Published online Sep 6, 2024. doi: 10.12998/wjcc.v12.i25.5697
Revised: May 31, 2024
Accepted: July 4, 2024
Published online: September 6, 2024
Processing time: 186 Days and 18.4 Hours
Bariatric and metabolic surgery have been routinely performed following the rapid increase in obesity and metabolic diseases worldwide. Of all evolving procedures, Roux-en-Y gastric bypass (RYGB) is considered the gold standard for surgical treatment of patients with type 2 diabetes mellitus (T2DM) and obesity. RYGB was introduced in China nearly 20 years ago, but the number of RYGB surgeries only accounts for 3.1% of the total number of weight loss and metabolic surgeries in China, it’s effect on Chinese people still needs further study.
To investigate the effect and safety of a modified gastric bypass performed in Chinese patients with T2DM.
Patients with obesity and T2DM who underwent modified gastric bypass, with > 5-year follow-up data, were analyzed.
All 37 patients underwent uneventful laparoscopic surgery, no patient was switched to laparotomy during the surgery, and no severe complications were reported. Average weight and body mass index of the patients reduced from 84.6 ± 17.3 (60.0–140.0) kg and 30.9 ± 5.0 (24.7–46.2) kg/m2 to 67.1 ± 12.2 (24.7–46.2) kg and 24.6 ± 3.9 (17.7–36.5) kg/m2, respectively, and fasting plasma glucose and glycated hemoglobin decreased from 7.4 ± 3.4 mmol/L and 8.2% ± 1.7% preoperatively to 6.5 ± 1.3 mmol/L and 6.5% ± 0.9% 5-years postoperatively, respectively. Only 29.7% (11/37) of the patients used hypoglycemic drugs 5-years postoperatively, and the complete remission rate of T2DM was 29.7% (11/37). Triglyceride level reduced significantly but high-density lipoprotein increased significantly (both P < 0.05) compared with those during the preoperative period. Liver and renal function improved significantly postoperatively, and binary logistic regression analysis revealed that the patients’ preoperative history of T2DM and fasting C-peptide were significant prognostic factors influencing complete T2DM remission after RYGB (P = 0.006 and 0.012, respectively).
The modified gastric bypass is a safe and feasible procedure for Chinese patients with obesity and T2DM, exhibiting satisfactory amelioration of weight problems, hyperglycemia, and combination disease.
Core Tip: We described a modified gastric bypass procedure, we report the effect of such procedure in Chinese patients with type 2 diabetes mellitus (T2DM) patients and data of it’s 5 year follow up data. We found that average weight and body mass index of all 37 patients were reduced to 67.1 ± 12.2 kg and 24.6 ± 3.9 kg/m2 respectively, while fasting plasma glucose and glycated hemoglobin A1c also reduced to 6.5 ± 1.3 mmol/L and 6.5% ± 0.9% 5 years later. Complete remission rate of T2DM at was 29.7%. Blood lipid, liver and renal function were significantly improved, history of T2DM and fasting C-peptide were significant prognostic factors influencing complete remission of T2DM after Roux-en-Y gastric bypass.
- Citation: Xing Y, Bai RX, Li YG, Xu J, Zhong ZQ, Yan M, Yan WM. Analysis of long-term outcome of modified gastric bypass for type 2 diabetes mellitus in Chinese patients. World J Clin Cases 2024; 12(25): 5697-5705
- URL: https://www.wjgnet.com/2307-8960/full/v12/i25/5697.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i25.5697
Bariatric and metabolic surgery have been routinely performed following the rapid increase in obesity and metabolic diseases worldwide, and the surgical procedures are constantly changing. Of these evolving procedures, Roux-en-Y gastric bypass (RYGB) is considered the gold standard for surgical treatment of patients with type 2 diabetes mellitus (T2DM) and obesity[1-5]. RYGB was introduced in China nearly 20 years ago, but the number of RYGB surgeries only accounts for 3.1% of the total number of weight loss and metabolic surgeries in China[6], which is much lower than the global average level due to its relatively high operating difficulty. Part of the reason may be the high demand for gastrojejunostomy, which is crucial as it avoids complications.
The reports on the efficacy and safety of RYGB for T2DM in China have primarily focused on the results of the short- and medium-term postoperative period, and reports on long-term efficacy are still needed[7-9].
Here, we report the effect of a modified RYGB using a unique side-to-side anastomosis of the lesser curvature of the stomach and jejunum in patients with T2DM in Beijing Tiantan Hospital with 5-year follow-up data, with the aim of providing a basis for the further development of RYGB for T2DM in China.
Patients with T2DM who underwent RYGB were enrolled from Beijing Tiantan Hospital’s weight loss and metabolic surgery database, and cases with at least 5 years of postoperative follow-up and complete data were selected.
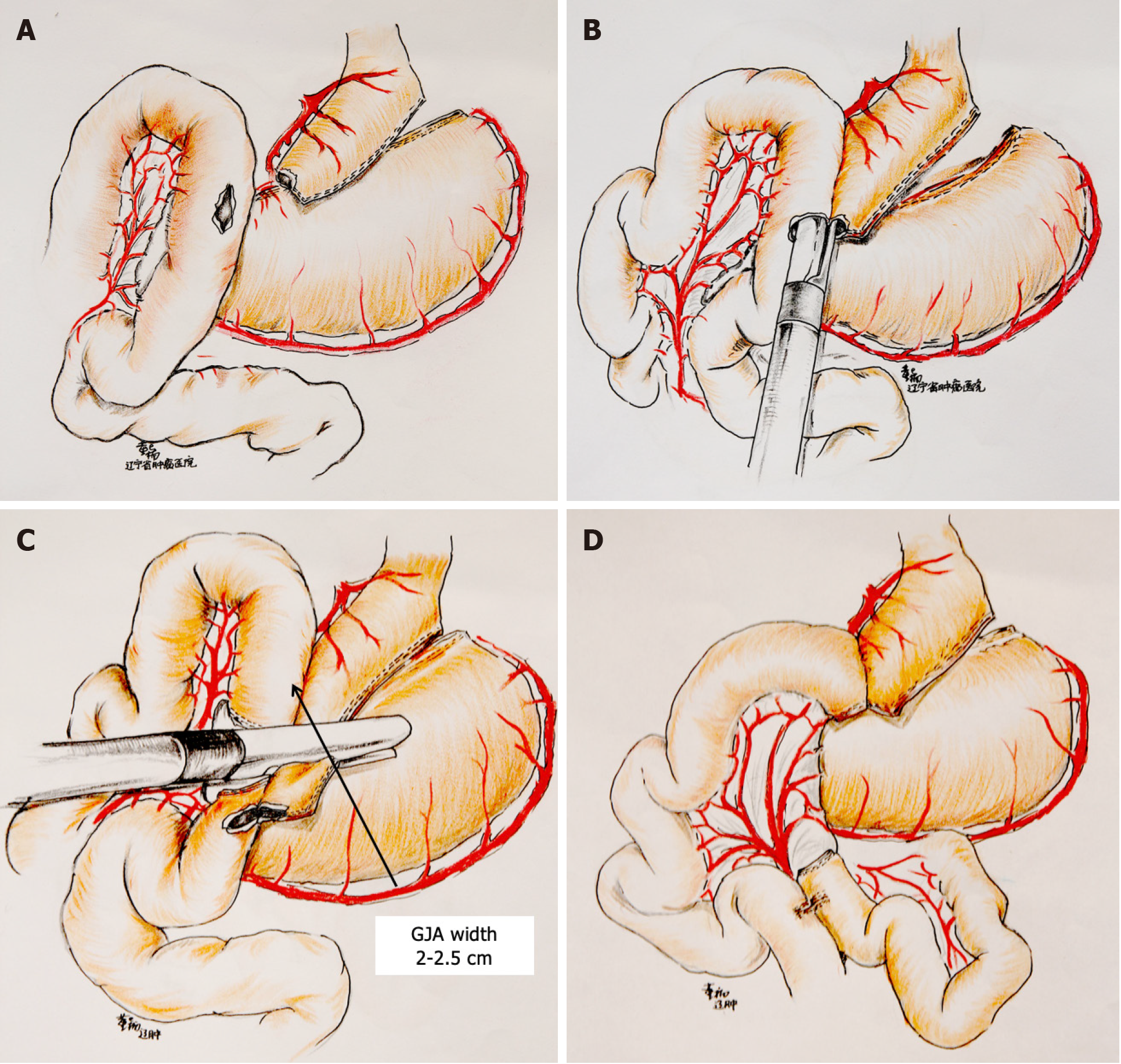
The patient was placed in a reverse Trendelenburg position with legs separated, the operator was located between the patient’s legs, and 3–5 trocars were used. The lesser curvature was dissected 9 cm below the cardia and continued for 2.5–3 cm toward the angle of His. The volume of the gastric pouch was approximately 20–30 mL.
Anterior colonic gastrointestinal anastomosis was performed with the diameter of the gastrointestinal anastomosis at 2.5 cm, and the length of the Roux limbs and biliopancreatic limbs was approximately 100 cm. Figure 1 illustrates the detailed surgical procedures[9].
Effectiveness: T2DM complete remission rate, weight, body mass index (BMI), total weight loss (TWL%), fasting plasma glucose (FPG), glycated hemoglobin (HbA1c), hypoglycemic drug administration, triglyceride (TG), total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (CREA), uric acid (UA), and concomitant disease (e.g., hypertension) amelioration were recorded.
Criteria for discontinuing hypoglycemic drugs: fasting blood glucose of ≤ 7.0 mmol/L, 2-h postprandial blood glucose of ≤ 10 mmol/L, and HbA1c of < 6.5% only through diet and life management[10].
Criteria for complete T2DM remission: no hypoglycemic drugs postoperatively, blood glucose controlled only through lifestyle changes, HbA1c of < 6.5%, and FPG of < 5.6 mmol/L maintained for > 1 year[11].
Safety: The main parameters observed were mid- and long-term complications, such as iron-deficiency anemia, intestinal obstruction, and secondary gallstones as well as nutritional indicators, including albumin (ALB), serum ferritin, folic acid (FA), and vitamin B12 (VB12).
Normally distributed quantitative data are presented as mean ± SD, and P-values were calculated using independent sample t-test; non-normally distributed quantitative data are presented as median, and P-values were calculated using Mann–Whitney U test. Qualitative data are presented as n (%) and compared using the Chi-square test or Fisher’s exact test. Univariate and multivariate logistic regression analyses were performed with variables, and a P < 0.05 was statistically significant for multivariate analysis; forward logistic regression was conducted. Two-sided tests were used for all analyses, and a P-value of < 0.05 was considered statistically significant. SPSS software package (version 22.0) was used.
A total of 197 patients with T2DM treated with RYGB were retrieved from the weight loss and metabolic surgery database of Beijing Tiantan Hospital by August 31, 2022. Postoperative follow-up of > 5 years, with an average follow-up duration of 6.2 ± 1.1 (5.0–9.0) years, and complete data were available for 37 cases. Among them, 14 were males and 23 were females, with average age, 45.3 ± 10.7 (22–62) years; weight, 84.6 ± 17.3 (60.0–140.0) kg; BMI, 30.9 ± 5.0 (24.7–46.2) kg/m2; T2DM duration, 7.1 ± 5.6 (0.1–15.0) years; preoperative FPG, 7.4 ± 3.4 (4.2–19.0) mmol/L; and preoperative HbA1c, 8.2% ± 1.7% (5.5%–13.3%).
The surgery was uneventful in all patients, and no conversion to open laparotomy or perioperative deaths were recorded. The surgery duration was 155.2 ± 43.2 (120.0-270.0) minutes, and two cholecystectomies were performed during the operation. The total length of hospital stay was 12.1 ± 2.8 (7.0-22.0) days, and the length of hospital stay postoperatively was 5.6 ± 2.3 (1-15) days.
Among the cases, 7 had hypertension preoperatively and were taking anti-hypertensive drugs, 18 cases had dyslipidemia, 35 cases had fatty liver, 9 cases had liver function abnormality, 1 case had renal function abnormality, 2 cases had hyperuricemia, and 2 cases had a history of cholecystectomy.
Average weight and BMI of the patients reduced from 84.6 ± 17.3 (60.0–140.0) kg and 30.9 ± 5.0 (24.7–46.2) kg/m2 to 67.1 ± 12.2 (24.7–46.2) kg and 24.6 ± 3.9 (17.7–36.5) kg/m2, respectively, which were significantly reduced compared with those during the preoperative period (all P < 0.01). TWL (%) reached 20.0% ± 7.8% (8.2%–41.5%). Among them, three cases had a BMI of > 30 kg/m2 5-years postoperatively (30.4, 34.3, and 36.5 kg/m2, respectively); TWL (%) of the first two cases were 28.6% and 25.7%, respectively, and glucose and lipid metabolisms returned to normal postoperatively. BMI in the third case reduced from 39.8 kg/m2 preoperatively to 36.5 kg/m2 5-years postoperatively, with a TWL (%) of 8.2%. Postoperatively, this patient had the lowest weight (80 kg; BMI, 32.5 kg/m2) for 1.5 years, and gradually rebounded over 3 years, with a constant BMI of 36.5 kg/m2 for > 6 years. The patient’s lipid levels had been normal postoperatively, and her blood glucose began to increase at 6 years postoperatively after metformin initiation (Table 1).
FPG and HbA1c reduced from 7.4 ± 3.4 mmol/L and 8.2% ± 1.7% preoperatively to 6.5 ± 1.3 mmol/L and 6.5% ± 0.9%, respectively, at 5-years postoperatively, with significantly pronounced HbA1c reduction (P < 0.05) (Table 2).
| Preoperative (n) | 5 years after surgery (n) | P value | |
| FPG (mmol/L) | 7.4 ± 3.4 (37) | 6.5 ± 1.3 (37) | 0.619 |
| HbA1c (%) | 8.2 ± 1.7 (36) | 6.5 ± 0.9 (36)a | 0.001 |
Only 29.7% (11/37) of the patients used hypoglycemic drugs 5-years postoperatively, which was significantly less compared with the preoperative level (100% used hypoglycemic drugs), and the difference was statistically significant (P < 0.01). Among the 26 cases who had not been using hypoglycemic drugs postoperatively, 17 had normal HbA1c levels and 13 had normal FPG levels; among them, 11 had FPG of < 5.6 mmol/L with HbA1c of < 6.5%. In addition, the rate of T2DM complete remission at 5-years postoperatively was 29.7% (11/37) (Tables 2 and 3).
| Abnormalities | Preoperative (%) | 5 years after surgery (%) | P value |
| Antihyperglycemic drug | 37 (100) | 11 (29.7) | 0.001a |
| Antihypertensive drug | 7 (18.9) | 2 (5.4) | 0.075 |
| Dyslipidemia | 18 (50.0) | 9 (2.05) | 0.028a |
| Transferase | 9 (25.0) | 0 (0) | 0.002a |
| CREA | 1 (2.8) | 1 (2.9) | 0.984 |
| UA | 2 (5.6) | 5 (13.9) | 0.429 |
| HGB | 1 (2.7) | 6 (18.2) | 0.038a |
| ALB | 1 (2.7) | 1 (2.9) | 1.000 |
| SF | 1 (3.0) | 8 (25.0) | 0.013a |
| FA | 0 (0) | 0 (0) | - |
| VB12 | 1 (3.0) | 3 (11.1) | 0.318 |
A total of 11 (29.7%) cases were taking hypoglycemic drugs; 3 cases were taking hypoglycemic drugs throughout the postoperative period and another eight cases were started on hypoglycemic drugs successively (two cases each at 3-, 4-, 5-, and 6-years postoperatively, respectively) during the follow-up period. Of the 11 cases, 3 cases received insulin to control blood glucose (1-, 4-, and 7-years postoperatively, respectively) (Table 3).
The results of this study revealed that 5 years after RYGB, TG decreased significantly and HDL increased significantly compared with the respective levels during the preoperative period (both P < 0.05). Cholesterol (CHO) and LDL also reduced compared with the levels during the preoperative period, but the difference was not statistically significant (both P > 0.05). Preoperative lipid metabolism abnormality was observed in 18 cases, whereas lipid metabolism abnormality was noted in 9 cases after > 5 years of postoperative follow-up, the number of which reduced significantly compared with that in the preoperative period, with a statistically significant difference (P < 0.05) (Tables 3 and 4).
ALT reduced significantly compared with that during the preoperative period (P < 0.05). Nine cases had liver function abnormality preoperatively, and all patients had normal liver function 5-years postoperatively, with a statistically significant difference (P < 0.05).
At 5-years postoperatively, CREA was slightly elevated and UA was slightly reduced compared with preoperative levels, but none of the differences were statistically significant (all P > 0.05). One patient had abnormal CREA preoperatively, which was slightly elevated 5-years postoperatively. Preoperatively, two cases had abnormally elevated UA, which returned to normal 5-years postoperatively; however, five cases demonstrated elevated UA. Of the 37 cases, two were taking anti-hypertensive drugs 5-years postoperatively, and the number reduced from preoperative period (seven cases) but with no statistically significant difference (P = 0.075) (Tables 3 and 5).
| Preoperative (n) | 5 years after surgery (n) | P value | |
| ALT (U/L) | 33.5 ± 18.7 (36) | 20.7 ± 6.8 (36)a | 0.004 |
| AST (U/L) | 21.6 ± 9.0 (36) | 22.1 ± 7.2 (35) | 0.694 |
| CREA (μmol/L) | 54.3 ± 16.2 (36) | 58.4 ± 15.8 (35) | 0.601 |
| UA (μmol/L) | 320.1 ± 115.0 (36) | 313.4 ± 95.0 (36) | 0.336 |
| HGB (g/L) | 133.9 ± 14.3 (37) | 126.3 ± 19.2 (33) | 0.366 |
| ALB (g/L) | 44.9 ± 2.8 (37) | 44.5 ± 2.7 (34) | 0.665 |
| SF (ng/mL) | 137.6 ± 138.5 (33) | 32.1 ± 29.4 (32)a | 0.001 |
| FA (ng/mL) | 18.1 ± 6.7 (32) | 17.1 ± 8.6 (28) | 0.308 |
| VB12 (pg/mL) | 563.3 ± 496.4 (33) | 420.7 ± 304.6 (27) | 0.196 |
One (2.7%, 1/37) case developed symptoms of incomplete intestinal obstruction several times after 5 years, which resolved completely after conservative symptomatic management. Two (6.0%, 2/33) cases developed new gallbladder stones, of which one underwent cholecystectomy 5-years postoperatively. One (3.0%, 1/33) case developed mild iron-deficiency anemia 1-year postoperatively, whereas six (18.2%, 6/33) developed iron-deficiency anemia 5-years postoperatively, including one case with moderate anemia, which improved after iron supplementation.
The mean ferritin reduced significantly at 5-years postoperatively, with a statistically significant difference (P < 0.05). HbA1C, ALB, FA, and VB12 Levels reduced compared with those during the preoperative period, but the difference was not statistically significant (P = 0.366, 0.665, 0.308, and 0.196, respectively).
Compared with the preoperative period, anemia and ferritin decompensation increased significantly (both P < 0.05). VB12 hypoplasia increased slightly compared with preoperative level, but the difference was not statistically significant (P = 0.318). The number of hypoproteinemia cases did not change from the preoperative period. All patients exhibited normal FA 5-years postoperatively (Tables 1 and 2).
Binary logistic regression analysis revealed that the patients’ preoperative T2DM history and fasting C-peptide were significant prognostic factors that influenced complete remission of T2DM after RYGB (P = 0.006 and 0.012, respectively), whereas the patients’ sex, age, height, weight, BMI, preoperative insulin use, hemoglobin, FPG, AST, ALT, TG, CHO, HDL, LDL, CREA, UA, surgery duration, intraoperative bleeding, postoperative hospital stay, and TWL were not significantly associated with T2DM remission after RYGB. The higher the preoperative fasting C-peptide level and the shorter the T2DM duration, the greater the likelihood of T2DM complete remission after RYGB.
RYGB is considered the gold standard for the surgical treatment of patients with T2DM and obesity. In recent years, several prospective randomized control trials have further demonstrated that RYGB has excellent long-term hypoglycemic efficacy in patients with T2DM[1,3,12]. Schauer et al[1] revealed a 30.6% complete remission rate of T2DM 5-years after RYGB, whereas Mingrone et al[12] reported a 37% complete remission rate. The results of our study revealed a 30.6% complete remission rate of T2DM 5-years after RYGB, which is consistent with the results of the aforementioned well-known prospective studies.
Our study revealed that RYGB led to a significant reduction in FPG and HbA1c levels and the number of patients using glucose-lowering drugs, which is consistent with the results of the study conducted by Schauer et al[1] and Mingrone et al[12]. Our results indicate that RYGB has a good effect on long-term glycemic control in Chinese patients with T2DM. Moreover, this study revealed that patients’ preoperative history of T2DM and fasting C-peptide were important prognostic factors that affect T2DM complete remission after RYGB. This is consistent with the results of a previous study at our center[13,14], revealing that the shorter the patient’s preoperative history of diabetes and higher the fasting C-peptide level, the greater is the likelihood of T2DM complete remission after RYGB. This finding indicates that the earlier the RYGB procedure is performed in patients with T2DM and obesity, the better the postoperative glycemic control will be.
Our study revealed that three patients are still receiving hypoglycemic medication after RYGB, but at significantly lower dosages and with easier glycemic control than that preoperatively. Three-years postoperatively, eight more patients were started on additional hypoglycemic medication, of which three required insulin for glycemic control. This indicates a rebound of blood glucose after RYGB. Moreover, Mingrone et al[12] revealed that approximately 53% of patients experienced a rebound of blood glucose 5-years postoperatively. Therefore, a balanced diet, regular exercise, routine follow-up, consistent glucose monitoring, and timely and correct use of glucose-lowering drugs for patients postoperatively may be effective in delaying glucose rebound.
The results of this study revealed that weight was still significantly lower 5-years postoperatively compared with preoperative value, and TWL (%) reached an average of 20.0%, indicating that RYGB demonstrated a good and lasting weight loss effect. A prospective study on patients with a BMI of 47.3 kg/m2 reported TWL (%) of 27.7% at 6 years after RYGB[15]. Schauer et al[1] and Mingrone et al[12] also revealed that TWL (%) at 5 years after RYGB was 21.7% and 28.4%, respectively. The TWL (%) in our study was slightly lower than that reported in literature, which may be associated with the significantly lower preoperative BMI of the patients enrolled in the present study (30.9 kg/m2) than that in the two studies mentioned above (37 kg/m2 and 44 kg/m2, respectively). In addition, in our results, three cases still had a BMI of > 30 kg/m2 5-years postoperatively, of which two cases (with preoperative BMI of 47 kg/m2 and 42 kg/m2, respectively) exhibited a postoperative TWL (%) of 28.6% and 25.7% with normal levels of glycemia and lipid metabolism postoperatively. Preoperatively, the BMI in the other case reduced from 39.8 kg/m2 to a minimum of 32.5 kg/m2 at 1.5-years postoperatively, which gradually began to rebound 3-years postoperatively and was maintained at 36.5 kg/m2 for > 6-years postoperatively; lipid levels were normal, and the patient began to use hypoglycemic drugs to control his blood glucose in the sixth year.
Combining the above results with the results of our center’s previous study[13,14], we conclude that (1) RYGB exhibits a very good and long-lasting effect on weight loss, and, generally, the weight was lowest approximately 1-year postoperatively, followed by a slight weight rebound, and was then maintained in a satisfied state for a long period; (2) the patient’s blood glucose and lipid levels were remarkably ameliorated, although the body weight failed to reach normal level postoperatively; and (3) patients with a preoperative high BMI cannot rely only on RYGB to achieve good weight loss; postoperative diet control, strengthening exercise, medication, and other ways of integrated treatment may help better reduce weight and improve obesity-related complications[16].
RYGB demonstrates a good improvement of lipid metabolism. This study revealed that TG reduced significantly 5 years after RYGB, HDL increased significantly, and CHO and LDL decreased compared with the preoperative period levels, although with no statistical difference. This result is consistent with the relevant literature[1,12,17]. In addition, 18 patients with combined lipid metabolism abnormality preoperatively were followed up for > 5 years, and only nine cases exhibited lipid metabolism abnormality, which is a remarkable decrease. This indicates that RYGB is beneficial in improving lipid metabolism abnormality. Moreover, nine cases had liver function abnormalities preoperatively, and all 36 patients demonstrated normal liver function > 5 years postoperatively, which may be associated with lipid metabolism improvement and fatty liver reduction or even disappearance after RYGB.
Most studies revealed that RYGB improves blood glucose and blood pressure in patients. A randomized control study by Mingrone et al[18] revealed blood pressure relief in 80% of patients 2 years after RYGB. Another randomized controlled study by Courcoulas[19] reported improvement in hypertension in patients 3 years after gastric bypass compared to conventional medical therapy. Our study revealed 7 cases with hypertension preoperatively and only 2 cases postoperatively, indicating that RYGB has a better long-term amelioration effect on hypertension.
Our study used a modified RYGB procedure, in which the anastomosis was located at the lesser curvature side of the gastric pouch, instead of the posterior side as in traditional procedure. The diameter of the anastomosis is more easily controlled at approximately 2.5 cm in this approach, which requires a shorter learning curve compared with gastrojejunostomy using a circular stapler, as used by Torres et al[20]. Furthermore, the shape of the gastric pouch as well as less tension and better blood supply by the anastomosis further guarantees the success of the modified procedure.
In our previous study[9], 77 patients underwent modified RYGB. No patient was switched to laparotomy and no mortality was recorded, surgery time was 1.5–2 h, blood loss reduced to a minimum of 10 mL, average hospital stay was 4 days, and no severe surgical complication was recorded in all 77 patients. This indicates that the modified RYGB is a safe and feasible procedure for Chinese patients.
The main mid- and long-term RYGB-related complications include intestinal obstruction, marginal ulceration of gastrointestinal anastomoses, and nutritional disorders[11]. The incidence of postoperative intestinal obstruction after RYGB has been reported in the literature at 0.5%–2%[21], whereas our study revealed one (2.7%) patient having recurrent incomplete intestinal obstruction postoperatively, which was due to postoperative intestinal adhesions or internal hernia. The occurrence of marginal ulcers was not observed owing to the limited number of patients enrolled. Moreover, this study revealed six (18.2%) cases of combined iron-deficiency anemia 5 years after RYGB, which was significantly higher compared with preoperative number (one case, 2.7%), and the incidence of anemia was consistent with the relevant literature reports[1,12]; all six cases were menstruating women, and the reason for anemia may be related to excessive menstruation and insufficient iron supplementation.
Moreover, this study revealed a significantly lower mean ferritin value 5-years postoperatively and a significantly higher number of people with reduced ferritin. Postoperative HbA1c, ALB, FA, and VB12 levels were all reduced compared with preoperative period levels, and the number of cases with VB12 deficiency increased slightly compared with those during the preoperative period. The number of hypoproteinemia cases remained unchanged. All patients had normal FA level 5-years postoperatively. Therefore, the occurrence of mid- and long-term complications after RYGB is acceptable, and the modified RYGB is considered safe. Patients are instructed to routinely take prophylactic supplements of iron, vitamins, and various micronutrients and to regularly monitor blood counts and related nutritional indexes, which is particularly important for female patients with heavy menstrual flow.
In conclusion, the current modified RYGB is a safe and effective surgical procedure for Chinese patients with T2DM and obesity. RYGB not only effectively controls blood glucose in the long term, reduces the use of hypoglycemic drugs, and maintains low body weight in the long term, but also improves obesity-related complications, such as lipid metabolism, hepatic function anomalies, and hypertension. However, patients are at risk of weight gain, blood glucose rebound, and nutritional disorders after RYGB; therefore, postoperative follow-up, diet, and medication guidance should be emphasized, and prophylactic supplementation of iron, vitamins, and various trace elements should be routinely performed in patients after RYGB.
The limitation of this study is its single-center, small-sample, retrospective design with a high rate of loss to follow-up beyond 5 years. Furthermore, the effect of modified RYGB on microvascular complications due to T2DM, such as diabetic retinopathy, ophthalmopathy, and peripheral neuropathy, could not be assessed owing to the paucity of follow-up data regarding diabetic microvascular complications.
| 1. | Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR; STAMPEDE Investigators. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med. 2017;376:641-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1626] [Cited by in RCA: 2013] [Article Influence: 223.7] [Reference Citation Analysis (0)] |
| 2. | Cummings DE, Arterburn DE, Westbrook EO, Kuzma JN, Stewart SD, Chan CP, Bock SN, Landers JT, Kratz M, Foster-Schubert KE, Flum DR. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomised controlled trial. Diabetologia. 2016;59:945-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 223] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 3. | Ikramuddin S, Billington CJ, Lee WJ, Bantle JP, Thomas AJ, Connett JE, Leslie DB, Inabnet WB 3rd, Jeffery RW, Chong K, Chuang LM, Sarr MG, Jensen MD, Vella A, Ahmed L, Belani K, Schone JL, Olofson AE, Bainbridge HA, Laqua PS, Wang Q, Korner J. Roux-en-Y gastric bypass for diabetes (the Diabetes Surgery Study): 2-year outcomes of a 5-year, randomised, controlled trial. Lancet Diabetes Endocrinol. 2015;3:413-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 4. | Dixon JB, Zimmet P, Alberti KG, Mbanya JC, Rubino F; International Diabetes Federation Taskforce on Epidemiology and Prevention. Bariatric surgery for diabetes: the International Diabetes Federation takes a position. J Diabetes. 2011;3:261-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Cummings DE, Rubino F. Response to Comment on Rubino et al Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Diabetes Care 2016;39:861-877. Diabetes Care. 2016;39:e202-e203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Team of researchers from the Greater China Weight Loss and Metabolic Surgery Database. [Greater China metabolic and bariatric surgery database registry report (2021)]. Zhongguo Shiyong Waike Zazhi. 2022;42:550-560. |
| 7. | Yang JG, Wang CC, Hu YZ, Li JY, Pan YL, Shen YY, Li YX, Huang J, Yu CL, Liu XM. [Laparoscopic Roux-en-Y gastric bypass surgery for obesity and type 2 diabetes]. Zhonghua Weichang Waike Zazhi. 2010;13:594-597. [DOI] [Full Text] |
| 8. | Bai RX, Li YG, Qin C, Xu J, Yan WM, Yan M, Wang X, Yuan HS, Song MM. [Efficacy of laparoscopic gastric bypass surgery in the treatment of type 2 diabetes]. Zhonghua Putong Waike Zazhi. 2015;30:957-960. [DOI] [Full Text] |
| 9. | Bai RX, Yan WM, Li YG, Xu J, Zhong ZQ, Yan M. Application of side-to-side anastomosis of the lesser curvature of stomach and jejunum in gastric bypass. World J Gastroenterol. 2016;22:8398-8405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Diabetes Branch of Chinese Medical Association. [Guidelines for the Prevention and Treatment of Type 2 diabetes in China (2013)]. Zhonghua Tangniaobing Zazhi. 2014;6:447-498. [DOI] [Full Text] |
| 11. | Surgeons Branch of Chinese Medical Doctor Association; Obesity and Diabetes Surgeons Committee. [Chinese Guidelines for Surgical Treatment of Obesity and Type 2 diabetes (2019 Edition)]. Zhongguo Shiyong Waike Zazhi. 2019;39:301-306. [DOI] [Full Text] |
| 12. | Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, Castagneto M, Bornstein S, Rubino F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386:964-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 917] [Article Influence: 83.4] [Reference Citation Analysis (0)] |
| 13. | Yan W, Bai R, Yan M, Song M. Preoperative Fasting Plasma C-Peptide Levels as Predictors of Remission of Type 2 Diabetes Mellitus after Bariatric Surgery: A Systematic Review and Meta-Analysis. J Invest Surg. 2017;30:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Yan W, Bai R, Li Y, Xu J, Zhong Z, Xing Y, Yan M, Lin Y, Song M. Analysis of Predictors of Type 2 Diabetes Mellitus Remission After Roux-en-Y Gastric Bypass in 101 Chinese Patients. Obes Surg. 2019;29:1867-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Adams TD, Davidson LE, Litwin SE, Kolotkin RL, LaMonte MJ, Pendleton RC, Strong MB, Vinik R, Wanner NA, Hopkins PN, Gress RE, Walker JM, Cloward TV, Nuttall RT, Hammoud A, Greenwood JL, Crosby RD, McKinlay R, Simper SC, Smith SC, Hunt SC. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012;308:1122-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 483] [Article Influence: 34.5] [Reference Citation Analysis (1)] |
| 16. | Yang PW, Yan WM. [Sharing China's experience in preventing and treating obesity with African countries]. Zhonghua Yufang Yixue Zazhi. 2022;56:1136-1141. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Bhasker AG, Remedios C, Batra P, Sood A, Shaikh S, Lakdawala M. Predictors of Remission of T2DM and Metabolic Effects after Laparoscopic Roux-en-y Gastric Bypass in Obese Indian Diabetics-a 5-Year Study. Obes Surg. 2015;25:1191-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, Rubino F. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1340] [Cited by in RCA: 1306] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 19. | Courcoulas AP, Goodpaster BH, Eagleton JK, Belle SH, Kalarchian MA, Lang W, Toledo FG, Jakicic JM. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg. 2014;149:707-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 20. | Torres JC, Oca CF, Garrison RN. Gastric Bypass. Southern Med J. 1983;76:1217-1221. [RCA] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 35] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Schauer PR, Nor Hanipah Z, Rubino F. Metabolic surgery for treating type 2 diabetes mellitus: Now supported by the world's leading diabetes organizations. Cleve Clin J Med. 2017;84:S47-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/