Published online Aug 6, 2024. doi: 10.12998/wjcc.v12.i22.4913
Revised: May 10, 2024
Accepted: June 7, 2024
Published online: August 6, 2024
Processing time: 120 Days and 16.1 Hours
Idiopathic pulmonary fibrosis (IPF) is classified under fibrotic interstitial pneu
To assess the effects of pirfenidone in the early treatment of IPF on lung function in patients.
A retrospective analysis was performed on 113 patients with IPF who were trea
The observation group exhibited significantly higher rates than the control group after therapy, with a clear distinction (P < 0.05). After treatment, the observation group experienced significantly fewer adverse reactions than the control group, with a noticeable difference (P < 0.05). When analyzing the symptom severity scores between the two groups of patients after treatment, the observation group had significantly lower scores than the control group, with a distinct difference (P < 0.05). When comparing the pulmonary function index levels between the two groups of patients after therapy, the observation group displayed significantly higher levels than the control group, with a noticeable difference (P < 0.05). Evaluating the inflammatory marker data (C-reactive protein, interleukin-2 [IL-2], and IL-8) between the two groups of patients after therapy, the observation group exhibited significantly lower levels than the control group, with significant disparities (P < 0.05). Comparison of the 6-min walking distance data between the two groups of patients after treatment showed that the observation group achieved significantly greater distances than the control group, with a marked difference (P < 0.05).
Prompt initiation of pirfenidone treatment in individuals diagnosed with IPF can enhance pulmonary function, elevate inflammatory factor levels, and increase the distance covered in the 6-min walk test. This intervention is conducive to effectively decreasing the occurrence of adverse reactions in patients.
Core Tip: This study assessed the effects of pirfenidone in the early treatment of idiopathic pulmonary fibrosis (IPF). A retrospective analysis was conducted on 113 patients with IPF, who were divided into control and observation groups. The control group received routine therapy in combination with methylprednisolone tablets, while the observation group received routine therapy along with pirfenidone. The results showed that the prompt initiation of pirfenidone treatment in individuals diagnosed with IPF can enhance pulmonary function, elevate inflammatory factor levels, and increase the distance covered in the 6-min walk test. This intervention is conducive to effectively decreasing the occurrence of adverse reactions.
- Citation: Lei Y, Sheng JH, Jin XR, Liu XB, Zheng XY, Xu XH. Study on the efficacy of early treatment with pirfenidone on the lung function of patients with idiopathic pulmonary fibrosis. World J Clin Cases 2024; 12(22): 4913-4923
- URL: https://www.wjgnet.com/2307-8960/full/v12/i22/4913.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i22.4913
Idiopathic pulmonary fibrosis (IPF) is classified under fibrotic interstitial pneumonia, characterized by a chronic and progressive course, with the specific etiology remaining elusive[1-4]. Research suggests that chronic inhalation, viral infections, genetics, and other factors may act as predisposing factors for IPF. The incidence of IPF is on the rise in China, posing significant challenges to the well-being and productivity of affected individuals[5,6]. The predominant clinical features of IPF include dyspnea and pulmonary dysfunction[7,8]. While high-resolution computed tomography is commonly employed for diagnosis in clinical settings, its lack of specificity increases the likelihood of misdiagnosis[9,10]. Pirfenidone, an effective agent, mitigates the synthesis of inflammatory mediators such as transforming growth factor beta, thus impeding fibrocyte proliferation and collagen synthesis[11,12]. Accumulating data support the efficacy of pir
Against this backdrop, this study primarily evaluated the impact of early pirfenidone intervention on pulmonary function in individuals with IPF, aiming to provide valuable insights into the clinical management of this condition.
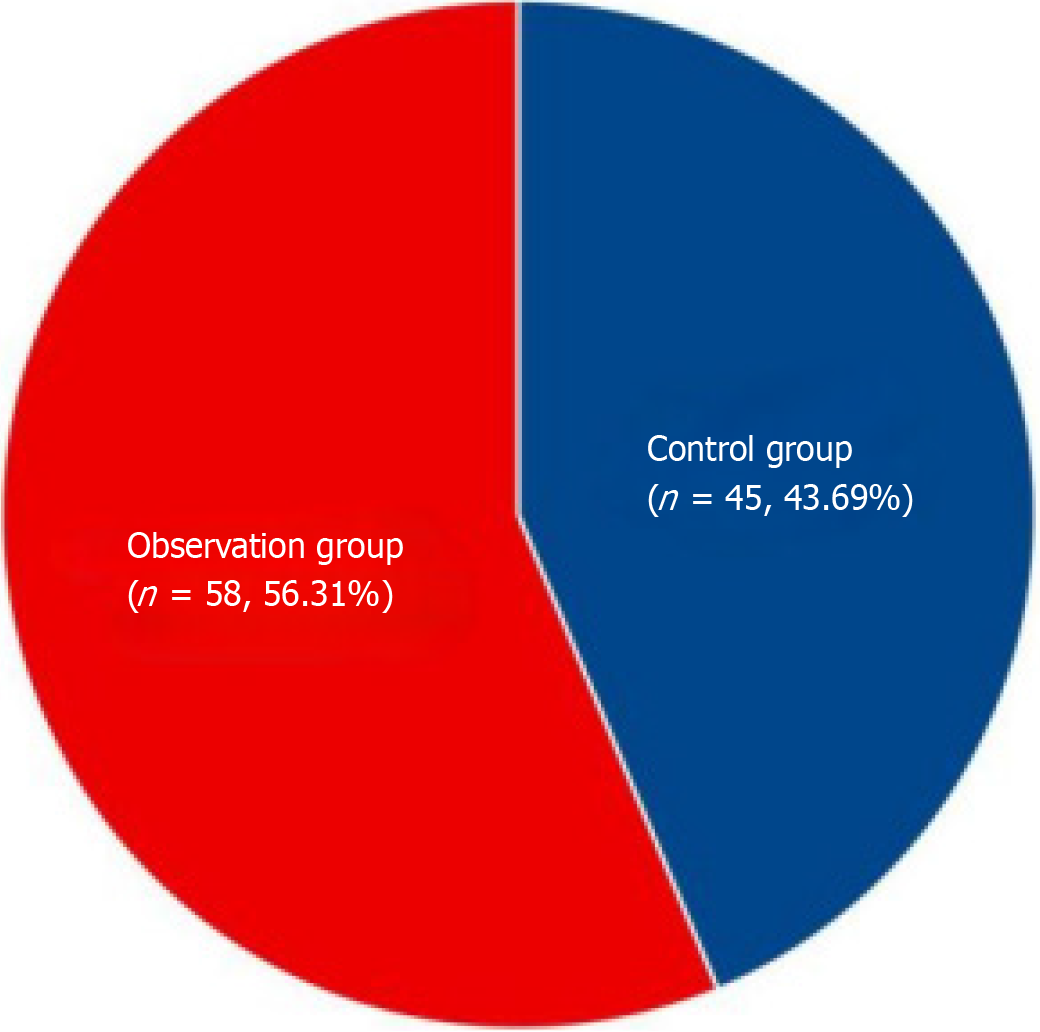
A retrospective analysis was conducted on 168 patients diagnosed with IPF who were admitted to our hospital between November 2017 and January 2023. These patients constituted the subjects of our study. They were divided into two groups: A control group receiving methylprednisolone treatment and an experimental group receiving combined pirfenidone treatment. Within the pirfenidone treatment group, patients were further categorized into subgroups based on their clinical presentation, lung function, chest X-rays, and chest computed tomography (CT) scans upon admission without prior medication. Patients exhibiting better lung function were classified into an observation group, indicating an earlier disease stage, while those with poorer lung function were placed into a later treatment group, indicative of a later disease stage. The control group comprised 53 patients, the observation group consisted of 60 patients, and the later treatment group included 55 patients. The age range in the control group was 45 years to 89 years, with an average age of 60.66 ± 10.51 years, including 45 males and 8 females. In the observation group, ages ranged from 34 years to 91 years, with an average age of 60.69 ± 10.59 years, comprising 45 males and 15 females. The later treatment group had ages ranging from 40 years to 88 years, with an average age of 60.58 ± 10.48 years, and consisted of 44 males and 11 females. Statistical analyses revealed no significant differences in demographic characteristics between the groups (P > 0.05).
The inclusion criteria for patient selection were: (1) Patients presenting clinical symptoms consistent with the dia
Exclusion criteria were: (1) Patients with severe allergic constitution; (2) Patients suffering from severe malnutrition; and (3) Patients with concurrent endocrine disorders such as hyperthyroidism and diabetes.
The control group underwent routine therapy in addition to methylprednisolone tablets. Routine therapy primarily co
The observation group received conventional treatment alongside pirfenidone. Conventional treatment included symptomatic measures like low-flow oxygen inhalation, cough suppression, and spasmolysis. Patients were provided with pirfenidone capsules (SFDA Approval No. H20133376) from Beijing Contini Pharmaceutical Co., Ltd. (Beijing, China). The dosage regimen entailed three administrations daily after meals, with each dose comprising 0.4 g. The duration of treatment for all patients spanned 30 wk.
Following the implementation of distinct treatment protocols in the two groups, various parameters were analyzed, including: (1) Total effective rate of clinical treatment; (2) Incidence of adverse reactions, such as nausea, vomiting, and anorexia; (3) Symptom scores encompassing shortness of breath, fatigue, cough, and spontaneous perspiration; (4) Pulmonary function index levels, including 1-s forced expiratory volume (FEV1), forced vital capacity (FVC), and the ratio of FEV1 to FVC; (5) Inflammatory factor indicators such as C-reactive protein (CRP), interleukin 2 (IL-2), and IL-8; and (6) 6-min walking distance before and after treatment in both groups. These analyses aimed to comprehensively evaluate the efficacy and safety of the respective treatment regimens employed in the study.
The total effective rate of clinical treatment, comprising markedly effective and effective rates, was evaluated based on predefined criteria. Marked effectiveness denoted a significant improvement in clinical symptoms such as cough and dyspnea, accompanied by notable improvement in lung-related symptoms observed through CT examination. Effectiveness indicated an improvement in clinical symptoms such as cough and dyspnea, with concurrent improvement in lung-related symptoms evident on CT examination. Ineffectiveness signified the absence of improvement or even wor
Symptom scores for shortness of breath, fatigue, cough, and spontaneous perspiration were assessed on a scale ranging from 0 to 6. Higher scores indicated greater severity of the corresponding symptom. For shortness of breath, scores of 0-2 indicated occasional shortness of breath after activity, 3-4 indicated shortness of breath upon slight exertion, and 5-6 indicated persistent shortness of breath. Fatigue scores of 0-2 denoted patients' ability to engage in activities despite feeling low in spirits, 3-4 indicated mental fatigue hindering activity, and 5-6 indicated severe mental fatigue impeding movement. Cough scores of 0-2 represented occasional dry cough or expectoration of small amounts of white sputum, 3-4 indicated easily triggered cough following exposure to cold or irritants, and 5-6 indicated prolonged cough with white sputum. Spontaneous perspiration scores of 0-2 indicated slight dampness of the skin at rest, worsening with slight activity, 3-4 indicated moist skin at rest with visible sweat after mild activity, and 5-6 indicated excessive sweating under normal conditions.
The levels of lung function indexes, including FEV1, FVC, and the ratio of FEV1 to FVC, were measured using a lung function tester (ST-150; Shanghai Yimu Medical Instrument Co., Ltd., Shanghai, China).
Inflammatory factor indicators such as CRP, IL-2, and IL-8 were assessed by collecting secretions from the patients' respiratory tract for testing. The enzyme-linked immunosorbent assay was employed for testing, utilizing the LD-96A fully automatic enzyme-linked immunosorbent analyzer (Shandong Lainde Intelligent Technology Co., Ltd., Shandong, China). The testing kit used was specifically matched for these assays.
All indicator data were inputted into SPSS 23.0 for statistical analysis. The χ² test was utilized to verify counting data expressed as percentages, while one-way analysis of variance was employed for measurement data expressed as means and standard deviations (SDs). P < 0.05 was considered statistically significant for both types of tests. This rigorous statistical analysis aimed to discern any significant differences between groups and evaluate the efficacy of the treatment interventions.
In the comparison of the clinical treatment total effective rates among the three groups of patients after therapy, the data in the observation group were significantly higher than those in both the later treatment group and control group, demonstrating a clear difference (P < 0.05). This information is summarized in Table 1 and illustrated in Figure 1.
| Group | Number of cases | Markedly effective | Effective | Invalid | Total effective rate |
| Observation group | 60 | 28 | 30 | 2 | 96.67 |
| Later treatment group | 55 | 26 | 23 | 6 | 89.09 |
| Control group | 53 | 25 | 20 | 8 | 84.91 |
| χ² | 5.279 | ||||
| P value | 0.509 |
In comparing the frequency of adverse reactions (vomiting, nausea, anorexia) among the three groups of patients after therapy, the observation group exhibited lower incidence compared to both the later treatment group and the control group, indicating a significant difference (P < 0.05). This information is summarized in Table 2 and depicted in Figure 2.
| Group | Number of cases | Nausea | Vomiting | Anorexia | Frequency of adverse reactions |
| Observation group | 60 | 1 | 1 | 0 | 3.33 |
| Later treatment group | 55 | 2 | 1 | 2 | 9.09 |
| Control group | 53 | 3 | 3 | 3 | 16.98 |
| χ² | 5.864 | ||||
| P value | 0.021 |
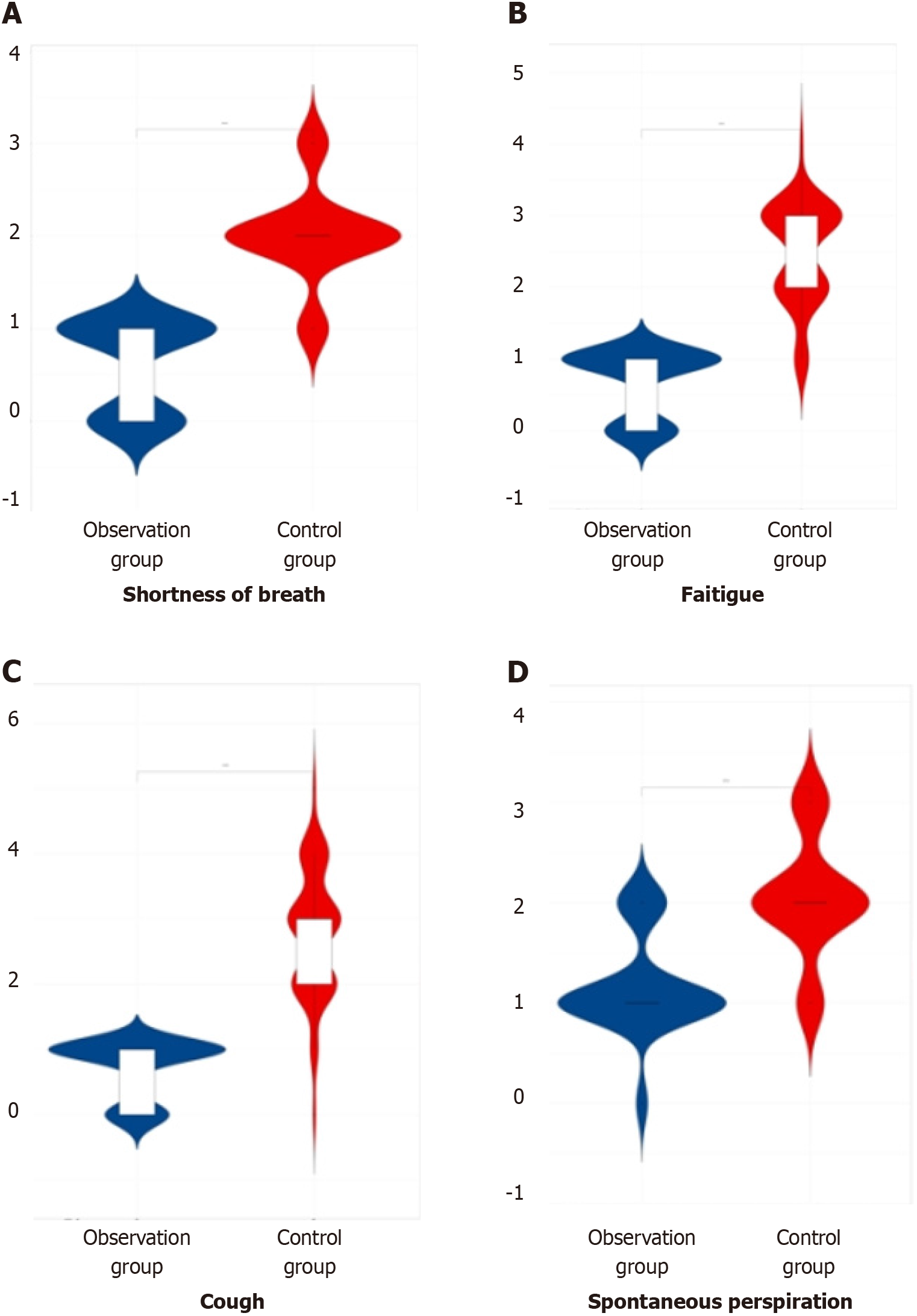
In the comparison of symptom scores (shortness of breath, fatigue, cough, and spontaneous perspiration) among the three groups of patients after therapy, the observation group demonstrated lower scores compared to both the later treatment group and the control group, indicating a significant difference (P < 0.05). This information is summarized in Table 3 and illustrated in Figure 3.
| Group | Number of cases | Shortness of breath | Fatigue | Cough | Spontaneous perspiration |
| Observation group | 60 | 0.54 ± 0.18 | 0.61 ± 0.21 | 0.62 ± 0.23 | 1.12 ± 0.43 |
| Later treatment group | 55 | 1.56 ± 0.21 | 1.66 ± 0.32 | 1.85 ± 0.11 | 1.84 ± 0.12 |
| Control group | 53 | 1.94 ± 0.52 | 2.45 ± 0.63 | 2.83 ± 0.92 | 2.05 ± 0.53 |
| F value | 289.83 | 278.74 | 215.92 | 91.95 | |
| P value | 0.000 | 0.000 | 0.000 | 0.000 |
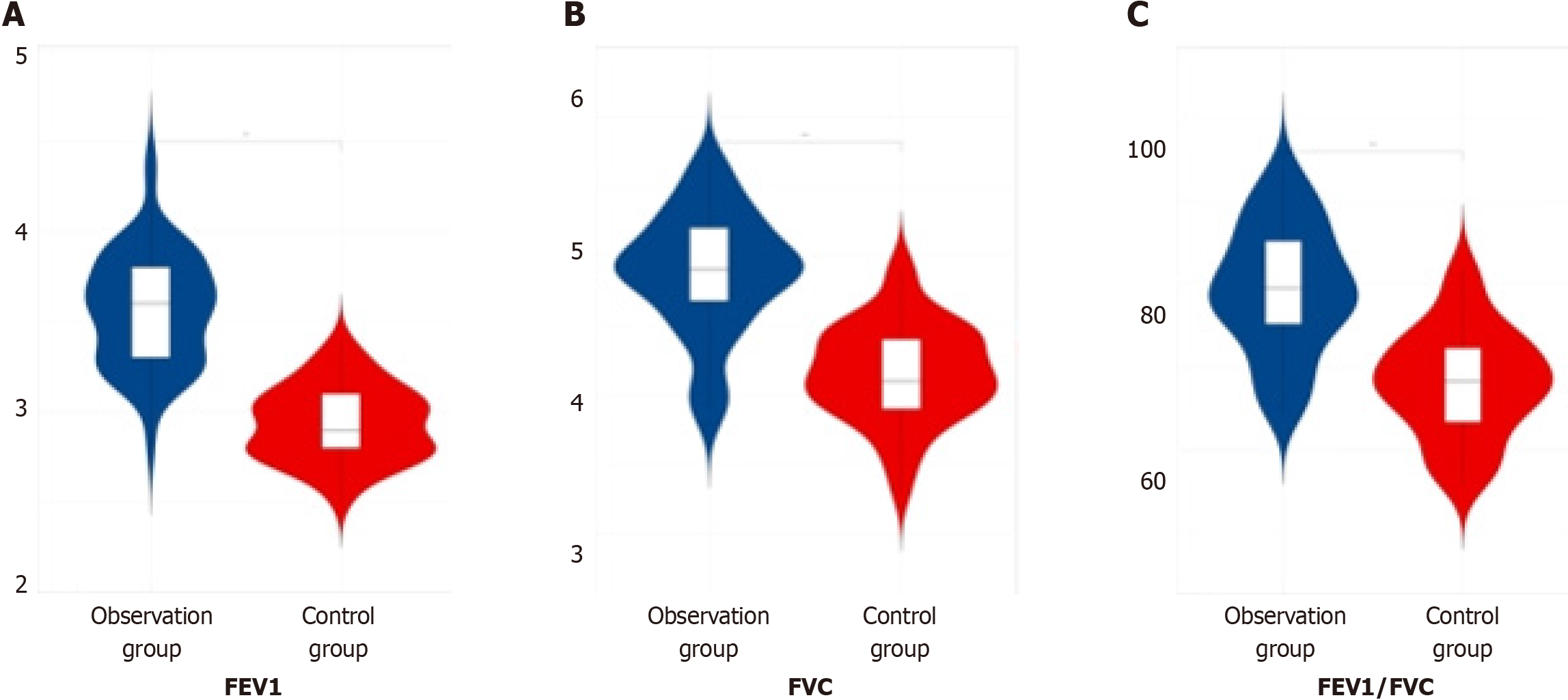
In assessing the pulmonary function index levels, including the FEV1, FVC, and the ratio of FEV1 to FVC, among the three patient groups post-therapy, it was observed that the observation group displayed notably higher values compared to both the later treatment group and the control group, indicating a significant difference (P < 0.05). This finding is summarized in Table 4 and depicted in Figure 4.
| Group | Number of cases | Forced expiratory volume in 1 s in L | Forced vital capacity in L | Ratio of forced expiratory volume in one second to forced vital capacity |
| Observation group | 60 | 3.56 ± 0.32 | 4.88 ± 0.44 | 79.39 ± 7.61 |
| Later treatment group | 55 | 3.14 ± 0.22 | 4.75 ± 0.13 | 70.36 ± 4.57 |
| Control group | 53 | 2.93 ± 0.21 | 4.15 ± 0.35 | 68.13 ± 6.41 |
| F value | 115.50 | 93.79 | 56.00 | |
| P value | 0.000 | 0.000 | 0.000 |
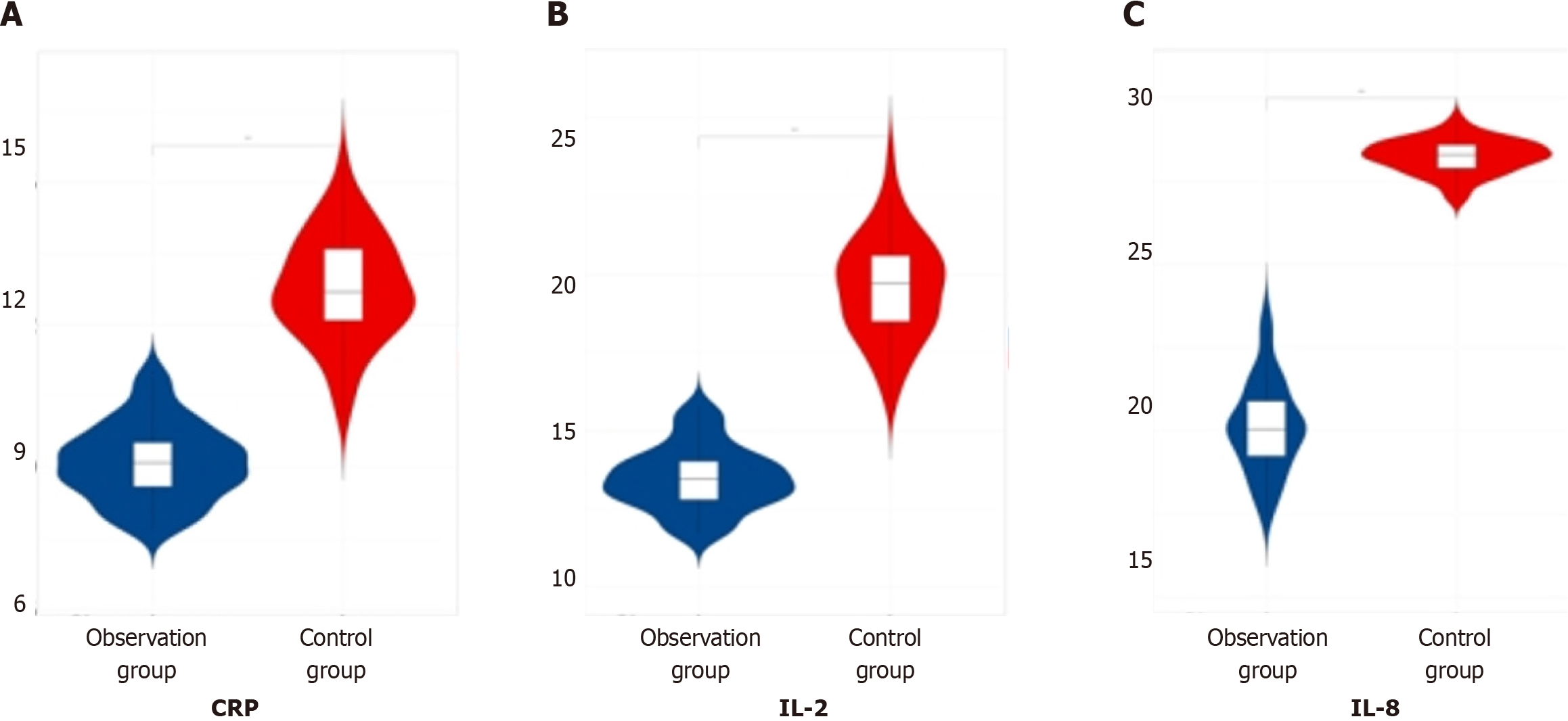
When comparing the data of inflammatory factor indicators (CRP, IL-2, IL-8) among the three patient groups post-therapy, it was evident that the observation group exhibited lower values compared to both the later treatment group and the control group, indicating a significant difference (P < 0.05). This finding is summarized in Table 5 and visualized in Figure 5.
| Group | Number of cases | C-reactive protein in mg/L | Interleukin-2 in ng/L | Interleukin-8 in ng/L |
| Observation group | 60 | 9.11 ± 0.81 | 13.47 ± 1.02 | 20.11 ± 1.38 |
| Later treatment group | 55 | 11.21 ± 0.31 | 18.63 ± 0.99 | 26.31 ± 1.43 |
| Control group | 53 | 12.78 ± 1.12 | 19.57 ± 1.63 | 28.25 ± 2.51 |
| F value | 267.26 | 267.26 | 263.35 | |
| P value | 0.000 | 0.000 | 0.000 |
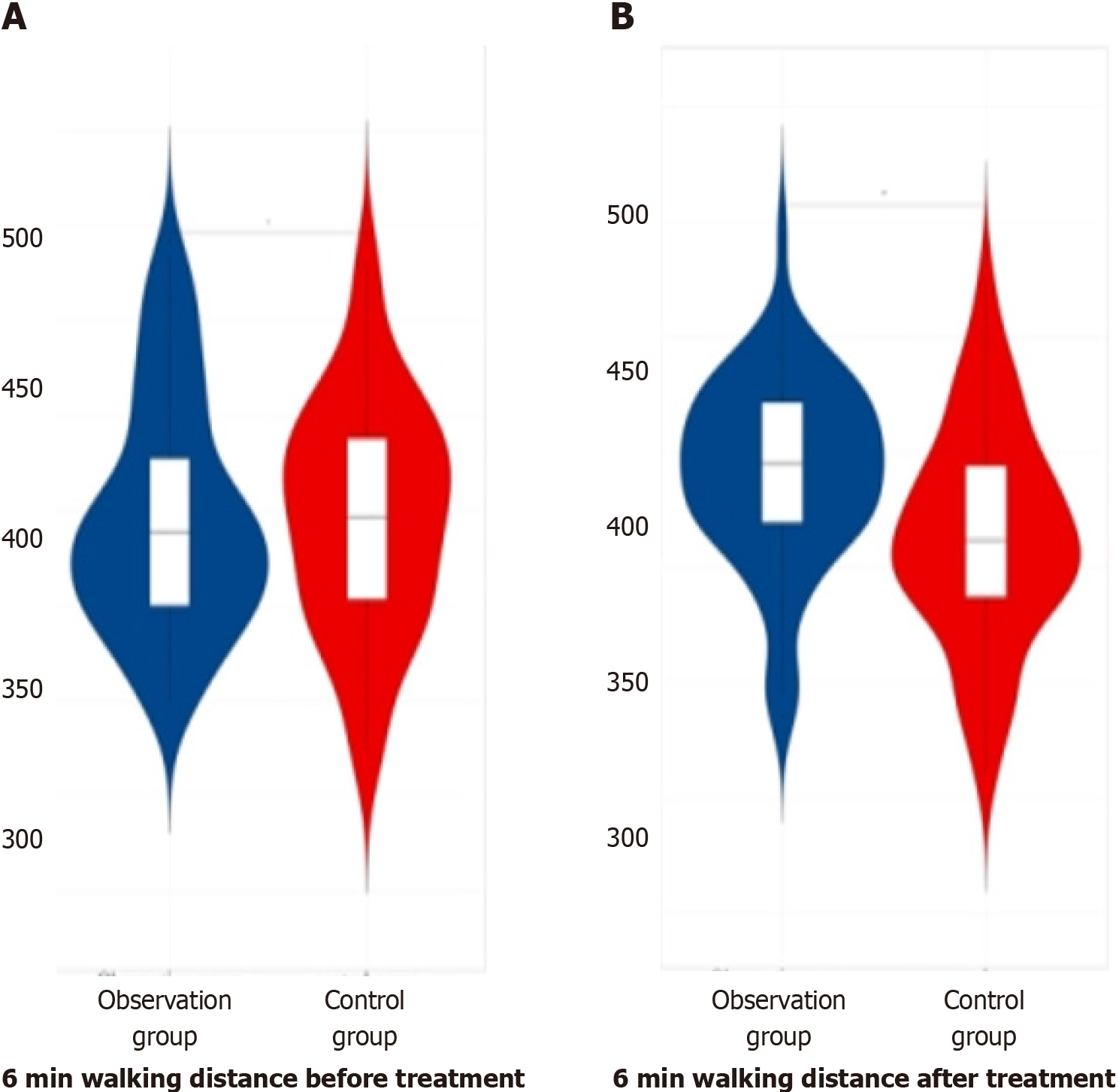
Before therapy, there was no obvious distinction in the 6-min walking distance data among the three groups of patients (P > 0.05). In the comparison of the data of 6-min walking distance among the three groups of patients after therapy, the observation group had greater data than the later treatment group and control group, with obvious differences (P < 0.05). This finding is summarized in Table 6 and depicted in Figure 6.
| Group | Number of cases | Before treatment | After treatment |
| Observation group | 60 | 397.77 ± 30.22 | 420.69 ± 20.31 |
| Later treatment group | 55 | 398.21 ± 30.16 | 410.35 ± 21.67 |
| Control group | 53 | 398.61 ± 30.21 | 406.66 ± 23.33 |
| F value | 0.34 | 7.44 | |
| P value | 0.711 | 0.001 |
Previous research has predominantly focused on assessing the overall therapeutic efficacy of pirfenidone in IPF. However, it has become increasingly evident that IPF is a chronic disease prone to acute exacerbations, emphasizing the importance of early intervention to prevent such exacerbations. Therefore, this study highlights the potential benefits of early pirfenidone administration by comparing treatment outcomes among the early, late, and control groups. The goal is to underscore the significance of prompt initiation of pirfenidone treatment upon detection of IPF, thereby enhancing clinical management of the disease.
IPF represents the most prevalent form of fibrotic interstitial lung diseases, characterized by a progressive and irreversible nature[15,16]. Patients with IPF typically experience symptoms such as dyspnea, dry cough, and limited mobility, which worsen over time. The incidence and fatality rates of the disease tend to rise with age[17,18]. While some IPF patients may rapidly progress to death, others may face a gradual decline in lung function, significantly impacting their quality of life[19-22]. A subset of IPF patients may also experience acute respiratory deterioration, often termed acute exacerbations, which are associated with high mortality rate[23,24]. Pirfenidone, acting on fibrogenesis as a multi-target cellular drug, is among the approved therapeutic options for IPF patients[25,26].
Pirfenidone is an orally administered small molecule compound, initially synthesized in the United States in 1974. It exhibits pleiotropic effects, including anti-inflammatory and antioxidant properties. In a clinical trial conducted in 2005, it was observed that pirfenidone had the potential to delay the decline in vital capacity and mitigate acute exacerbations in patients with IPF[27,28]. Furthermore, the change in FVC served as the primary endpoint in Phase III clinical trials, wherein pirfenidone demonstrated its ability to delay the progression of IPF[29].
Research has consistently demonstrated that pirfenidone, compared to placebo, exhibits efficacy in delaying the progression of IPF, reducing disease severity, and significantly delaying the decline in FVC, ultimately leading to reduced mortality rates among patients. The conventional formulation of pirfenidone is in capsule form, intended for oral administration, typically recommended after meals to minimize gastrointestinal adverse reactions. Interestingly, some clinical experimental data[30] suggest that taking pirfenidone with food can notably decrease the occurrence of adverse reactions.
Indeed, recent clinical experimental data[30] underscore the potential benefits of pirfenidone in the treatment of pulmonary fibrosis. Researchers induced pulmonary fibrosis in rats and administered pirfenidone orally and via inhalation for 14 consecutive days to assess their efficacy and pharmacokinetics. The findings revealed that inhaled pirfenidone can be directly delivered to the lungs, achieving higher drug concentrations in lung tissue with a smaller dose compared to oral administration. Specifically, inhalation of 100 mg pirfenidone aerosol resulted in a 35-fold increase in maximum drug concentration in alveolar epithelial cell fluid and a 20% increase in the area under the plasma concentration-time curve compared to oral administration of the same dose. Importantly, no adverse effects on respiratory rate or lung function were observed with pirfenidone aerosol inhalation. Furthermore, both inhalation and oral routes demonstrated similar therapeutic efficacy, indicating the potential of inhaled pirfenidone as an alternative administration route. The clinical data further support the therapeutic potential of pirfenidone in pulmonary fibrosis management. The observation group exhibited superior outcomes in terms of total effective rate of clinical therapy, pulmonary function indicators (including FEV1, FVC, and the ratio of FEV1 to FVC), and the 6-min walking distance, compared to the control group, with significant differences (P < 0.05). Additionally, after therapy, the observation group experienced fewer adverse reactions (such as nausea, vomiting, and anorexia), lower symptom scores (for shortness of breath, fatigue, cough, and spontaneous perspiration), and reduced levels of inflammatory factors (CRP, IL-2, IL-8) compared to the control group, again with significant differences (P < 0.05). These findings underscore the significant clinical value of early pirfenidone intervention in IPF treatment. The approach demonstrates high safety and feasibility, significantly alleviating clinical symptoms, improving lung function, and slowing disease progression.
It is essential to note that the findings of this study had certain limitations, including the relatively small scope of research, limited number of selected cases, and absence of long-term follow-up. Consequently, there may be some degree of deviation in the research data. Therefore, future research endeavors should aim to broaden the scope of study selection, increase the number of selected cases, and incorporate long-term follow-up assessments. By doing so, the reliability of the research conclusions can be improved, providing a more robust foundation for clinical decision-making and patient management in IPF.
The implementation of early pirfenidone intervention in patients with IPF appears to several favorable outcomes. These include improvements in pulmonary function, reductions in inflammatory factor levels, enhancements in the 6-min walking distance, and a decrease in the frequency of adverse reactions, ultimately promoting faster patient recovery.
| 1. | Finnerty JP, Ponnuswamy A, Dutta P, Abdelaziz A, Kamil H. Efficacy of antifibrotic drugs, nintedanib and pirfenidone, in treatment of progressive pulmonary fibrosis in both idiopathic pulmonary fibrosis (IPF) and non-IPF: a systematic review and meta-analysis. BMC Pulm Med. 2021;21:411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 2. | Lu ZH, Yang CL, Yang GG, Pan WX, Tian LG, Zheng JX, Lv S, Zhang SY, Zheng PY, Zhang SX. Efficacy of the combination of modern medicine and traditional Chinese medicine in pulmonary fibrosis arising as a sequelae in convalescent COVID-19 patients: a randomized multicenter trial. Infect Dis Poverty. 2021;10:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Zhang F, Ayaub EA, Wang B, Puchulu-Campanella E, Li YH, Hettiarachchi SU, Lindeman SD, Luo Q, Rout S, Srinivasarao M, Cox A, Tsoyi K, Nickerson-Nutter C, Rosas IO, Low PS. Reprogramming of profibrotic macrophages for treatment of bleomycin-induced pulmonary fibrosis. EMBO Mol Med. 2020;12:e12034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 4. | Rajan SK, Cottin V, Dhar R, Danoff S, Flaherty KR, Brown KK, Mohan A, Renzoni E, Mohan M, Udwadia Z, Shenoy P, Currow D, Devraj A, Jankharia B, Kulshrestha R, Jones S, Ravaglia C, Quadrelli S, Iyer R, Dhooria S, Kolb M, Wells AU. Progressive pulmonary fibrosis: an expert group consensus statement. Eur Respir J. 2023;61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 158] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 5. | Cottin V, Hirani NA, Hotchkin DL, Nambiar AM, Ogura T, Otaola M, Skowasch D, Park JS, Poonyagariyagorn HK, Wuyts W, Wells AU. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur Respir Rev. 2018;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 433] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 6. | Xiong Y, Cui X, Zhou Y, Chai G, Jiang X, Ge G, Wang Y, Sun H, Che H, Nie Y, Zhao P. Dehydrocostus lactone inhibits BLM-induced pulmonary fibrosis and inflammation in mice via the JNK and p38 MAPK-mediated NF-κB signaling pathways. Int Immunopharmacol. 2021;98:107780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Käsmann L, Dietrich A, Staab-Weijnitz CA, Manapov F, Behr J, Rimner A, Jeremic B, Senan S, De Ruysscher D, Lauber K, Belka C. Radiation-induced lung toxicity - cellular and molecular mechanisms of pathogenesis, management, and literature review. Radiat Oncol. 2020;15:214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 168] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 8. | Wei Y, Tanaka M, Sakurai T, Kamiyoshi A, Ichikawa-Shindo Y, Kawate H, Cui N, Kakihara S, Zhao Y, Aruga K, Sanjo H, Shindo T. Adrenomedullin Ameliorates Pulmonary Fibrosis by Regulating TGF-ß-Smads Signaling and Myofibroblast Differentiation. Endocrinology. 2021;162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Gao Q, Chang X, Yang M, Zheng J, Gong X, Liu H, Li K, Wang X, Zhan H, Li S, Feng S, Sun X, Sun Y. LncRNA MEG3 restrained pulmonary fibrosis induced by NiO NPs via regulating hedgehog signaling pathway-mediated autophagy. Environ Toxicol. 2022;37:79-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Vásquez-Garzón VR, Ramírez-Cosmes A, Reyes-Jiménez E, Carrasco-Torres G, Hernández-García S, Aguilar-Ruiz SR, Torres-Aguilar H, Alpuche J, Pérez-Campos Mayoral L, Pina-Canseco S, Arellanes-Robledo J, Villa-Treviño S, Baltiérrez-Hoyos R. Liver damage in bleomycin-induced pulmonary fibrosis in mice. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:1503-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | D'Amico R, Monaco F, Fusco R, Siracusa R, Impellizzeri D, Peritore AF, Crupi R, Gugliandolo E, Cuzzocrea S, Di Paola R, Genovese T, Cordaro M. Atrazine Inhalation Worsen Pulmonary Fibrosis Regulating the Nuclear Factor-Erythroid 2-Related Factor (Nrf2) Pathways Inducing Brain Comorbidities. Cell Physiol Biochem. 2021;55:704-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Takao S, Nakashima T, Masuda T, Namba M, Sakamoto S, Yamaguchi K, Horimasu Y, Miyamoto S, Iwamoto H, Fujitaka K, Hamada H, Takahashi S, Nakashima A, Hattori N. Human bone marrow-derived mesenchymal stromal cells cultured in serum-free media demonstrate enhanced antifibrotic abilities via prolonged survival and robust regulatory T cell induction in murine bleomycin-induced pulmonary fibrosis. Stem Cell Res Ther. 2021;12:506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Al-Mutairy EA, Imtiaz FA, Khalid M, Al Qattan S, Saleh S, Mahmoud LM, Al-Saif MM, Al-Haj L, Al-Enazi A, AlJebreen AM, Mohammed SF, Mobeireek AF, Alkattan K, Chisti MA, Luzina IG, Al-Owain M, Weheba I, Abdelsayed AM, Ramzan K, Janssen LJ, Conca W, Alaiya A, Collison KS, Meyer BF, Atamas SP, Khabar KS, Hasday JD, Al-Mohanna F. An atypical pulmonary fibrosis is associated with co-inheritance of mutations in the calcium binding protein genes S100A3 and S100A13. Eur Respir J. 2019;54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Zhou W, Li L, Tao J, Ma C, Xie Y, Ding L, Hou S, Zhang Z, Xue D, Luo J, Zhu Y. Autophagy inhibition restores CD200 expression under IL-1β microenvironment in placental mesenchymal stem cells of fetal origin and improves its pulmonary fibrosis therapeutic potential. Mol Immunol. 2022;151:29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Marchioni A, Tonelli R, Cerri S, Castaniere I, Andrisani D, Gozzi F, Bruzzi G, Manicardi L, Moretti A, Demurtas J, Baroncini S, Andreani A, Cappiello GF, Busani S, Fantini R, Tabbì L, Samarelli AV, Clini E. Pulmonary Stretch and Lung Mechanotransduction: Implications for Progression in the Fibrotic Lung. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | Lee JYT, Tikellis G, Corte TJ, Goh NS, Keir GJ, Spencer L, Sandford D, Khor YH, Glaspole I, Price J, Hey-Cunningham AJ, Maloney J, Teoh AKY, Watson AL, Holland AE. The supportive care needs of people living with pulmonary fibrosis and their caregivers: a systematic review. Eur Respir Rev. 2020;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 17. | Norman O, Koivunen J, Mäki JM, Pihlajaniemi T, Heikkinen A. Identification of suitable reference genes for normalization of reverse transcription quantitative real-time PCR (RT-qPCR) in the fibrotic phase of the bleomycin mouse model of pulmonary fibrosis. PLoS One. 2022;17:e0276215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 18. | Liu J, Gao D, Ding Q, Zhang B, Zhu W, Shi Y. Sparganii Rhizoma alleviates pulmonary fibrosis by inhibiting fibroblasts differentiation and epithelial-mesenchymal transition mediated by TGF-β1/ Smad2/3 pathway. J Ethnopharmacol. 2023;309:116305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 19. | Cheresh P, Kim SJ, Huang LS, Watanabe S, Joshi N, Williams KJN, Chi M, Lu Z, Harijith A, Yeldandi A, Lam AP, Gottardi C, Misharin AV, Budinger GRS, Natarajan V, Kamp DW. The Sphingosine Kinase 1 Inhibitor, PF543, Mitigates Pulmonary Fibrosis by Reducing Lung Epithelial Cell mtDNA Damage and Recruitment of Fibrogenic Monocytes. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Hesse C, Beneke V, Konzok S, Diefenbach C, Bülow Sand JM, Rønnow SR, Karsdal MA, Jonigk D, Sewald K, Braun A, Leeming DJ, Wollin L. Nintedanib modulates type III collagen turnover in viable precision-cut lung slices from bleomycin-treated rats and patients with pulmonary fibrosis. Respir Res. 2022;23:201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (1)] |
| 21. | Samareh Fekri M, Mandegary A, Sharififar F, Poursalehi HR, Nematollahi MH, Izadi A, Mehdipour M, Asadi A, Samareh Fekri M. Protective effect of standardized extract of Myrtus communis L. (myrtle) on experimentally bleomycin-induced pulmonary fibrosis: biochemical and histopathological study. Drug Chem Toxicol. 2018;41:408-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Chu M, Wu S, Wang W, Mao L, Yu Y, Jiang L, Yuan W, Zhang M, Sang L, Huang Q, Tian T, Han L, Zhuang X, Zhang ZF, Wu J. miRNA sequencing reveals miRNA-4508 from peripheral blood lymphocytes as potential diagnostic biomarker for silica-related pulmonary fibrosis: A multistage study. Respirology. 2020;25:511-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Yagasaki H, Takekoshi S, Kitatani K, Kato C, Yamasaki H, Shioyama K, Tsuboi T, Matsuzaki T, Inagaki Y, Masuda R, Iwazaki M. Protective effect of ebselen on bleomycin-induced lung fibrosis: analysis of the molecular mechanism of lung fibrosis mediated by oxidized diacylglycerol. Free Radic Res. 2022;56:473-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | Lv YQ, Cai GF, Zeng PP, Dhlamini Q, Chen LF, Chen JJ, Lyu HD, Mossahebi-Mohammadi M, Ahmadvand N, Bellusci S, Li X, Chen C, Zhang JS. FGF10 Therapeutic Administration Promotes Mobilization of Injury-Activated Alveolar Progenitors in a Mouse Fibrosis Model. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Mecozzi L, Mambrini M, Ruscitti F, Ferrini E, Ciccimarra R, Ravanetti F, Sverzellati N, Silva M, Ruffini L, Belenkov S, Civelli M, Villetti G, Stellari FF. In-vivo lung fibrosis staging in a bleomycin-mouse model: a new micro-CT guided densitometric approach. Sci Rep. 2020;10:18735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 26. | Liang J, Liu N, Liu X, Mena JM, Xie T, Geng Y, Huan C, Zhang Y, Taghavifar F, Huang G, Kurkciyan A, Barron V, Jiang D, Noble PW. Mitogen-activated Protein Kinase-activated Protein Kinase 2 Inhibition Attenuates Fibroblast Invasion and Severe Lung Fibrosis. Am J Respir Cell Mol Biol. 2019;60:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Rago F, Melo EM, Kraemer L, Galvão I, Cassali GD, Santos RAS, Russo RC, Teixeira MM. Effect of preventive or therapeutic treatment with angiotensin 1-7 in a model of bleomycin-induced lung fibrosis in mice. J Leukoc Biol. 2019;106:677-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Dekoster K, Decaesteker T, Berghen N, Van den Broucke S, Jonckheere AC, Wouters J, Krouglov A, Lories R, De Langhe E, Hoet P, Verbeken E, Vanoirbeek J, Vande Velde G. Longitudinal micro-computed tomography-derived biomarkers quantify non-resolving lung fibrosis in a silicosis mouse model. Sci Rep. 2020;10:16181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Zohny MH, Cavalu S, Youssef ME, Kaddah MMY, Mourad AAE, Gaafar AGA, El-Ahwany E, Amin NA, Arakeep HM, Shata A, Saleh S, Hafez MM, Elazab ST, Abdelhady R, El Shahat RM, Yahya G, Saber S. Coomassie brilliant blue G-250 dye attenuates bleomycin-induced lung fibrosis by regulating the NF-κB and NLRP3 crosstalk: A novel approach for filling an unmet medical need. Biomed Pharmacother. 2022;148:112723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 30. | Derrett-Smith E, Clark KEN, Shiwen X, Abraham DJ, Hoyles RK, Lacombe O, Broqua P, Junien JL, Konstantinova I, Ong VH, Denton CP. The pan-PPAR agonist lanifibranor reduces development of lung fibrosis and attenuates cardiorespiratory manifestations in a transgenic mouse model of systemic sclerosis. Arthritis Res Ther. 2021;23:234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/