Published online Sep 6, 2024. doi: 10.12998/wjcc.v12.i25.5749
Revised: June 27, 2024
Accepted: July 3, 2024
Published online: September 6, 2024
Processing time: 56 Days and 16.1 Hours
The prevalence of nonalcoholic fatty liver disease (NAFLD) in patients with chronic hepatitis B (CHB) has increased in recent clinical practice; however, the relationship between CHB and hepatic steatosis (HS) remains controversial.
To shed light on the potential association between NAFLD and hepatitis B virus (HBV) infection.
We conducted a systematic literature search using multiple databases, including PubMed, the Cochrane Library, Web of Science, and EMBASE, to identify relevant studies. Predefined inclusion criteria were used to determine the eligibility of the studies for further analysis.
Comprehensive meta-analysis software was used for statistical analysis, which covered 20 studies. The results indicated a lower NAFLD susceptibility in HBV-infected individuals (pooled OR = 0.87; 95%CI = 0.69-1.08; I2 = 91.1%), with diabetes (P = 0.015), body mass index (BMI; P = 0.010), and possibly age (P = 0.061) as heterogeneity sources. Of note, in four studies (6197 HBV patients), HBV-infected individuals had a reduced NAFLD risk (OR = 0.68, 95%CI = 0.51-0.89, P = 0.006). A positive link between hyperlipidemia and metabolic syndrome emerged in hepatitis B patients, along with specific biochemical indicators, including BMI, creatinine, uric acid, fasting blood glucose, and homeostasis model assessment of insulin resistance.
HBV infection may provide protection against HS; however, the occurrence of HS in patients with HBV infection is associated with metabolic syndrome and specific biochemical parameters.
Core Tip: Investigating chronic hepatitis B (CHB) and nonalcoholic fatty liver disease (NAFLD) was reviewed in 20 studies. The results suggest reduced NAFLD susceptibility in hepatitis B virus (HBV)-infected individuals (OR = 0.87, 95%CI = 0.69-1.08, I2 = 91.1%). In HBV patients, a positive correlation emerged between hyperlipidemia, metabolic syndrome, and indicators, such as body mass index, creatinine, uric acid, fasting blood glucose, and homeostasis model assessment of insulin resistance. HBV may protect from hepatic steatosis, but its occurrence is associated with metabolic syndrome and specific factors. This study describes the complex interplay between CHB, NAFLD, and its associated factors.
- Citation: Zhang L, Wu HD, Qian YF, Xu HY. Prevalence of nonalcoholic fatty liver disease in patients with hepatitis B: A meta-analysis. World J Clin Cases 2024; 12(25): 5749-5760
- URL: https://www.wjgnet.com/2307-8960/full/v12/i25/5749.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i25.5749
Nonalcoholic fatty liver disease (NAFLD) is an acquired metabolic liver injury in adults. It is characterized by hepatic steatosis (HS), except alcohol and other definite liver damage factors, and is commonly associated with obesity, hyperlipidemia, and insulin resistance[1,2]. The global incidence of NAFLD is increasing, in part, because of improved living standards and lifestyle changes. It has emerged as a leading cause of chronic liver disease worldwide[3]. The prevalence of NAFLD ranges from 6% to 35% globally, with a median of 20%[4]. In clinical practice, there is an increasing occurrence of NAFLD and chronic hepatitis B (CHB) presenting together[5], which affects approximately 29.6% of CHB patients worldwide[6]. Because both hepatitis B virus (HBV) infection and NAFLD independently contribute to chronic liver injury, exacerbated liver damage, and an increased risk of cirrhosis and hepatocellular carcinoma, understanding their relationship is of paramount importance[7-9].
The association between CHB and HS has been a subject of debate[10]. Although a previous meta-analysis indicated a negative association between hepatitis B and the risk of NAFLD[11], a subsequent study by Azarkar et al[12] demon
This study was conducted according to the Meta-analysis of Observational Studies in Epidemiology criteria[15]. To ensure transparency and credibility, we prospectively registered the study protocol at PROSPERO (CRD42022376132). The PubMed, the Cochrane Library, Web of Science, and EMBASE databases were searched through December 30, 2022. The search terms included “hepatitis b virus” OR “b virus, hepatitis” OR “hepatitis b viruses” OR “viruses, hepatitis b” OR “HBV,” and “nonalcoholic fatty liver disease” OR “NAFLD” OR “fatty live, nonalcoholic” OR “live, nonalcoholic fatty” OR “nonalcoholic fatty live” OR “nonalcoholic steatohepatitis” OR “steatohepatitis, nonalcoholic.” In addition, we reviewed the references of the retrieved articles to ensure the inclusion of all relevant studies.
For inclusion in the meta-analysis, studies had to meet the following criteria: (1) Written in English or Chinese; (2) Cross-sectional, case-control, or cohort study design; (3) involved adult participants aged 18 years and older; and (4) Provided data that allowed an assessment of NAFLD risk (e.g., number of NAFLD cases in the HBV and non-HBV groups, or hazard ratios with their 95%CIs). Studies were excluded if they (1) investigated alcoholic liver disease; (2) were letters, review articles, case reports, case series, in vitro studies, or animal studies; (3) were duplicate publications; or (4) provided ambiguous or insufficient information.
To ensure accuracy, two researchers independently collected data, including details regarding study design, location, author names, year of publication, the number of NAFLD cases, age and gender distribution, follow-up duration, and RR or OR with 95%CI in the HBV-positive and HBV-negative groups. A positive HBsAg test was used for the identification of HBV infection.
The risk of bias across the included studies was assessed using the Newcastle-Ottawa Scale (NOS)[16], and the results were categorized as low quality (NOS score < 4), medium quality (NOS score 4-6), and high quality (NOS score 7-9)[17]. Any discrepancies were resolved through consensus.
Dichotomous variables were expressed as OR RR with 95%CI. Continuous variables were presented as the weighted mean difference (SMD) with its CI. The heterogeneity between included studies was assessed using the Q statistic and I2 index, where I2 25% indicated a small degree of heterogeneity and I2 50% indicated significant heterogeneity[18]. In the presence of statistically significant heterogeneity, meta-regression was used to analyze potential sources of heterogeneity, such as age, year, study quality, study design, diagnosis of fatty liver, body mass index (BMI), diabetes, and study location. A factor with a meta-regression coefficient of P < 0.05 was considered a source of heterogeneity. Because of the observed significant heterogeneity, random-effects models were preferred over fixed-effects models. The use of random-effects models allows for possible variations in study procedures and settings, making the findings more generalizable[19].
Egger’s and Begg’s P values were utilized for the assessment of publication bias, along with funnel plot asymmetry, where P < 0.05 pointed to a significant publication bias. Symmetrical funnel plots upon visual inspection suggest no publication bias. Statistical analysis was conducted using STATA software version 16 (Stata Corp., College Station, TX, United States), with a threshold of P < 0.05 indicating statistical significance.
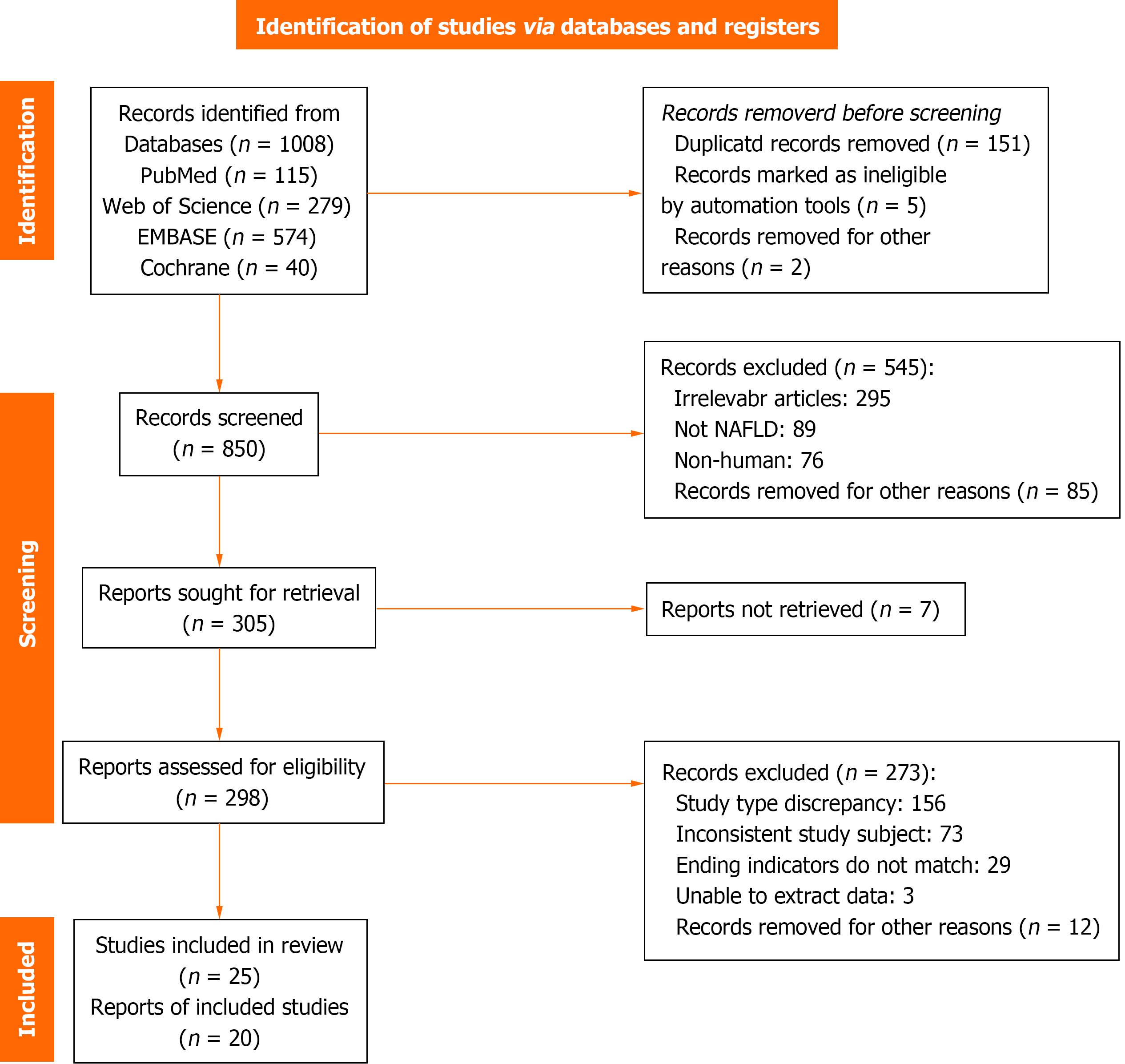
Figure 1 shows the strategy for selecting the studies. An initial search of the databases yielded 1008 articles, including 40 from Cochrane, 279 from Web of Science, 115 from PubMed, and 574 from EMBASE. After removing 151 duplicates, we screened the titles and abstracts of the remaining articles, leading to the identification of 298 full-text articles. A final set of 20 articles[10,12,13,20-36] were considered eligible for the meta-analysis after excluding studies that did not meet the eligibility criteria.
All of the included studies were observational and consisted of seven cross-sectional[22,23,25,31,34-36], five case-control[12,20,21,24,28], and eight cohort studies[10,13,26,27,29,30,32,33]. The characteristics of the studies are summarized in Table 1. In total, data from 157734 patients were evaluated, with 50.05% being male. Of these patients, 49598 (31.44%) were diagnosed with NAFLD. The studies were conducted in various regions of the world, including China, Iran, Israel, South Korea, Pakistan, and Turkey. The NOS scores of the analyzed studies ranged from 6 to 9, with 18 studies rated as high quality and two as medium quality (Table 2).
| Ref. | Study location | Number of NAFLD | Sample size (HBV+ /HBV-) | Age (year) | Gender (F/M) | Subtype of study | Diagnosis of fatty liver | Follow | Adjusted OR/RR (95%CI) |
| Wang et al[20], 2008 | Taiwan | 257 | 50/457 | 44.6 ± 1.4/46.8 ± 0.4 | 264/243 | Case-control | Ultrasonography | NA | 0.97 (0.48, 1.95) |
| Yun et al[21], 2009 | Korea | 44 | 68/18 | 21.0 (20-26) | 0/86 | Case-control | Liver biopsy | 2005-2006 | 1.19 (1.17, 2.83) |
| Wong et al[22], 2012 | Hong Kong | 273 | 91/922 | 49 ± 10/48 ± 11 | 580/433 | Cross-sectional | MRS | 2008-2010 | 0.42 (0.20, 0.88) |
| Cheng et al[23], 2013 | Taiwan | 14671 | 3642/29797 | 51.9 ± 13.1 | 15182/18257 | Cross-sectional | Ultrasonography | 2002-2009 | 0.66 (0.59, 0.72) |
| Peng et al[24], 2013 | China | 527 | 253/922 | 20-74 | 438/1131 | Case-control | Ultrasonography | 2007-2008 | 3.96 (2.10, 7.48) |
| Yilmaz et al[25], 2015 | Turkey | 28 | 88/0 | 31 ± 1.1 | 27/61 | Cross-sectional | Liver biopsy | NA | 0.21 (0.11, 0.41) |
| Chan et al[26], 2017 | Hong Kong | 107 | 270/0 | 43.6 ± 11.3 | 67/203 | Cohort | Liver biopsy | 2006-2009 | 0.43 (0.30, 0.60) |
| Joo et al[27], 2017 | Korea | 20200 | 3926/79413 | 38.5 (7.2)/ 37.5 (7.6) | 40856/42483 | Cohort | Ultrasonography | 2002-2014 | 0.78 (0.72, 0.84) |
| Zhong et al[28], 2018 | China | 631 | 291/2697 | 45-66 | 1849/1139 | Case-control | Ultrasonography | 2015-2016 | 0.64 (0.42, 0.95) |
| Azarkar et al[12], 2019 | Iran | 336 | 373/447 | 41.1 ± 12.9/39.8 ± 13.9 | 403/420 | Case-control | Ultrasonography | 2013-2014 | 0.62 (0.455, 0.845) |
| Lee et al[29], 2019 | Korea | 70 | 321/0 | 41 (33-49) | 125/196 | Cohort | Liver biopsy | 2007-2015 | 1.56 (0.90, 2.71) |
| Peleg et al[30], 2019 | Israel | 241 | 524/0 | 42.32 (18.5-83.5) | 209/315 | Cohort | Ultrasonography | 2007-2017 | 0.08 (0.02, 0.28) |
| Sharif et al[31], 2019 | Pakistan | 166 | 230/0 | 36.8 ± 10.84 | 27/203 | Cross-sectional | Transient elastography | 2018-2019 | 5.4 (3.62, 8.03) |
| Wang et al[13], 2019 | China | 308 | 152/1714 | 46.42 ± 10.291 | 1866/0 | Cohort | Ultrasonography | 2011-2014 | 0.656 (0.379, 1.134) |
| Zhu et al[32], 2019 | China | 283 | 2393/0 | 50.7 ± 13.2 | 1767/626 | Cohort | Ultrasonography | 2012-2015 | 0.89 (0.69, 1.15) |
| Huang et al[33], 2020 | China | 4917 | 2110/12342 | 43.84 ± 13.03 | 6710/7742 | Cohort | Ultrasonography | 2016-2018 | 0.717 (0.608, 0.846) |
| Su et al[34], 2020 | Taiwan | 104 | 30/74 | 49.97 ± 14.76 | 47/57 | Cross-sectional | Liver biopsy | 2009-2018 | 3.55 (1.46, 8.58) |
| Zhuang et al[35], 2020 | China | 88 | 46/42 | 37.37 ± 10.11/39.64 ± 12.31 | 13/75 | Cross-sectional | MRS | 2013-2016 | 0.322 (0.095, 0.550) |
| Lv et al[10], 2021 | China | 6315 | 5680/10771 | 42.6 ± 11.2/46.9 ± 10.6 | 6519/9932 | Cohort | Ultrasonography | 2013-2017 | NA |
| Zhou et al[36], 2022 | China | 32 | 78/105 | 45.41 ± 11.59 | 54/129 | Cross-sectional | Ultrasonography | 2019-2020 | 1.95 (0.90, 4.22) |
| Ref. | Selection | Comparability | Exposure | Total score | |||||
| Fully defined cases | Define the study design | Selection of controls | Described the general characteristics | Controlling the important factors or confounding factors | List inclusion and exclusion criteria for all the participants | Provided Enrollment duration for all the participants | Indicate study period and follow-up duration | ||
| Wang et al[20], 2008 | * | * | * | 2 | 1 | 1 | 7 | ||
| Yun et al[21], 2009 | 1 | 1 | 1 | 2 | 1 | 1 | 7 | ||
| Wong et al[22], 2012 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | |
| Cheng et al[23], 2013 | 1 | 1 | 1 | 2 | 1 | 1 | 7 | ||
| Peng et al[24], 2013 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | ||
| Yilmaz et al[25], 2015 | 1 | 1 | 1 | 2 | 1 | 1 | 7 | ||
| Chan et al[26], 2017 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Joo et al[27], 2017 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Zhong et al[28], 2018 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 8 | |
| Azarkar et al[12], 2019 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 8 | |
| Lee et al[29], 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |
| Peleg et al[30], 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |
| Sharif et al[31], 2019 | 1 | 1 | 1 | 2 | 1 | 1 | 7 | ||
| Wang et al[13], 2019 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Zhu et al[32], 2019 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | |
| Huang et al[33], 2020 | 1 | 1 | 1 | 2 | 1 | 6 | |||
| Su et al[34], 2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |
| Zhuang et al[35], 2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |
| Lv et al[10], 2021 | 1 | 1 | 1 | 2 | 1 | 1 | 7 | ||
| Zhou et al[36], 2022 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |
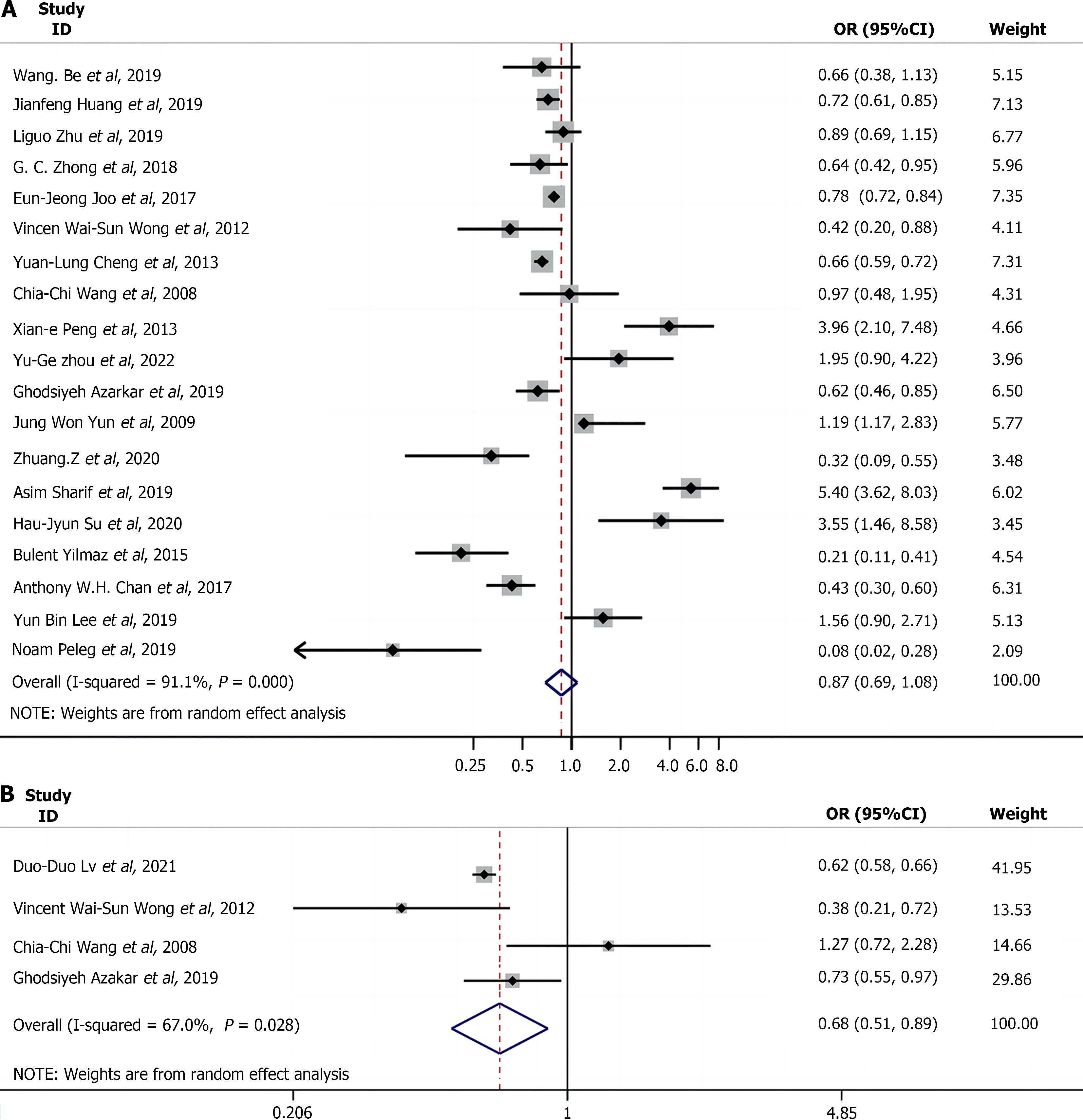
We performed a meta-analysis on 20 studies to determine a link between NAFLD and hepatitis B infection. Of these studies, 13[10,12,13,22,23,25-28,30,32,33] indicated that hepatitis B infection was associated with a lower risk of NAFLD, whereas seven[20,21,24,29,31,34,36] showed a slightly higher risk of NAFLD among patients with HBV infection compared with those without. A meta-analysis of 19 of the studies[10,12,13,20-24,26-36] revealed that the OR/RR with 95%CIs using a random-effects model and a Q and I2 test, suggested significant heterogeneity among them (P = 0.000, I2 = 91.1%). The analysis revealed that patients with CHB did not show significant differences in the risk for NAFLD compared with those without CHB, but there was a possible reduction of 12.7% in the risk of NAFLD in patients with CHB (OR = 0.87, 95%CI = 0.69-1.08, P = 0.209; Figure 2A). Nex, the incidence of NAFLD was compared between patients with and without hepatitis B infection by analyzing four of the included studies[10,12,20,22] consisting of 6197 patients with CHB (including 1947 patients with NAFLD) and 12597 non-CHB patients (including 2534 patients with NAFLD). Because of the heterogeneity across the four studies in terms of the incidence of NAFLD, a random-effects model was used (P = 0.028, I2 = 67.0%) for data analysis, which indicated a lower incidence of NAFLD in patients with CHB compared with non-CHB controls (OR = 0.68, 95%CI = 0.51-0.89, P = 0.006; Figure 2B). This suggests that HBV may have a protective effect against HS.
Univariate meta-regression was used to identify potential causes of heterogeneity among factors that could influence the results, including age, year, study quality, study design, diagnosis of fatty liver, BMI, diabetes, study location, and follow-up duration. To ensure comprehensive consideration of potential influential factors, the test level α was relaxed to 0.1. The results indicated that BMI (P = 0.010) and diabetes (P = 0.015) were significant contributors to heterogeneity, whereas age (P = 0.061) was identified as a possible factor (Table 3). A further subgroup analysis based on age revealed a higher risk of developing NAFLD in patients with CHB older than 45 years (RR = 1.04, 95%CI = 0.72-1.48) compared with those with CHB under 45 years (RR = 0.72, 95%CI = 0.50-1.05).
| Heterogeneous factors | Coef. | SE | 95%CI | P value | |
| Lower | Upper | ||||
| Age | 0.477 | 0.391 | -0.347 | 1.302 | 0.061 |
| Year | -0.020 | 0.055 | -0.136 | 0.096 | 0.723 |
| Study quality | -0.527 | 0.734 | -2.075 | 1.021 | 0.718 |
| Study design | -0.226 | 0.265 | -0.784 | 0.332 | 0.959 |
| Diagnosis of fatty liver | -0.095 | 0.223 | -0.565 | 0.375 | 0.827 |
| BMI | 0.848 | 0.389 | 0.233 | 1.673 | 0.010 |
| Diabetes | 0.790 | 0.417 | -0.112 | 1.691 | 0.015 |
| Study location | 0.992 | 0.082 | 0.832 | 1.182 | 0.208 |
| Follow | -0.068 | 0.057 | -0.191 | 0.054 | 0.603 |
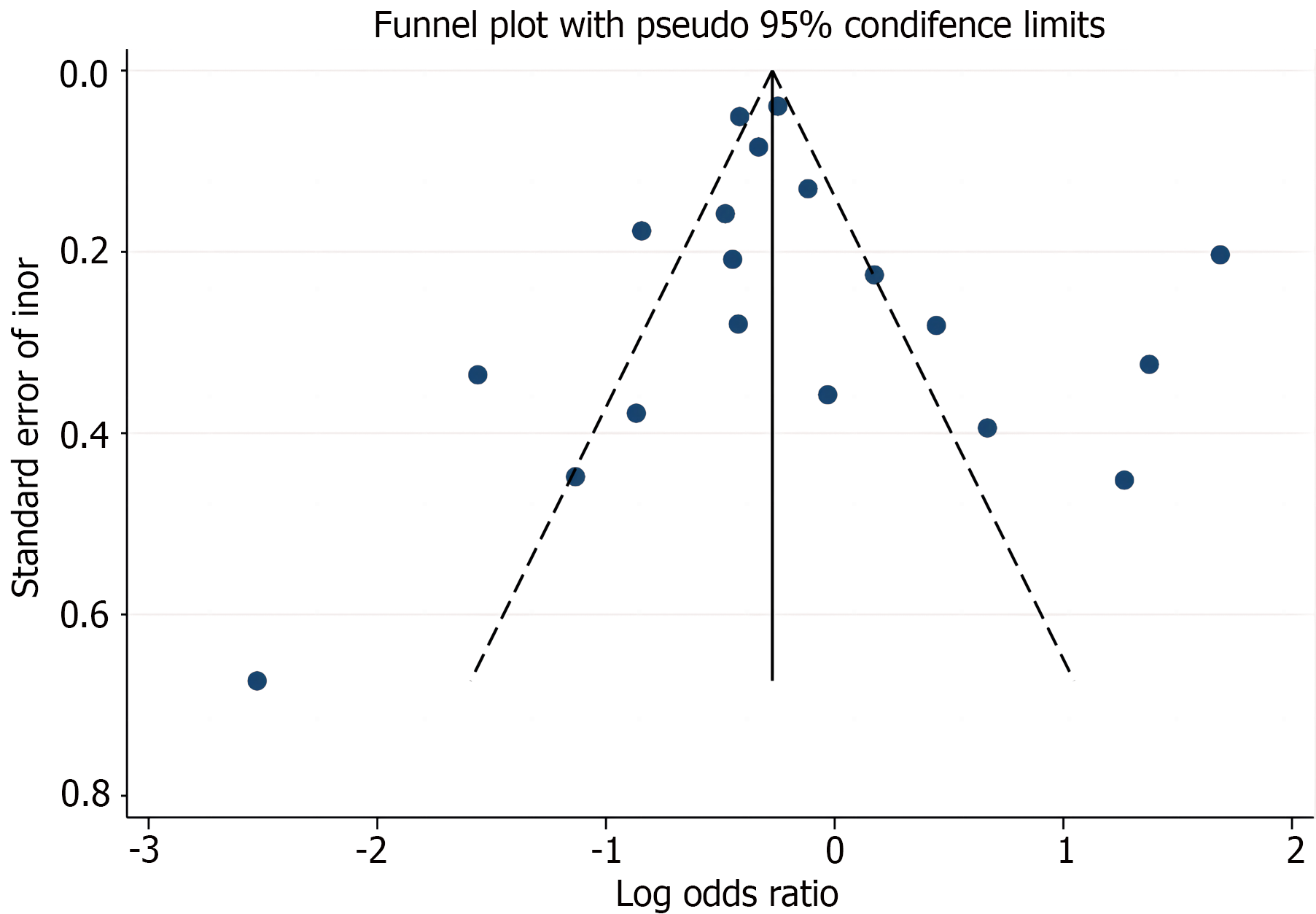
An assessment of publication bias was performed using a funnel plot, which displayed a largely symmetrical pattern (Figure 3). The results of Egger’s linear regression (P = 0.486) and Begg’s rank correlation (P = 0.944) tests suggested no significant publication bias.
The evaluation of risk factors for HS in patients with CHB revealed a strong association between BMI and the incidence of HS (in five studies: Pooled SMD = 2.40, 95%CI = 1.20-3.61, P < 0.001; in seven studies: OR = 1.24, 95%CI = 1.21-1.27, P < 0.001). In addition, metabolic disorders indicated by abnormal levels of fasting blood glucose (FBG), homeostatic model assessment-insulin resistance (HOMA-IR), and low-density lipoprotein cholesterol (LDL-C) were also contributing factors to HS in these patients. A statistical analysis revealed that these biochemical abnormalities were significant risk factors for liver steatosis among patients with CHB (Table 4).
| Variable | Number of studies | Random effects model | Heterogeneity | Publication bias | ||||
| OR or SMD (95%CI) | P value | Q value | P value | I2 | Egger’s P value | Begg’s P value | ||
| BMI (kg/m2) | 7 | OR: 1.24 (1.21, 1.27) | < 0.01 | 27.79 | < 0.01 | 78.40 | 0.69 | 0.88 |
| 5 | SMD: 2.40 (1.20, 3.61) | < 0.01 | 34.11 | < 0.01 | 88.30 | 0.94 | 0.73 | |
| ALT | 5 | OR: 1.01 (1.00, 1.03) | 0.05 | 140.35 | < 0.01 | 97.10 | 0.38 | 0.81 |
| 5 | SMD: 6.76 (0.92, 12.60) | 0.02 | 24.28 | < 0.01 | 83.50 | 0.01 | 0.46 | |
| Creatinine | 2 | SMD: 4.73 (4.15, 5.31) | < 0.01 | 0.17 | 0.68 | 0.00 | NA | 0.32 |
| UA | 3 | SMD: 64.24 (60.75, 67.74) | < 0.01 | 0.59 | 0.74 | 0.00 | 0.16 | 0.60 |
| FBG | 6 | OR: 1.58 (1.13, 1.77) | < 0.01 | 114.14 | < 0.01 | 95.60 | 0.49 | 0.85 |
| 5 | SMD: 1.55 (1.43, 1.68) | < 0.01 | 9.86 | 0.04 | 59.40 | 0.92 | 0.81 | |
| Cholesterol | 7 | SMD: 0.37 (0.12, 0.62) | < 0.01 | 37.01 | < 0.01 | 83.80 | 0.01 | 0.76 |
| LDL-C | 6 | SMD: 0.28 (0.03, 0.52) | 0.03 | 66.65 | < 0.01 | 92.50 | 0.06 | 0.71 |
| Triglyceride | 6 | OR: 1.71 (1.14, 2.55) | 0.01 | 59.19 | < 0.01 | 91.60 | 0.04 | 0.45 |
| HOMA-IR | 3 | OR: 1.46 (1.17, 1.83) | < 0.01 | 6.68 | 0.03 | 70.90 | 0.40 | 0.60 |
Nearly 350 million people have chronic HBV infection worldwide[37] and approximately 25% of the population is affected by NAFLD[7]. When these two conditions coexist, they exert significant damage to the liver, accelerating the progression of liver fibrosis and liver cancer. To systematically examine the correlation between CHB and NAFLD, we used a meta-analysis approach. Interestingly, the results suggest that CHB infection may reduce the risk of NAFLD.
A meta-analysis published in 2017, which examined how CHB affects the occurrence of NAFLD, reported similar results to our study; however, only five publications were included. It found that CHB infection was linked to a decreased incidence of NAFLD (OR = 0.71, 95%CI = 0.53-0.90, I2 = 75.20%, P < 0.05), but did not confirm a negative correlation in case-control studies[11]. In contrast, our analysis of 20 studies confirmed a negative correlation between CHB infection and the risk of NAFLD in cross-sectional studies (OR = 0.96, 95%CI = 0.40-2.29) and cohort studies (OR = 0.71, 95%CI = 0.56-0.89). However, in case-control studies, this negative correlation was not confirmed (OR = 1.09, 95%CI = 0.61-1.93), possibly because of recall and selection biases inherent in case-control designs, leading to insufficient data to determine the association between CHB and the risk of NAFLD. The majority of the included studies were cross-sectional studies and cohort studies, which may also contribute to the discrepancy in the results.
The results of our analysis suggest that having an HBV infection may decrease the chances of developing NAFLD, although the precise underlying mechanisms remain unclear. Several possible biological explanations could account for this phenomenon. First, HBV infection appears to affect fat distribution. Studies have found associations between HBV infection and alterations in cholesterol, triglycerides, high-density lipoprotein cholesterol, and LDL-C[38-40]. Second, HBV infection can affect fat metabolism, possibly by reducing the levels of apolipoprotein A5[41]. Decreased expression of the apolipoprotein A5 gene is associated with reduced intrahepatic triglyceride levels[42], potentially affecting the development of NAFLD. This may result in a minor degree of hepatocyte steatosis that is insufficient to form a fatty liver because of an inability to accumulate more hepatocytes[39]. Third, HBV-X protein may also be involved in the development of NAFLD by hindering the synthesis of apolipoprotein B, which is essential for the formation of (very) low-density lipoprotein[43]. The HBV-X protein interacts with liver X receptor-a and TNF receptor 1, leading to lipid accumulation, impaired apolipoprotein secretion, and inflammation induction, all of which increase the risk of NAFLD in HBV-infected patients[44]. Finally, some studies have reported associations from various perspectives. For example, HBV infection was found to be linked to a lower risk of other health problems, including heart disease and hypertension[45,46], which are positively correlated with NAFLD[47]. This suggests that HBV infection may not only lower the risk of NAFLD but also reduce the risk of cardiovascular diseases.
A meta-regression analysis was performed to determine the source of heterogeneity among the studies. BMI and diabetes were identified as significant factors contributing to the observed heterogeneity, whereas age may also have a role in variation between studies. Several studies have highlighted the importance of host factors, such as BMI and fasting glucose, in the development of NAFLD in CHB patients[23,25,48-50]. For example, Viganò et al[51] found that 62% of CHB patients with steatosis exhibited high BMI and hyperglycemia. A subgroup analysis based on age indicated that patients with CHB over the age of 45 years had an increased risk of developing NAFLD compared with those younger than 45 (RR = 1.04, 95%CI = 0.72-1.48). This may be the result of a higher risk of complications, such as cardiovascular disease[47] and metabolic syndrome[45], which are positively correlated with NAFLD and tend to increase with age. The observed heterogeneity across the included studies may have been caused by differences in study design and demographic characteristics, such as age, BMI, and glycemia.
Although we used a statistical analysis to explore the sources of heterogeneity, there may still be other factors that we did not consider, such as differences in sample size (ranging from 88 to 83339 cases) and variations in the study population (including differences in ethnicity, physical activity, and dietary factors) in the literature.
Interestingly, our findings suggest that BMI is a significant factor for HS in patients with HBV infection (OR 1.24; SMD 2.40). A similar study found that BMI was a significant factor for HS in people with both HBV infection and NAFLD compared with those with HBV infection alone[52]. Metabolic factors also contribute to a higher risk of HS. For example, FBG and HOMA-IR conferred a 58% and 46% increased risk of HS, respectively. Moreover, our results showed that viral hepatitis-infected patients had significantly higher levels of creatinine, cholesterol, LDL-C, and triglycerides, which are risk factors for HS, compared with those without viral hepatitis. These results are consistent with the importance of metabolic factors in the development of NAFLD and metabolic syndrome[53]. Furthermore, we found that uric acid and alanine transaminase levels were significantly increased in patients with both HS and CHB. An earlier study[30] indicated that the co-occurrence of liver steatosis and CHB had a synergistic impact, leading to a quicker advancement of advanced liver disease and related complications, compared with the presence of NAFLD alone. This finding may help to clarify our findings. Nevertheless, additional studies comparing the long-term outcomes of patients with CHB and liver steatosis with those who have NAFLD, but no CHB, are necessary to confirm this finding.
The present study has some limitations. First, only Chinese and English literature studies were included. Second, the sample sizes varied widely among the included studies. Third, the studies used different methods to diagnose NAFLD, ranging from ultrasound to magnetic resonance imaging and pathological examination. In addition, the studies varied in type, including cross-sectional studies, cohort studies, and case-control studies. As a result of these differences in diagnostic methods and study design, we assessed the heterogeneity across the various studies. Although we considered and analyzed multiple factors for the heterogeneity, some potential risk factors, including those related to physical activity and diet, besides alcohol, were not considered. Studies have demonstrated that individuals infected with HBV may prioritize physical activity to enhance their dietary habits, which may affect the incidence and progression of NAFLD in humans[54,55].
To summarize, our meta-analysis indicated that hepatitis B infection protects against the occurrence of NAFLD; however, this association was observed only in cross-sectional and cohort studies, not in case-control studies. To further examine this relationship and understand the underlying reasons, additional prospective studies and basic research are needed. Specifically, if it can be established that a substance produced during HBV infection reduces the incidence of NAFLD, drugs containing this substance could be developed to prevent or treat NAFLD.
| 1. | Wang TY, Wang RF, Bu ZY, Targher G, Byrne CD, Sun DQ, Zheng MH. Association of metabolic dysfunction-associated fatty liver disease with kidney disease. Nat Rev Nephrol. 2022;18:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 151] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 2. | Das UN. Bioactive lipids in intervertebral disc degeneration and its therapeutic implications. Biosci Rep. 2019;39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Calzadilla-Bertot L, Vilar-Gomez E, Wong VW, Romero-Gomez M, Aller-de la Fuente R, Wong GL, Castellanos M, Eslam M, Desai AP, Jeffrey GP, George J, Chalasani N, Adams LA. ABIDE: An Accurate Predictive Model of Liver Decompensation in Patients With Nonalcoholic Fatty Liver-Related Cirrhosis. Hepatology. 2021;73:2238-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7872] [Article Influence: 787.2] [Reference Citation Analysis (2)] |
| 5. | Huang Y, Gan Q, Lai R, Wang W, Guo S, Sheng Z, Chen L, Guo Q, Cai W, Wang H, Zhao G, Cao Z, Xie Q. Application of Fatty Liver Inhibition of Progression Algorithm and Steatosis, Activity, and Fibrosis Score to Assess the Impact of Non-Alcoholic Fatty Liver on Untreated Chronic Hepatitis B Patients. Front Cell Infect Microbiol. 2021;11:733348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Wong SW, Chan WK. Epidemiology of non-alcoholic fatty liver disease in Asia. Indian J Gastroenterol. 2020;39:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Tao X, Chen L, Zhao Y, Liu Y, Shi R, Jiang B, Mi Y, Xu L. A Novel Noninvasive Diagnostic Model of HBV-Related Inflammation in Chronic Hepatitis B Virus Infection Patients With Concurrent Nonalcoholic Fatty Liver Disease. Front Med (Lausanne). 2022;9:862879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Kim GW, Imam H, Khan M, Mir SA, Kim SJ, Yoon SK, Hur W, Siddiqui A. HBV-Induced Increased N6 Methyladenosine Modification of PTEN RNA Affects Innate Immunity and Contributes to HCC. Hepatology. 2021;73:533-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 9. | Spradling PR, Bulkow L, Teshale EH, Negus S, Homan C, Simons B, McMahon BJ. Prevalence and causes of elevated serum aminotransferase levels in a population-based cohort of persons with chronic hepatitis B virus infection. J Hepatol. 2014;61:785-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Lv DD, Wang YJ, Wang ML, Chen EQ, Tao YC, Zhang DM, Tang H. Effect of silibinin capsules combined with lifestyle modification on hepatic steatosis in patients with chronic hepatitis B. Sci Rep. 2021;11:655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Xiong J, Zhang H, Wang Y, Wang A, Bian J, Huang H, Zheng Y, Sang X, Xu Y, Lu X, Zhao H. Hepatitis B virus infection and the risk of nonalcoholic fatty liver disease: a meta-analysis. Oncotarget. 2017;8:107295-107302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Azarkar G, Doosti Z, Osmani F, Ziaee M. Analysis Of Risk Factors For Nonalcoholic Fatty-Liver Disease In Hepatitis B Virus Infection: A Case-Control Study. Hepat Med. 2019;11:153-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Wang B, Li W, Fang H, Zhou H. Hepatitis B virus infection is not associated with fatty liver disease: Evidence from a cohort study and functional analysis. Mol Med Rep. 2019;19:320-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Yang M, Wei L. Impact of NAFLD on the outcome of patients with chronic hepatitis B in Asia. Liver Int. 2022;42:1981-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14425] [Cited by in RCA: 17179] [Article Influence: 660.7] [Reference Citation Analysis (0)] |
| 16. | Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, Niu Y, Du L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8:2-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1492] [Cited by in RCA: 1515] [Article Influence: 137.7] [Reference Citation Analysis (0)] |
| 17. | Zhang YP, Li WQ, Sun YL, Zhu RT, Wang WJ. Systematic review with meta-analysis: coffee consumption and the risk of gallstone disease. Aliment Pharmacol Ther. 2015;42:637-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 26882] [Article Influence: 1120.1] [Reference Citation Analysis (0)] |
| 19. | Trikalinos TA, Salanti G, Zintzaras E, Ioannidis JP. Meta-analysis methods. Adv Genet. 2008;60:311-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Wang CC, Hsu CS, Liu CJ, Kao JH, Chen DS. Association of chronic hepatitis B virus infection with insulin resistance and hepatic steatosis. J Gastroenterol Hepatol. 2008;23:779-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Yun JW, Cho YK, Park JH, Kim HJ, Park DI, Sohn CI, Jeon WK, Kim BI, Son BH, Shin JH. Hepatic steatosis and fibrosis in young men with treatment-naïve chronic hepatitis B. Liver Int. 2009;29:878-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Wong VW, Wong GL, Chu WC, Chim AM, Ong A, Yeung DK, Yiu KK, Chu SH, Chan HY, Woo J, Chan FK, Chan HL. Hepatitis B virus infection and fatty liver in the general population. J Hepatol. 2012;56:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 197] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 23. | Cheng YL, Wang YJ, Kao WY, Chen PH, Huo TI, Huang YH, Lan KH, Su CW, Chan WL, Lin HC, Lee FY, Wu JC. Inverse association between hepatitis B virus infection and fatty liver disease: a large-scale study in populations seeking for check-up. PLoS One. 2013;8:e72049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Peng XE, Chen FL, Wu YL, Lu QQ, Chen WN, Xu L. The roles of HBV infection and host factors in ultrasound-diagnosed fatty liver: a case-control study. Hepatogastroenterology. 2013;60:1698-1704. [PubMed] |
| 25. | Yilmaz B, Koklu S, Buyukbayram H, Yalçin K, Korkmaz U, Posul E, Can G, Kurt M. Chronic hepatitis B associated with hepatic steatosis, insulin resistance, necroinflammation and fibrosis. Afr Health Sci. 2015;15:714-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Chan AW, Wong GL, Chan HY, Tong JH, Yu YH, Choi PC, Chan HL, To KF, Wong VW. Concurrent fatty liver increases risk of hepatocellular carcinoma among patients with chronic hepatitis B. J Gastroenterol Hepatol. 2017;32:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 159] [Article Influence: 17.7] [Reference Citation Analysis (1)] |
| 27. | Joo EJ, Chang Y, Yeom JS, Ryu S. Hepatitis B virus infection and decreased risk of nonalcoholic fatty liver disease: A cohort study. Hepatology. 2017;65:828-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 28. | Zhong GC, Wu YL, Hao FB, Rao XW, Yuan XW, Zhao Y, Gong JP. Current but not past hepatitis B virus infection is associated with a decreased risk of nonalcoholic fatty liver disease in the Chinese population: A case-control study with propensity score analysis. J Viral Hepat. 2018;25:842-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Lee YB, Ha Y, Chon YE, Kim MN, Lee JH, Park H, Kim KI, Kim SH, Rim KS, Hwang SG. Association between hepatic steatosis and the development of hepatocellular carcinoma in patients with chronic hepatitis B. Clin Mol Hepatol. 2019;25:52-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 30. | Peleg N, Issachar A, Sneh Arbib O, Cohen-Naftaly M, Braun M, Leshno M, Barsheshet A, Shlomai A. Liver steatosis is a strong predictor of mortality and cancer in chronic hepatitis B regardless of viral load. JHEP Rep. 2019;1:9-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 31. | Sharif A, Abbas Z, Ahmed S, Ali Samjo S, Baqai K. Effect of Non-alcoholic Fatty Liver Disease on Transaminase Levels and Transient Elastography in Patients with Chronic Hepatitis B. Cureus. 2019;11:e5995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Zhu L, Jiang J, Zhai X, Baecker A, Peng H, Qian J, Zhou M, Song C, Zhou Y, Xu J, Liu H, Hang D, Hu Z, Shen H, Zhang ZF, Zhu F. Hepatitis B virus infection and risk of non-alcoholic fatty liver disease: A population-based cohort study. Liver Int. 2019;39:70-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 33. | Huang J, Jing M, Wang C, Wang M, You S, Lin S, Zhu Y. The impact of hepatitis B virus infection status on the prevalence of nonalcoholic fatty liver disease: A population-based study. J Med Virol. 2020;92:1191-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Su HJ, Kao JH, Tseng TC, Yang HC, Su TH, Chen PJ, Liu CJ. Pathologic findings of patients with nonalcoholic fatty liver disease and the impact of concurrent hepatitis B virus infection in Taiwan. J Formos Med Assoc. 2020;119:1476-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Zhuang Z, Qu H, Yang W, Liu J, Wang F, Liu Y, Ding J, Shi J. Comparing hepatic steatosis distribution patterns between non-alcoholic fatty liver disease and fatty liver disease with chronic hepatitis B by second-harmonic generation/two-photon excited fluorescence method. Ann Hepatol. 2020;19:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Zhou YG, Tian N, Xie WN. Total cholesterol to high-density lipoprotein ratio and nonalcoholic fatty liver disease in a population with chronic hepatitis B. World J Hepatol. 2022;14:791-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9500] [Cited by in RCA: 9716] [Article Influence: 694.0] [Reference Citation Analysis (0)] |
| 38. | Chiang CH, Yang HI, Jen CL, Lu SN, Wang LY, You SL, Su J, Iloeje UH, Chen CJ; REVEAL-HBV Study Group. Association between obesity, hypertriglyceridemia and low hepatitis B viral load. Int J Obes (Lond). 2013;37:410-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Liu J, Yang HI, Lee MH, Lu SN, Jen CL, Wang LY, You SL, Iloeje UH, Chen CJ; REVEAL-HBV Study Group. Incidence and determinants of spontaneous hepatitis B surface antigen seroclearance: a community-based follow-up study. Gastroenterology. 2010;139:474-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 40. | Chen JY, Wang JH, Lin CY, Chen PF, Tseng PL, Chen CH, Chang KC, Tsai LS, Chen SC, Lu SN. Lower prevalence of hypercholesterolemia and hyperglyceridemia found in subjects with seropositivity for both hepatitis B and C strains independently. J Gastroenterol Hepatol. 2010;25:1763-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Zhu C, Gao G, Song H, Xu F, Wu K, Liu X. Hepatitis B virus inhibits apolipoprotein A5 expression through its core gene. Lipids Health Dis. 2016;15:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Ress C, Moschen AR, Sausgruber N, Tschoner A, Graziadei I, Weiss H, Schgoer W, Ebenbichler CF, Konrad RJ, Patsch JR, Tilg H, Kaser S. The role of apolipoprotein A5 in non-alcoholic fatty liver disease. Gut. 2011;60:985-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Yang MH, Sung J, Gwak GY. The associations between apolipoprotein B, A1, and the B/A1 ratio and nonalcoholic fatty liver disease in both normal-weight and overweight Korean population. J Clin Lipidol. 2016;10:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 44. | Kim JY, Song EH, Lee HJ, Oh YK, Choi KH, Yu DY, Park SI, Seong JK, Kim WH. HBx-induced hepatic steatosis and apoptosis are regulated by TNFR1- and NF-kappaB-dependent pathways. J Mol Biol. 2010;397:917-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Razi B, Alizadeh S, Omidkhoda A, Imani D, Rezaei R. Association of chronic hepatitis B infection with metabolic syndrome and its components: Meta-analysis of observational studies. Diabetes Metab Syndr. 2017;11 Suppl 2:S939-S947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 46. | Tseng CH, Muo CH, Hsu CY, Kao CH. Association of hepatitis B virus infection with decreased ischemic stroke. Acta Neurol Scand. 2016;134:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 47. | Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol. 2016;65:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1050] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 48. | Pokorska-Śpiewak M, Kowalik-Mikołajewska B, Aniszewska M, Pluta M, Walewska-Zielecka B, Marczyńska M. Liver steatosis in children with chronic hepatitis B and C: Prevalence, predictors, and impact on disease progression. Medicine (Baltimore). 2017;96:e5832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Chen XL, Han YD, Wang H. Relations of hepatic steatosis with liver functions, inflammations, glucolipid metabolism in chronic hepatitis B patients. Eur Rev Med Pharmacol Sci. 2018;22:5640-5646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 50. | Nau AL, Soares JC, Shiozawa MB, Dantas-Corrêa EB, Schiavon Lde L, Narciso-Schiavon JL. Clinical and laboratory characteristics associated with dyslipidemia and liver steatosis in chronic HBV carriers. Rev Soc Bras Med Trop. 2014;47:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Viganò M, Valenti L, Lampertico P, Facchetti F, Motta BM, D'Ambrosio R, Romagnoli S, Dongiovanni P, Donati B, Fargion S, Colombo M. Patatin-like phospholipase domain-containing 3 I148M affects liver steatosis in patients with chronic hepatitis B. Hepatology. 2013;58:1245-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2643] [Article Influence: 188.8] [Reference Citation Analysis (1)] |
| 53. | Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1725] [Cited by in RCA: 1759] [Article Influence: 70.4] [Reference Citation Analysis (5)] |
| 54. | Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 378] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 55. | Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67:829-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 969] [Article Influence: 107.7] [Reference Citation Analysis (0)] |