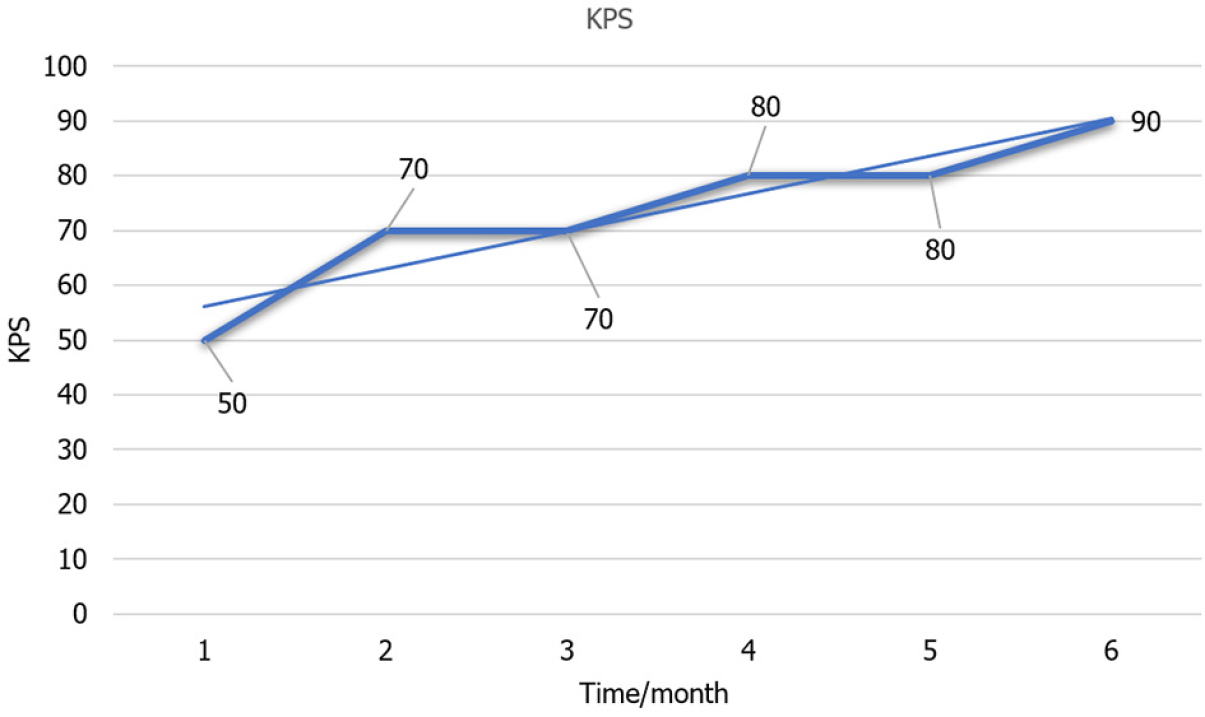
Thereafter, recombinant human endostatin targeted therapy and tislelizumab immunotherapy were combined and administered for five courses. On 15 November 2022, the final course of treatment was administered, which included symptomatic measures such as bone protection. During the course of the illness, the patient’s Karnofsky Performance Status (KPS) score dropped below 50. She was unable to take care of herself, spent most days in bed, and her blood pressure decreased below the baseline level. Considering the patient’s frail constitution, high susceptibility to intravenous chemotherapy, immune-related factors, and the tumor’s ongoing progression, as well as the patient’s young age and refusal to discontinue treatment, it was ultimately recommended that the patient undergo independent sotorasib treatment. This recommendation is based on the presence of a KRASG12C target mutation. Due to the patient’s limited financial resources and poor physical condition, it was recommended that she be administered half the recommended dose.
The patient began taking sotorasib 240 mg twice a day on 15 March 2023. The whole treatment flow chart of this patient is shown in Figure 2.
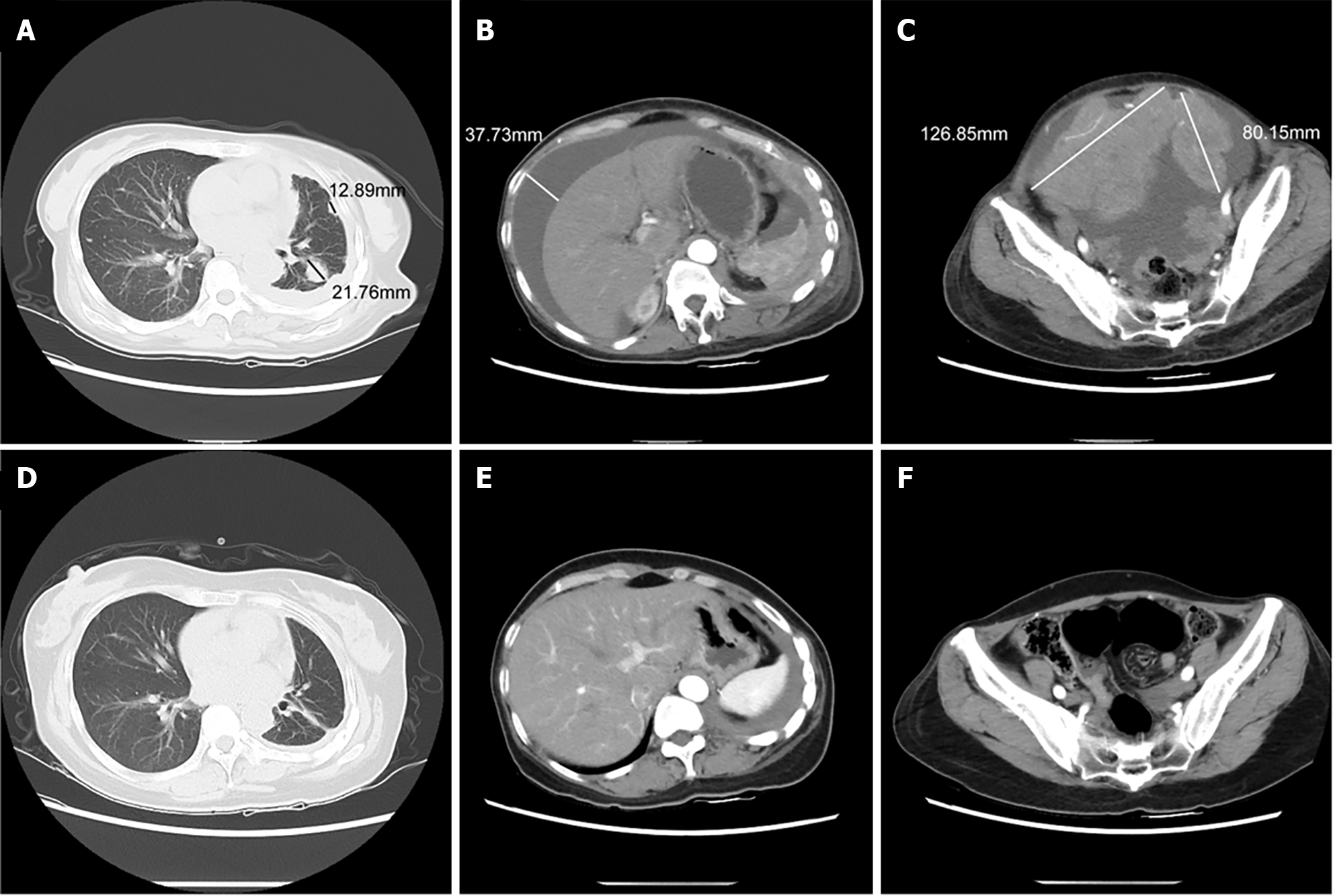
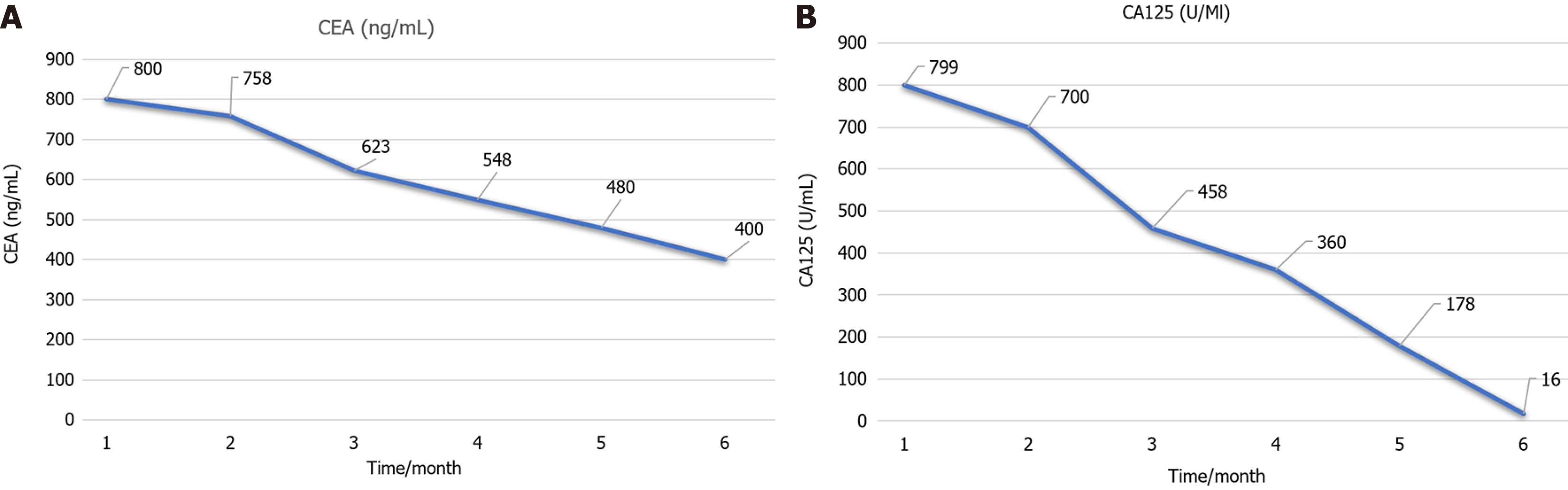
After a week on sotorasib, the patient’s quality of life score gradually improved. After a 4-month observation period, a CT scan showed a decrease in the size of the left lung lesion, a reduction in pulmonary metastases, the absence of pelvic metastases, and the resolution of peritoneal effusion (Figure 3). Furthermore, the patient experienced a significant decrease in CEA and CA125 tumor markers (Figure 4A and Figure 4B).
The patient is now fully self-sufficient, and the KPS score has increased from 50 to 90 points (Figure 5). The patient did not experience significant toxic side effects during the treatment with sotorasib, and her liver and kidney functions, as well as blood routine, were normal.
Significant progress has been made in the treatment of NSCLC over the last decade. This includes the development of immune checkpoint inhibitors, anti-angiogenic therapy, and personalized therapeutics for actionable genetic alterations[13-15]. The standard of care for patients with advanced illness and no modifiable changes is to administer immunotherapy either sequentially or simultaneously with platinum-based chemotherapy. However, there are few approved therapeutic options available as the cancer progresses, with docetaxel being a recommended choice[16,17]. There is a pressing need to develop safe and effective therapy options for patients with advanced NSCLC, as some patients may not be able to tolerate more aggressive treatments to achieve beneficial results.
Approximately 25%-39% of non-squamous NSCLCs have activating mutations in KRAS, while 13%-16% of lung adenocarcinomas have the KRAS G12C mutation[18-21]. With respect to well-established actionable driver genomic alterations (such as EGFR, ALK, ROS1, BRAF, MET, RET, NTRK, and HER2), the KRASG12C mutation is nearly always exclusive[22-24]. For over 40 years, KRAS has been considered undruggable, underscoring the unmet need for these patients to receive targeted therapy rather than chemotherapy. The KRAS G12C protein contains a targetable regulatory pocket, which was discovered in 2013[25]. Sotorasib, a small molecule, specifically and irreversibly inhibits the KRAS G12C protein. This action traps KRAS G12C in the inactive GDP-bound state, inhibits downstream signaling in cancer cells, and reduces oncogenesis (Appendix p4)[26].
The first randomized phase 3 trial for a KRAS G12C inhibitor, CodeBreaK 200, demonstrates that sotorasib significantly improved progression-free survival compared to docetaxel[27]. Sotorasib also decreased the relative risk of disease progression or death by 34%. When sotorasib was used instead of docetaxel, there was a significant increase in overall response, as well as a faster and more enduring response in the sotorasib group. The overall response rate and progression-free survival favored sotorasib in all subgroups. The experimental results sufficiently establish the basis for the clinical use of sotorasib.
Although sotorasib’s effectiveness in treating NSCLC has been recognized internationally, there is no substantial experimental data from China. A 52-year-old female patient diagnosed with stage IV cT4N2M1 lung adenocarcinoma, as well as pelvic and bone metastases, was selected by our department. Imaging results indicated that the largest diameter of pelvic lesions was approximately 126 mm. The tumor measured 85 mm before treatment with sotorasib. Following treatment, there was no evidence of pelvic metastasis, the left lung lesion decreased in size, the pulmonary metastasis reduced, and the peritoneal fluid disappeared. Leukocyte count, alanine aminotransferase, aspartate aminotransferase, creatinine, and estimated glomerular filtration rate were monitored during sotorasib therapy. The results showed that there was no noticeable decrease in the mentioned variables. In comparison to docetaxel, sotorasib was found to be better tolerated and had fewer major adverse events associated with treatment (grade 3 or worse) in the CodeBreaK 200 study. Hepatotoxicity was the most frequent treatment-related adverse event that resulted in the withdrawal of sotorasib. Patients who received immunotherapy less than 2.6 months before starting sotorasib treatment experienced a higher incidence of this side effect. On 15 November 2022, 5 months before receiving sotorasib, the selected case for this trial received the final dose of tirelizumab 200 mg q3w vaccination + Enduranti-vascular targeted therapy. So far, there have been no signs of hepatotoxicity, indicating that the patient is tolerating the medication well. Furthermore, the patient exhibited no obvious signs of medication side effects. Consequently, sotorasib may yield unexpected clinical outcomes without inducing severe side effects in patients undergoing multi-line therapy for advanced lung adenocarcinoma with a G12c mutation. However, sotorasib has not been extensively utilized in clinical trials and requires more data samples to support its usage due to its high cost and the fact that only 20% of KRAS protein Gly12 mutations in lung cancer are Cys12 (KRAS G12C).
Combined with the current research progress of targeted therapy, we believe that the reasons why sotorasib has not been widely expanded in China are as follows: sotorasib has not been marketed in China, and there is no relevant medication experience and guidance for such a large population, which is obviously not suitable. In addition, the cost of genetic testing is relatively high, and most Chinese patient’s families cannot afford the high cost of treatment and testing, which will affect the use of targeted drugs. Sotorasib, a novel anticancer drug, is increasingly used worldwide, especially in patients with advanced NSCLC specific gene mutations. However, sotorasib has not been officially launched in the Chinese market, which means that doctors and patients in China lack practical experience and professional guidance on this drug. Without sufficient clinical data to support it, blind promotion may pose unforeseen risks. In this context, reporting on the response and efficacy of sotorasib in Chinese patients is particularly important. By sharing specific cases, reports can provide valuable reference information for domestic counterparts to help them better understand the application prospects of sotorasib in Chinese individuals. Furthermore, this will encourage the relevant domestic authorities to expedite the approval process of sotorasib, enabling more patients to benefit from this innovative drug promptly. Therefore, it is important to report the efficacy and safety of sotorasib in Chinese patients. By gaining insight into the use of sotorasib in the Chinese population, physicians can better tailor treatment regimens to prolong patient survival and improve quality of life. In the next 5 years, as sotorasib expands in the Chinese market, the drug will be more widely used. If the effect is significant, the targeted drugs for the same site will be continuously updated to better serve patients.
There are limitations to this study. (1) Sample size limitations: Since this is a single case study, the results may not be generalized to all NSCLC patients with KRAS G12C mutations; (2) Individual differences in patients: A patient’s response may be influenced by their unique genetic background, lifestyle, environmental factors, and other health conditions that may influence the effectiveness and tolerability of treatment; (3) Under-observation of treatment duration: Case studies may not provide sufficient information to assess long-term efficacy and safety, including long-term side effects and the likelihood of disease recurrence; (4) Lack of control group: There was no control group to compare the effects of sotorasib treatment; and (5) Lack of statistical rigor: Due to the lack of statistical comparisons, it is difficult to determine whether the observed efficacy is statistically significant. To address these limitations, future work should include multicenter, large-scale clinical trials to improve the universality and reliability of results. Focus should be on individualized medicine, considering individual differences in patients, and personalize treatment protocols through genomics, biomarkers, and other clinical data. At the same time, more long-term follow-up studies should be carried out to evaluate the long-term efficacy and safety of sotorasib, to better improve clinical efficacy and enhance patient quality of life.
The patient’s distant metastases were significantly reduced with sotorasib in this case of stage IV left lung adenocarcinoma (T4N2M1). The patient, who had advanced lung cancer and was at risk of serious tumor-related complications, achieved a long-term remission because of this. Five months prior to starting sotorasib, the patient underwent immunotherapy and did not experience any serious side effects, such as hepatotoxicity, while receiving the medication. Sotorasib’s impact on quality of life in a first-line setting is currently being evaluated worldwide, either in combination with chemotherapy or as a precursor to immunotherapy prior to initiating treatment. This situation could inspire some thoughts. Additionally, many nations, such as China, have not yet adopted sotorasib. Clinicians may be encouraged by this example to identify and manage KRAS G12C.
The authors would like to express their sincere gratitude to the Lu’an Hospital of Traditional Chinese Medicine Affiliated to Anhui University of Chinese Medicine for their invaluable support and contributions throughout the course of this research. We extend our thanks to the hospital’s administration and staff for providing access to essential facilities and resources that have significantly aided our study.
| 1. | Bade BC, Dela Cruz CS. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin Chest Med. 2020;41:1-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 1279] [Article Influence: 213.2] [Reference Citation Analysis (0)] |
| 2. | Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584-594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 571] [Cited by in RCA: 1611] [Article Influence: 89.5] [Reference Citation Analysis (0)] |
| 3. | Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e1S-e29S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 494] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 4. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68022] [Article Influence: 13604.4] [Reference Citation Analysis (201)] |
| 5. | Rizzo A, Mollica V, Tateo V, Tassinari E, Marchetti A, Rosellini M, De Luca R, Santoni M, Massari F. Hypertransaminasemia in cancer patients receiving immunotherapy and immune-based combinations: the MOUSEION-05 study. Cancer Immunol Immunother. 2023;72:1381-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 134] [Reference Citation Analysis (0)] |
| 6. | Dall'Olio FG, Rizzo A, Mollica V, Massucci M, Maggio I, Massari F. Immortal time bias in the association between toxicity and response for immune checkpoint inhibitors: a meta-analysis. Immunotherapy. 2021;13:257-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 7. | Rizzo A. Identifying optimal first-line treatment for advanced non-small cell lung carcinoma with high PD-L1 expression: a matter of debate. Br J Cancer. 2022;127:1381-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 8. | Sahin TK, Rizzo A, Aksoy S, Guven DC. Prognostic Significance of the Royal Marsden Hospital (RMH) Score in Patients with Cancer: A Systematic Review and Meta-Analysis. Cancers (Basel). 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 149] [Reference Citation Analysis (0)] |
| 9. | Ostrem JM, Shokat KM. Direct small-molecule inhibitors of KRAS: from structural insights to mechanism-based design. Nat Rev Drug Discov. 2016;15:771-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 477] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 10. | Zimmermann G, Papke B, Ismail S, Vartak N, Chandra A, Hoffmann M, Hahn SA, Triola G, Wittinghofer A, Bastiaens PI, Waldmann H. Small molecule inhibition of the KRAS-PDEδ interaction impairs oncogenic KRAS signalling. Nature. 2013;497:638-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 501] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 11. | Lee A. Sotorasib: A Review in KRAS G12C Mutation-Positive Non-small Cell Lung Cancer. Target Oncol. 2022;17:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Wu X, Song W, Cheng C, Liu Z, Li X, Cui Y, Gao Y, Li D. Small molecular inhibitors for KRAS-mutant cancers. Front Immunol. 2023;14:1223433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 13. | Tan AC, Tan DSW. Targeted Therapies for Lung Cancer Patients With Oncogenic Driver Molecular Alterations. J Clin Oncol. 2022;40:611-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 520] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 14. | Perdrizet K, Leighl NB. The Role of Angiogenesis Inhibitors in the Era of Immune Checkpoint Inhibitors and Targeted Therapy in Metastatic Non-Small Cell Lung Cancer. Curr Treat Options Oncol. 2019;20:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Reck M, Remon J, Hellmann MD. First-Line Immunotherapy for Non-Small-Cell Lung Cancer. J Clin Oncol. 2022;40:586-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 617] [Article Influence: 154.3] [Reference Citation Analysis (4)] |
| 16. | Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2504] [Cited by in RCA: 2416] [Article Influence: 134.2] [Reference Citation Analysis (0)] |
| 17. | Modest DP, Ricard I, Heinemann V, Hegewisch-Becker S, Schmiegel W, Porschen R, Stintzing S, Graeven U, Arnold D, von Weikersthal LF, Giessen-Jung C, Stahler A, Schmoll HJ, Jung A, Kirchner T, Tannapfel A, Reinacher-Schick A. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann Oncol. 2016;27:1746-1753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 254] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 18. | Biernacka A, Tsongalis PD, Peterson JD, de Abreu FB, Black CC, Gutmann EJ, Liu X, Tafe LJ, Amos CI, Tsongalis GJ. The potential utility of re-mining results of somatic mutation testing: KRAS status in lung adenocarcinoma. Cancer Genet. 2016;209:195-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 19. | Riely GJ, Kris MG, Rosenbaum D, Marks J, Li A, Chitale DA, Nafa K, Riedel ER, Hsu M, Pao W, Miller VA, Ladanyi M. Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma. Clin Cancer Res. 2008;14:5731-5734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 463] [Cited by in RCA: 456] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 20. | Boch C, Kollmeier J, Roth A, Stephan-Falkenau S, Misch D, Grüning W, Bauer TT, Mairinger T. The frequency of EGFR and KRAS mutations in non-small cell lung cancer (NSCLC): routine screening data for central Europe from a cohort study. BMJ Open. 2013;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Garcia BNC, van Kempen LC, Kuijpers CCHJ, Schuuring E, Willems SM, van der Wekken AJ. Prevalence of KRAS p.(G12C) in stage IV NSCLC patients in the Netherlands; a nation-wide retrospective cohort study. Lung Cancer. 2022;167:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Spira AI, Tu H, Aggarwal S, Hsu H, Carrigan G, Wang X, Ngarmchamnanrith G, Chia V, Gray JE. A retrospective observational study of the natural history of advanced non-small-cell lung cancer in patients with KRAS p.G12C mutated or wild-type disease. Lung Cancer. 2021;159:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Scheffler M, Ihle MA, Hein R, Merkelbach-Bruse S, Scheel AH, Siemanowski J, Brägelmann J, Kron A, Abedpour N, Ueckeroth F, Schüller M, Koleczko S, Michels S, Fassunke J, Pasternack H, Heydt C, Serke M, Fischer R, Schulte W, Gerigk U, Nogova L, Ko YD, Abdulla DSY, Riedel R, Kambartel KO, Lorenz J, Sauerland I, Randerath W, Kaminsky B, Hagmeyer L, Grohé C, Eisert A, Frank R, Gogl L, Schaepers C, Holzem A, Hellmich M, Thomas RK, Peifer M, Sos ML, Büttner R, Wolf J. K-ras Mutation Subtypes in NSCLC and Associated Co-occuring Mutations in Other Oncogenic Pathways. J Thorac Oncol. 2019;14:606-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 191] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 24. | Gainor JF, Varghese AM, Ou SH, Kabraji S, Awad MM, Katayama R, Pawlak A, Mino-Kenudson M, Yeap BY, Riely GJ, Iafrate AJ, Arcila ME, Ladanyi M, Engelman JA, Dias-Santagata D, Shaw AT. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res. 2013;19:4273-4281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 509] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 25. | Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1939] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 26. | Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, Gaida K, Holt T, Knutson CG, Koppada N, Lanman BA, Werner J, Rapaport AS, San Miguel T, Ortiz R, Osgood T, Sun JR, Zhu X, McCarter JD, Volak LP, Houk BE, Fakih MG, O'Neil BH, Price TJ, Falchook GS, Desai J, Kuo J, Govindan R, Hong DS, Ouyang W, Henary H, Arvedson T, Cee VJ, Lipford JR. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 1629] [Article Influence: 232.7] [Reference Citation Analysis (1)] |
| 27. | de Langen AJ, Johnson ML, Mazieres J, Dingemans AC, Mountzios G, Pless M, Wolf J, Schuler M, Lena H, Skoulidis F, Yoneshima Y, Kim SW, Linardou H, Novello S, van der Wekken AJ, Chen Y, Peters S, Felip E, Solomon BJ, Ramalingam SS, Dooms C, Lindsay CR, Ferreira CG, Blais N, Obiozor CC, Wang Y, Mehta B, Varrieur T, Ngarmchamnanrith G, Stollenwerk B, Waterhouse D, Paz-Ares L; CodeBreaK 200 Investigators. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRAS(G12C) mutation: a randomised, open-label, phase 3 trial. Lancet. 2023;401:733-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 374] [Article Influence: 124.7] [Reference Citation Analysis (0)] |