Published online Aug 6, 2024. doi: 10.12998/wjcc.v12.i22.5124
Revised: May 29, 2024
Accepted: June 17, 2024
Published online: August 6, 2024
Processing time: 149 Days and 21.7 Hours
Eosinophilic solid and cystic (ESC) renal cell carcinoma (RCC), a unique and emerging subtype of RCC, has an indolent nature; in some rare instances, it may exhibit metastatic potential. Current cases are inadequate to precisely predict the clinical outcome of ESC RCC and determine treatment choices.
Herein, we report two patients with ESC RCC. Patient 1 was a young woman with classical pathological characteristics. Patient 2 was a 52-year-old man with multifocal metastases, involving the pulmonary hilar and mediastinal lymph nodes, liver, brain, mesosternum, vertebra, rib, femur, and symphysis pubis. Awareness of ESC RCC, along with its characteristic architecture and immuno
The discovery of ESC RCC molecular signatures may provide new therapeutic strategies in the future.
Core Tip: Most patients with eosinophilic solid and cystic (ESC) renal cell carcinoma (RCC) have an indolent nature, but 8 cases have exhibited metastatic potential. In this study, we made a clear diagnosis in 2 cases of ESC RCC, including a case with multifocal metastases, by pathological microscopy, immunohistochemistry, next-generation sequencing, or whole exome sequencing analysis, which played a crucial role in clinical treatment. Usually, ESC RCC has a solid and cystic growth pattern, a voluminous eosinophilic cytoplasm with coarse basophilic stippling, is CK20 positive and has TSC1/2 mutations.
- Citation: Cao HH, Li H, Guo XH, Cao ZX, Zhang BH. Eosinophilic solid and cystic renal cell carcinoma with aggressive behavior: Two case reports. World J Clin Cases 2024; 12(22): 5124-5130
- URL: https://www.wjgnet.com/2307-8960/full/v12/i22/5124.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i22.5124
Eosinophilic solid and cystic (ESC) renal cell carcinoma (RCC) is a recently described novel renal tumor entity; it was initially reported in 2016 by Trpkov et al[1]. It has unique morphologic, immunohistochemical, and molecular features, and is currently one of the new renal tumor subtypes included in the World Health Organization (WHO) 2022 classification of renal neoplasia; however, approximately 74 cases of ESC RCC have been reported so far, of which 8 were metastatic lesions[1-18].
In the initial investigation, all tumors occurred in female patients. The age range of the patients in the study was 14 to 79 years, and tumor sizes varied from 5 to 205 millimeters. Epidemiologically, ESC RCC often presents without symptoms and is incidentally diagnosed through imaging, typically as a solid and cystic mass. Surgical resection has proven to be curative for the majority of cases. Notably, one patient diagnosed with hematogenous liver metastases responded favorably to mammalian target of rapamycin (mTOR) pathway inhibitors, achieving a complete response and maintaining disease remission for a decade since the initial diagnosis[6]. Morphologically, the tumor has a cystic and solid growth structure; the tumor cells have shown an abundant granular eosinophilic cytoplasm, eosinophilic or basophilic particles are often observed in the cytoplasm. Immunohistochemistry (IHC) suggested that the tumor was frequently positive for CK20 and commonly harbored TSC1/2 mutations.
Herein, we report two patients with ESC RCC: A young woman with classical pathological characteristics (case 1) and a 52-year-old man with multifocal metastases in the pulmonary hilar and mediastinal lymph nodes, liver, brain, mesosternum, vertebra, rib, femur, and symphysis pubis (case 2).
Case 1: A 33-year-old Chinese woman presented to the urology clinic with the complaint of a suspicious mass in the right kidney for more than 3 months, found during routine annual examination.
Case 2: A 52-year-old Chinese man presented to the chest pain clinic with the complaint of right chest pain for more than 3 months.
Case 1: A suspicious renal mass was detected by ultrasonography during a routine annual examination more than 3 months ago.
Case 2: The patient experienced left upper arm pain more than 3 months ago due to falling while riding a bicycle, and then gradually developed right chest pain.
Case 1: The patient had a history of uterine leiomyoma and had undergone myomectomy 7 years ago.
Case 2: The patient had a history of hypertension for more than 5 years.
Both patients denied any family history of malignant tumors.
On physical examination, no abnormalities were found in the two patients.
Case 1: No abnormalities were found on routine blood and urine analyses.
Case 2: Levels of serum tumor markers were normal. No abnormalities were found on routine blood and urine analyses.
Case 1: Ultrasonography revealed a hypo-echoic mass, 3 cm × 2.4 cm in size, on the middle right kidney. Subsequent magnetic resonance imaging in the middle abdomen showed that a 3.1 cm × 1.8 cm nodular, well-circumscribed mass, protruding from the renal capsule; thus, a tentative diagnosis of malignancy was made.
Case 2: Computed tomographic imaging on the pectoral, abdominal and cranial regions revealed an 11.5-cm renal mass and multiple metastases. These multifocal metastases included the pulmonary hilar and mediastinal lymph nodes, liver, brain, mesosternum, 10th thoracic vertebra, right 1st and 5th ribs, 4th lumbar spine, upper part of the right femur, and left symphysis pubis.
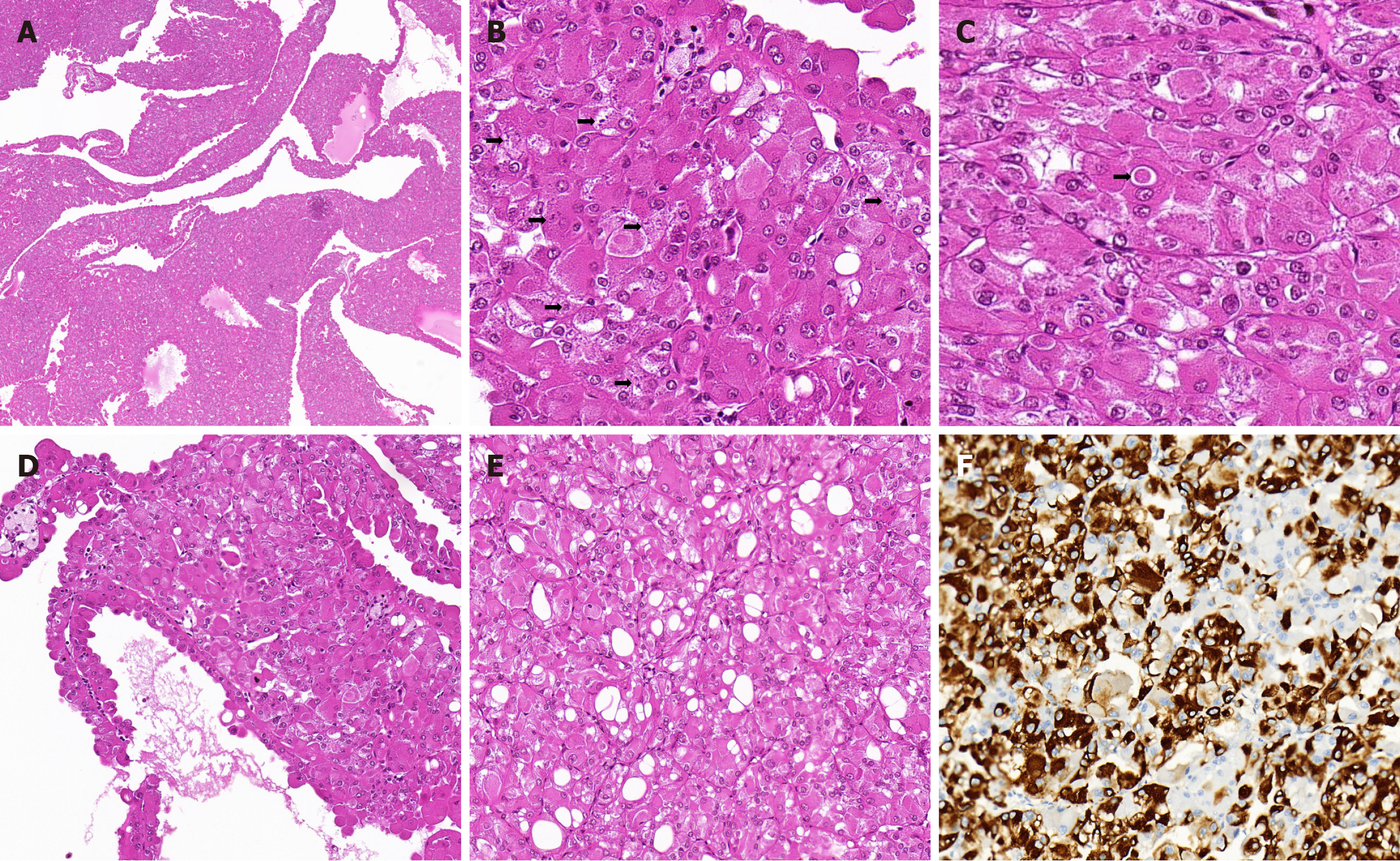
Case 1: The resected renal tumor specimen showed a 3 cm × 2 cm × 2 cm well-circumscribed, soft, tan-yellow, cystic and solid mass protruding from the renal capsule that was completely excised. Histopathological examination revealed that the mass was composed of eosinophilic cells that grew into macrocysts, microcysts, and a solid pattern, with no true papillary formation and no prominent vascular network (Figure 1A). The tumor cells had round nuclei with prominent focal nucleoli, an abundant granular eosinophilic cytoplasm with prominent fine or coarse basophilic granular cytoplasmic stippling (Figure 1B), and eosinophilic cytoplasmic globules (Figure 1C). The tumor cells lining the septa showed a hobnail arrangement (Figure 1D), and focal intracytoplasmic vacuoles were observed (Figure 1E). Tumor necrosis, substantial mitotic activity, and perinuclear clearing were not observed. Clusters of foamy histiocytes and lymphocytes were also identified in background samples. IHC demonstrated the presence of PAX8 (nuclear; strong and diffuse), CK20 (cytoplasmic; strong and focal (Figure 1F), succinate dehydrogenase B (SDHB) (cytoplasmic; strong and diffuse), fumarate hydratase (FH) (cytoplasmic; strong and diffuse), vimentin (cytoplasmic; strong and diffuse), and P504S (cytoplasmic; strong and focal) but the absence of CK7, CD10, CAIX, epithelial membrane antigen (EMA), CD117, lymphoma kinase (ALK), human elanoma black 45 (HMB-45), Melan-A, and Desmin. Transcription factor binding to IGHM enhancer 3 (TFE3)/transcription factor EB (TFEB) was negative on IHC and in situ hybridization. Next-generation sequencing of nephrectomy specimens showed a frame shift mutation in exon 7 of TSC2 [c.601del (p.M201fs)] and a splicing region mutation in exon 12 of TSC2 (c.1257+5G>A).
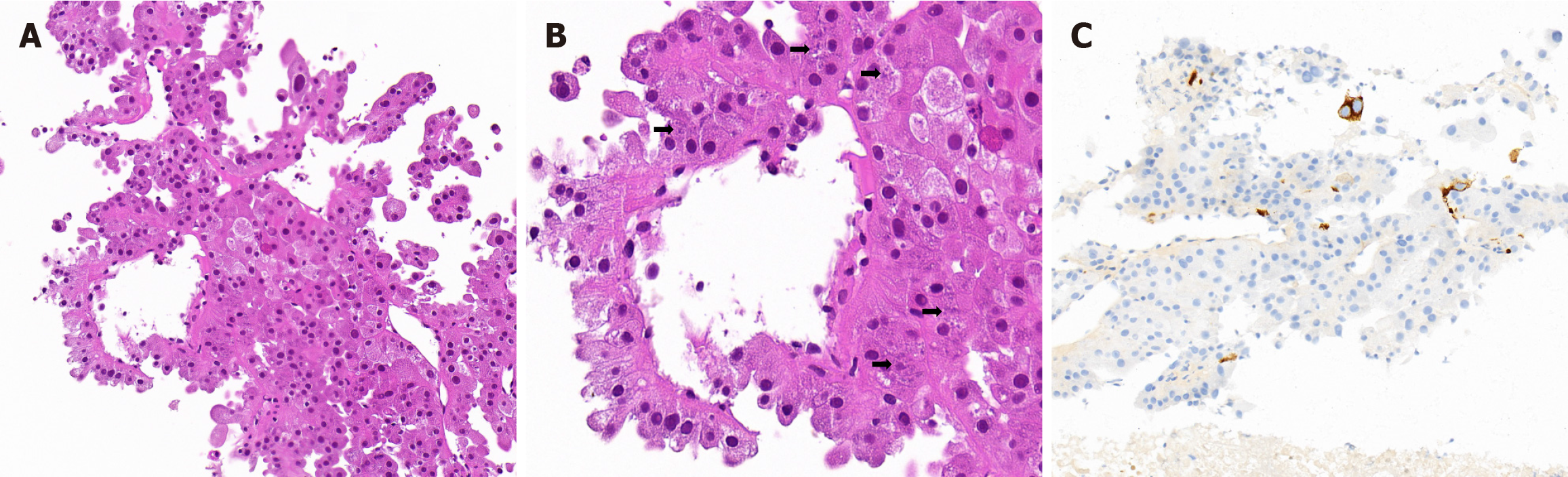
Case 2: A fine-needle aspiration biopsy of the liver was performed. The tumor cells were arranged in an acinar-like, microcyst, and pseudo-papillary pattern; cells lining septa had a hobnail morphology (Figure 2A). The cells exhibited a voluminous eosinophilic cytoplasm with various sizes of basophilic granular stippling (Figure 2B). Patchy tumor necrosis, substantial mitotic activity, and round to ovoid nuclei with conspicuous nucleoli were observed. Results of immunostaining indicated the presence of PAX8 (nuclear; strong and diffuse), CK20 (cytoplasmic; strong and scattered (Figure 2C), SDHB (cytoplasmic; strong and diffuse), FH (cytoplasmic; strong and diffuse), vimentin (cytoplasmic; strong and diffuse), P504S (cytoplasmic; weak and diffuse), and EMA (cytoplasmic; weak and focal) but the absence of RCC, CK7, CD10, CAIX, CD117, ALK, TFE3, TFEB, HepPar1, Arginase-1, Glypican-3, and alpha-fetoprotein. The whole exome sequencing analysis of this patient identified somatic deleterious mutations (insertion mutations) in TSC1.
Case 1: The final diagnosis was ESC RCC.
Case 2: Combined with the patient’s medical history, the final diagnosis was liver metastatic ESC RCC.
Case 1: Renal tumor resection was performed.
Case 2: The patient abandoned treatment due to multiple systemic metastases.
Case 1: Tumor progression was not detected within 40 months of follow-up.
Case 2: Due to the patient giving up treatment, follow-up information was not available.
ESC RCC was initially reported in 2016 by Trpkov et al[1]. They described a class of eosinophilic tumors with cystic and solid structures, and the morphology was highly similar to that of TSC-associated RCC. However, the patient did not have TSC. Because the morphological characteristics and immunophenotype of such tumors are difficult to include in the existing WHO classification of renal tumors, they are often diagnosed as “unclassified renal cell carcinoma” or descriptively diagnosed as “unclassified renal tumors with eosinophilic features”. The authors evaluated 16 patients with this tumor type and proposed that this may be an independent type of kidney cancer. Its common characteristics were summarized as follows and used as the diagnostic criteria: (1) Solid and cystic or mixed growth structure, hobnail arrangement of cells lining the septa, with absence of a capsule; (2) eosinophilic, voluminous cytoplasm with granular stippling, round to oval nuclei, and prominent nucleoli; (3) scattered foamy histiocytes, lymphocytes, and multinucleated cells; (4) positive immunohistochemical CK20 features and negative for CK7; and (5) no history of TSC. In 2017, Trpkov et al[1]. reported 19 patients with ESC RCC, who exhibited the molecular characteristics of this tumor[2]. In the first two reports, all tumors developed in female patients[1,2]. Subsequent cases were reported in individual male patients, but the majority of patients were women[3-18]. The age of onset ranged from 14 to 79 years, and the tumor sizes ranged from 5 to 205 mm. Approximately 16% of patients (12 cases/74 cases) had multifocal tumors. The patients were usually diagnosed at an early stage and had a good prognosis. To date, eight patients have been diagnosed with metastatic lesions. Immunohistochemical staining revealed that the PAX8+/CK20+/CK7− phenotype was the most common, but some cases are CK20− (approximately 20%) or CK7+ (approximately 16%, always focal). The tumors showed SDHB and FH expression, and were usually positive for CD10, P504S, Melan-A and cathepsin K, but some cases were negative. HMB-45 was occasionally positive, whereas CA9, CD117, TFE-3, TFEB and ALK were almost always negative. Most patients exhibited TSC1/TSC2 mutations, indicating that ESC RCC is closely associated with TSC gene dysfunction. This information is summarized in Supplementary Table 1.
Palsgrove et al[6] confirmed that of 15 ESC RCC patients, 14 had pathogenic mutations in the TSC 1 and TSC 2 genes. Mehra et al[8] evaluated seven patients with ESC RCC and demonstrated that the TSC 1 and TSC 2 genes have biallelic dysfunction, leading to the loss of inhibition and activation of the downstream mTOR signaling pathway. Munari et al[15] found four ESC RCC patients with TSC1 mutations (germline confirmed in one case) and one patient with a sporadic case. Although patients do not present systemic syndromes as the TSC gene dysfunction only occurs in the tumor tissues, the pathogenesis and signaling pathway changes in ESC RCC are similar to those of TSC-related renal cancer.
Palsgrove et al[6] found that 62.5% of ESC RCC patients expressed cathepsin K, and the rate of cathepsin K expression was up to 78% in all ESC RCC patients published. In addition, approximately 80% of ESC RCC patients published were positive for Melan-A. Since the inhibition of mTOR signaling can suppress cathepsin K expression in osteoclasts, cathepsin K expression may be upregulated in ESC RCC owing to activation of the mTOR signaling pathway. MiT family translocation-related renal cancer is also associated with activation of the mTOR signaling pathway, and is often accompanied by the expression of cathepsin K and the pigment markers Melan-A and HMB-45, which are widely used in the adjuvant diagnosis of MiT family kidney cancer. We speculate that ESC-RCC and MiT family translocation-related RCC have co-activated signaling pathways and can express cathepsin K, therefore, pigment markers may also be expressed simultaneously. This result further supports the molecular pathogenesis of ESC RCC while providing effective immune markers for differential diagnosis.
Moreover, due to activation of the mTOR signaling pathway in tumors, the mTOR pathway inhibitor rapamycin can be used not only for the treatment of TSC-related renal cancer but also for the targeted therapy of ESC RCC. One patient with ESC-RCC reported by Palsgrove et al[6] achieved complete remission 8 years after treatment with mTOR inhibitors.
The differential diagnoses of ESC-RCC included epithelioid angiomyolipoma (EAML) and other renal tumors with eosinophilic cytoplasm: (1) EAML is characterized by overlaps in two clinical, imaging, morphological, immuno
Although most patients with ESC RCC have an indolent nature, nine patients demonstrated aggressive behavior. Herein, we report two patients with ESC RCC, a unique and emerging subtype of RCC. Awareness of this recent tumor entity, along with its characteristic architecture and immunophenotype, would contribute to making a definitive diagnosis, even on core biopsy samples. The discovery of these molecular signatures may provide new therapeutic strategies in the future.
| 1. | Trpkov K, Hes O, Bonert M, Lopez JI, Bonsib SM, Nesi G, Comperat E, Sibony M, Berney DM, Martinek P, Bulimbasic S, Suster S, Sangoi A, Yilmaz A, Higgins JP, Zhou M, Gill AJ, Przybycin CG, Magi-Galluzzi C, McKenney JK. Eosinophilic, Solid, and Cystic Renal Cell Carcinoma: Clinicopathologic Study of 16 Unique, Sporadic Neoplasms Occurring in Women. Am J Surg Pathol. 2016;40:60-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 2. | Trpkov K, Abou-Ouf H, Hes O, Lopez JI, Nesi G, Comperat E, Sibony M, Osunkoya AO, Zhou M, Gokden N, Leroy X, Berney DM, Werneck Cunha I, Musto ML, Athanazio DA, Yilmaz A, Donnelly B, Hyndman E, Gill AJ, McKenney JK, Bismar TA. Eosinophilic Solid and Cystic Renal Cell Carcinoma (ESC RCC): Further Morphologic and Molecular Characterization of ESC RCC as a Distinct Entity. Am J Surg Pathol. 2017;41:1299-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 3. | Li Y, Reuter VE, Matoso A, Netto GJ, Epstein JI, Argani P. Re-evaluation of 33 'unclassified' eosinophilic renal cell carcinomas in young patients. Histopathology. 2018;72:588-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 4. | McKenney JK, Przybycin CG, Trpkov K, Magi-Galluzzi C. Eosinophilic solid and cystic renal cell carcinomas have metastatic potential. Histopathology. 2018;72:1066-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Parilla M, Kadri S, Patil SA, Ritterhouse L, Segal J, Henriksen KJ, Antic T. Are Sporadic Eosinophilic Solid and Cystic Renal Cell Carcinomas Characterized by Somatic Tuberous Sclerosis Gene Mutations? Am J Surg Pathol. 2018;42:911-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Palsgrove DN, Li Y, Pratilas CA, Lin MT, Pallavajjalla A, Gocke C, De Marzo AM, Matoso A, Netto GJ, Epstein JI, Argani P. Eosinophilic Solid and Cystic (ESC) Renal Cell Carcinomas Harbor TSC Mutations: Molecular Analysis Supports an Expanding Clinicopathologic Spectrum. Am J Surg Pathol. 2018;42:1166-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 7. | Tretiakova MS. Eosinophilic solid and cystic renal cell carcinoma mimicking epithelioid angiomyolipoma: series of 4 primary tumors and 2 metastases. Hum Pathol. 2018;80:65-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Mehra R, Vats P, Cao X, Su F, Lee ND, Lonigro R, Premkumar K, Trpkov K, McKenney JK, Dhanasekaran SM, Chinnaiyan AM. Somatic Bi-allelic Loss of TSC Genes in Eosinophilic Solid and Cystic Renal Cell Carcinoma. Eur Urol. 2018;74:483-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Cho WC, Collins K, Mnayer L, Cartun RW, Earle JS. Concurrent Eosinophilic Solid and Cystic Renal Cell Carcinoma and Angiomyolipoma With Epithelial Cysts in the Setting of Tuberous Sclerosis Complex: A Rare Synchronous Occurrence of 2 Distinct Entities. Int J Surg Pathol. 2019;27:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Mohaghegh Poor SM, Mathur S, Kassier K, Rossouw J, Wightman R, Saranchuk J, Gibson IW. Two Cases of Sporadic Eosinophilic Solid and Cystic Renal Cell Carcinoma in Manitoba Population. Int J Surg Pathol. 2021;29:747-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Lerma LA, Schade GR, Tretiakova MS. Co-existence of ESC-RCC, EVT, and LOT as synchronous and metachronous tumors in six patients with multifocal neoplasia but without clinical features of tuberous sclerosis complex. Hum Pathol. 2021;116:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Kamboj M, Gupta G, Pasricha S, Rawal S, Sharma A, Durga G, Mehta A. Eosinophilic solid and cystic renal cell carcinoma: A rare under-recognized indolent entity. Indian J Pathol Microbiol. 2021;64:799-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Wang L, Jiang J. Eosinophilic solid and cystic renal cell carcinoma: A new entity. Asian J Surg. 2021;44:1334-1335. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Sakhadeo U, Yadav SC, Bakshi GK, Prakash G, Katdare A, Menon S, Desai SB. Eosinophilic solid cystic renal cell carcinoma: A series of 3 cases elucidating the spectrum of morphological and clinical features of an emerging new entity. Indian J Urol. 2021;37:350-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Munari E, Settanni G, Caliò A, Segala D, Lonardi S, Sandrini S, Vacca P, Tumino N, Marconi M, Brunelli M, Gobbo S, Netto GJ, Moretta L, Zamboni G, Martignoni G. TSC loss is a clonal event in eosinophilic solid and cystic renal cell carcinoma: a multiregional tumor sampling study. Mod Pathol. 2022;35:376-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Aldera AP, Hes O. Eosinophilic Solid and Cystic Renal Cell Carcinoma With Melanin Pigment-Expanding the Morphological Spectrum. Int J Surg Pathol. 2022;30:295-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Lobo J, Rechsteiner M, Helmchen BM, Rupp NJ, Weber A, Moch H. Eosinophilic solid and cystic renal cell carcinoma and renal cell carcinomas with TFEB alterations: a comparative study. Histopathology. 2022;81:32-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Sharma R, Thirunavukkarasu B, Elhence P, Rodha MS, Sureka B. Eosinophilic Solid and Cystic Renal Cell Carcinoma: From Unclassified to Classified, A Case Report. Turk Patoloji Derg. 2022;38:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Trpkov K, Hes O. New and emerging renal entities: a perspective post-WHO 2016 classification. Histopathology. 2019;74:31-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/