Published online Jul 16, 2024. doi: 10.12998/wjcc.v12.i20.4412
Revised: May 4, 2024
Accepted: May 24, 2024
Published online: July 16, 2024
Processing time: 88 Days and 22.2 Hours
Cases of severe inflammatory renal disease and renal cell carcinoma (RCC) that occur simultaneously in the same kidney have been occasionally reported. However, extrarenal RCC that does not originate from the native kidney has rarely been reported. To our knowledge, this is the first reported case of RCC developing in the ipsilateral retroperitoneal space after a simple nephrectomy (SN) for inflammatory renal disease.
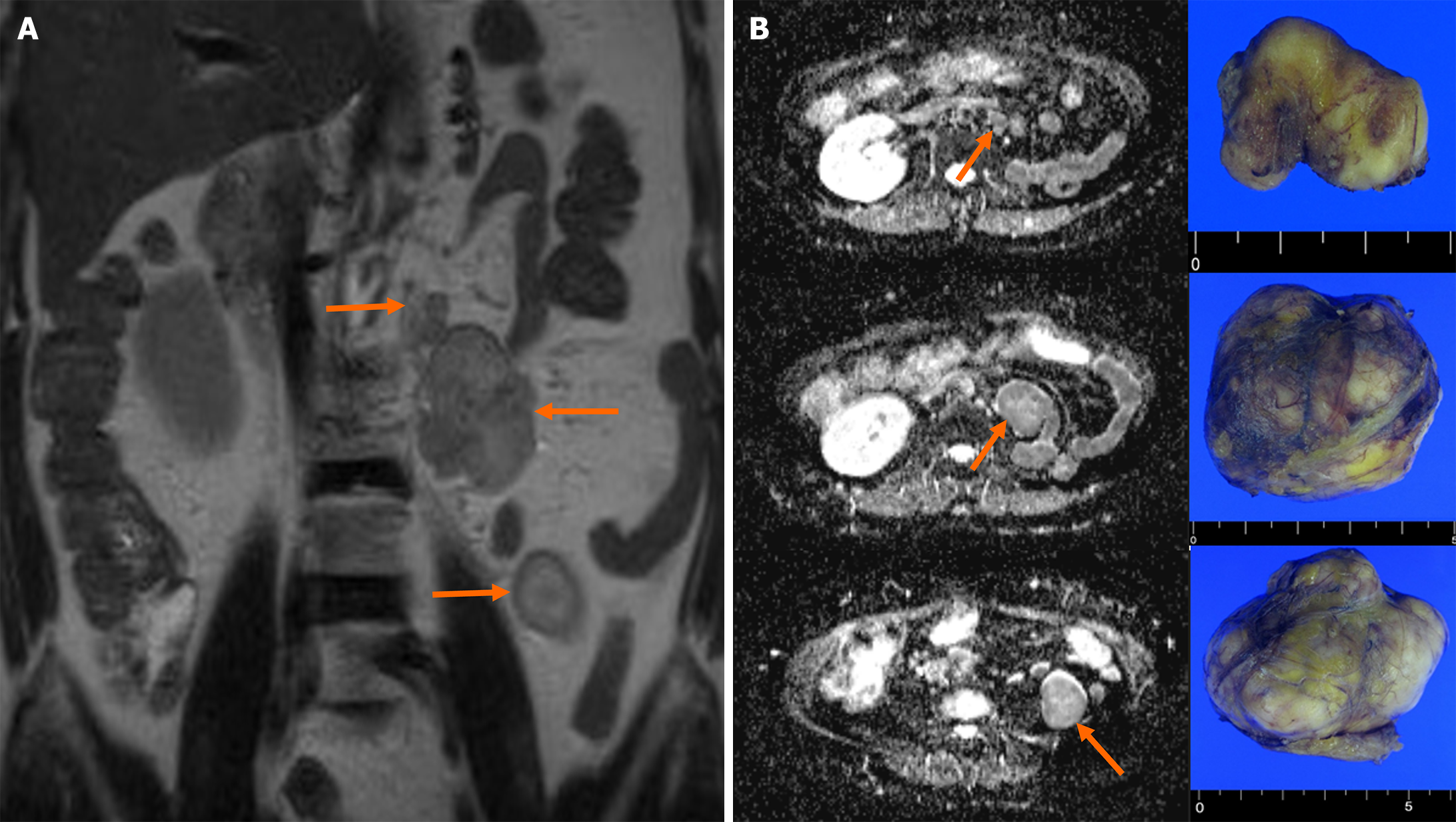
A 63-year-old woman was referred to our hospital following the incidental discovery of a left retroperitoneal mass without specific symptoms. Her medical history revealed a left SN 27 years ago due to a renal abscess. Magnetic resonance imaging of the abdomen revealed three oval masses in the left retroperitoneum. The masses were successfully excised, and subsequent pathology confirmed papillary RCC. After surgery, the patient remained disease-free for 11 years without adjuvant therapy.
Clinicians should be vigilant of RCC in patients with retroperitoneal masses, especially after SN for inflammatory renal disease.
Core Tip: To date, no cases of renal cell carcinoma (RCC) arising in the ipsilateral retroperitoneum following simple nephrectomy (SN) have been reported. We aimed to share our experience of treating RCC in the ipsilateral retroperitoneum of a 63-year-old Asian woman who underwent SN for a renal abscess 27 years ago. When evaluating retroperitoneal masses in patients with a positive clinical history of SN, clinicians should consider the possibility of late-onset RCC as a differential diagnosis.
- Citation: Kim TN, Kim A, Kim KB, Lee CH. Ipsilateral retroperitoneal papillary renal cell carcinoma 27 years after simple nephrectomy for a renal abscess: A case report. World J Clin Cases 2024; 12(20): 4412-4418
- URL: https://www.wjgnet.com/2307-8960/full/v12/i20/4412.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i20.4412
The synchronous occurrence of renal malignancies and inflammatory conditions such as xanthogranulomatous pyelonephritis in the same kidney is rare but has been reported occasionally[1-3]. However, late-onset and incidental detection of renal cell carcinoma (RCC) in the ipsilateral retroperitoneum following simple nephrectomy (SN) is a novel finding. When performing SN on a severely inflamed kidney harboring undiagnosed renal cancer, a risk of tumor cell spillage and inoculation in the peritoneal cavity exists. On the other hand, extrarenal RCC is extremely rare and has only been reported eight times in the literature. In all cases, the patients had intact bilateral kidneys, and the masses were located very close to the native kidney, presenting with a single cancer cell type[4-8]. Herein, we report a unique case of extrarenal papillary renal cell carcinoma (PRCC) located in the ipsilateral retroperitoneum 27 years after SN for the treatment of a renal abscess.
A 63-year-old Asian woman was referred to our hospital on April 23, 2013, following the incidental detection of a left retroperitoneal mass on abdominal computed tomography (CT).
CT findings obtained during health screening revealed three lesions in the left retroperitoneum. The patient was referred to our clinic for further evaluation and disease management, with no reported specific symptoms or pain.
Her medical history included a left SN due to a renal abscess 27 years ago and a cesarean delivery 41 years ago. At the time of SN, she was admitted to the intensive care unit for 3 d because of massive intraoperative bleeding. The surgeon informed the patient that the nephrectomy was performed incompletely because of severe perinephric inflammation and intraoperative bleeding.
There was nothing remarkable in the patient's personal and family history.
No positive signs were observed on abdominal, cardiopulmonary, or nervous system examinations.
The results of routine laboratory tests, including complete blood count, liver and kidney function tests, and urine analysis, were normal.
Magnetic resonance imaging of the abdomen revealed three oval masses in the left retroperitoneum (2.8 cm, 5.0 cm, and 6.0 cm, respectively) (Figure 1). The T2-weighted coronal image exhibited a high signal intensity and demonstrated a well-enhanced pattern. Diffusion-weighted transverse imaging showed a mildly restricted diffusion. The results of chest CT and bone scintigraphy for detecting metastasis were unremarkable.
Considering the above-mentioned clinical data and the absence of a history of RCC, the patient was diagnosed with multiple solitary fibrous tumors.
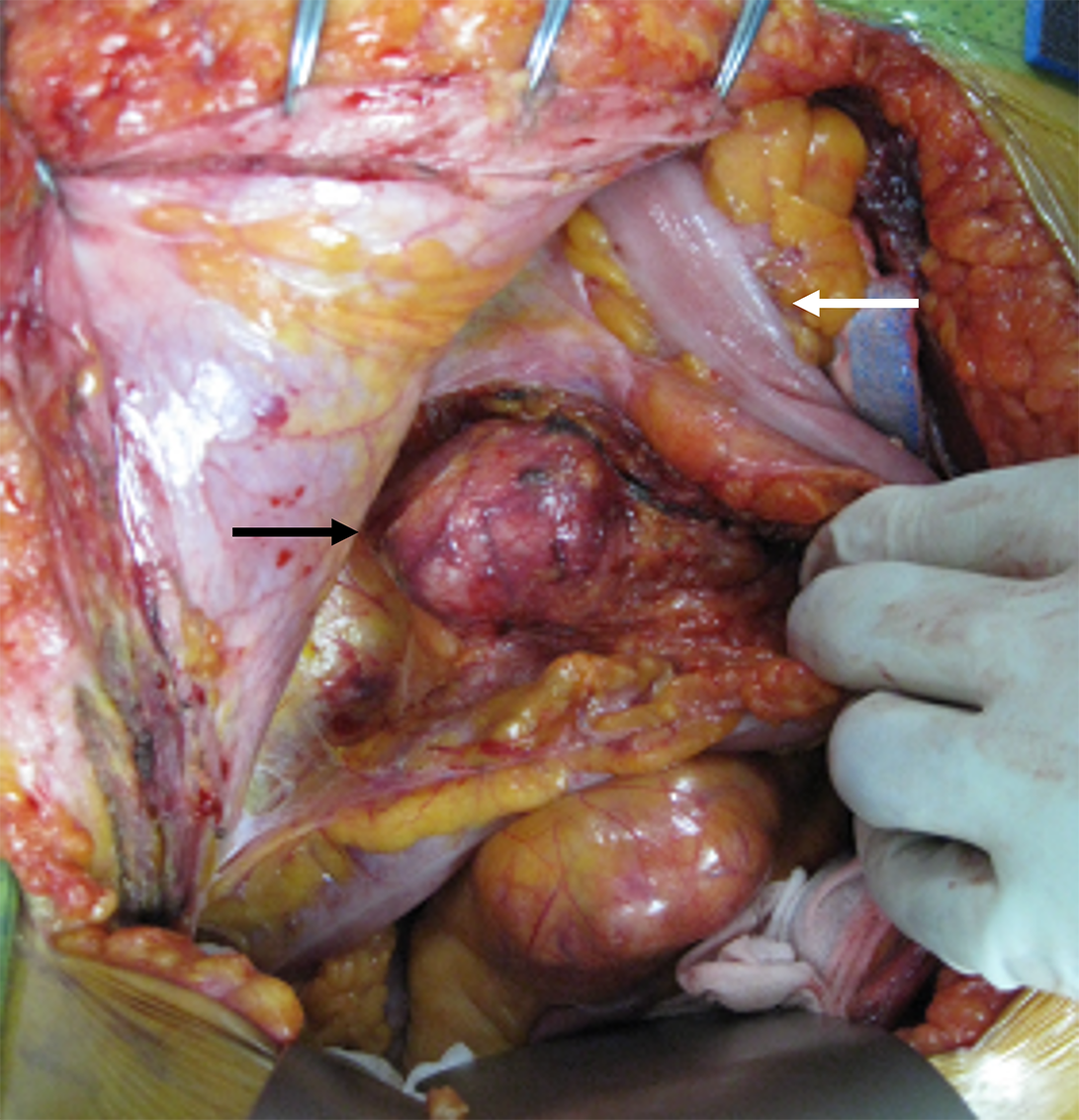
Following a detailed discussion, we decided to perform open surgery. Mild tissue adhesion was observed between the descending colon and the three retroperitoneal masses located in the psoas muscle between lumbar vertebral levels 1 and 4. The masses had clear boundaries with the surrounding tissue; therefore, complete resection was not difficult (Figure 2). The postoperative course was uneventful.
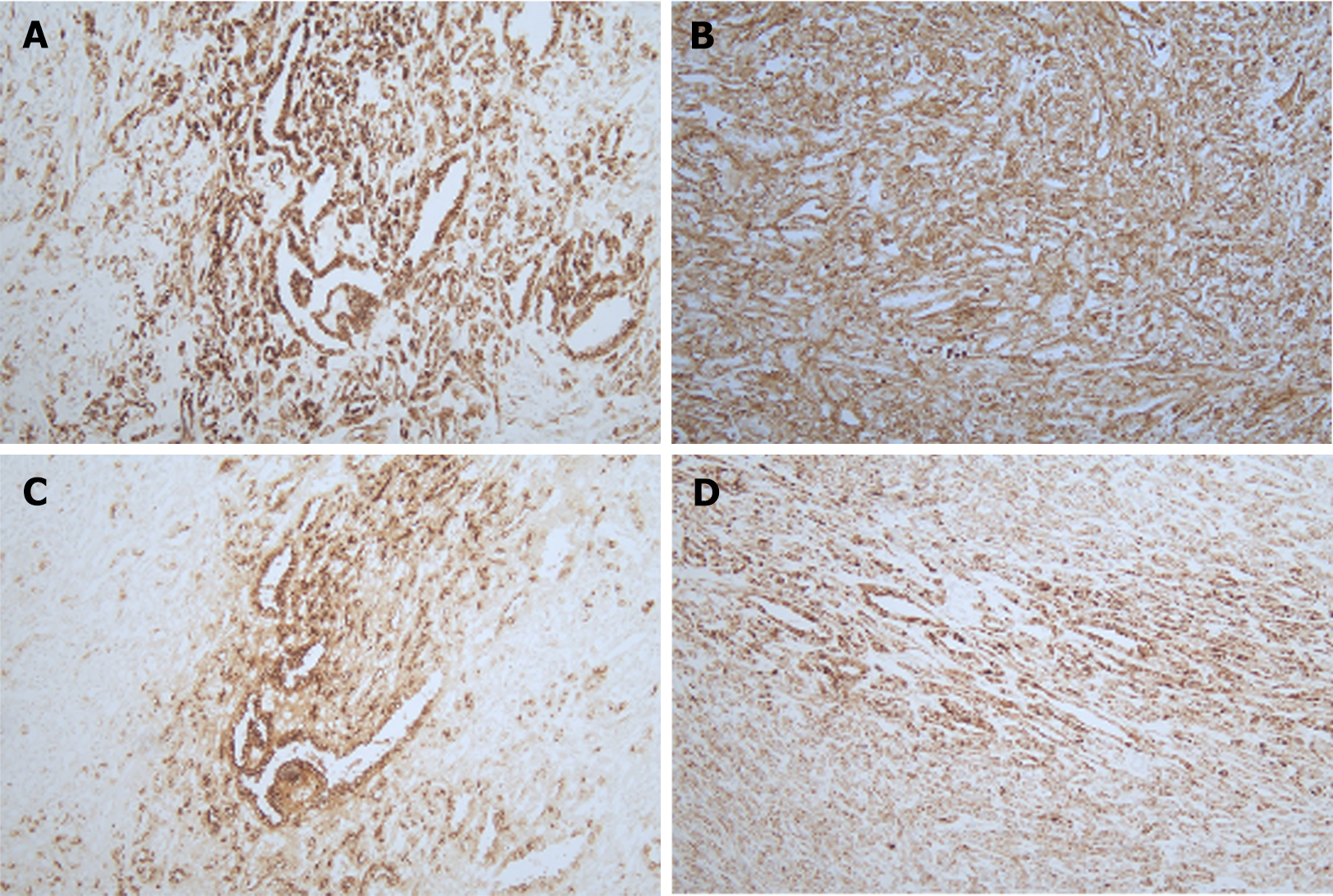
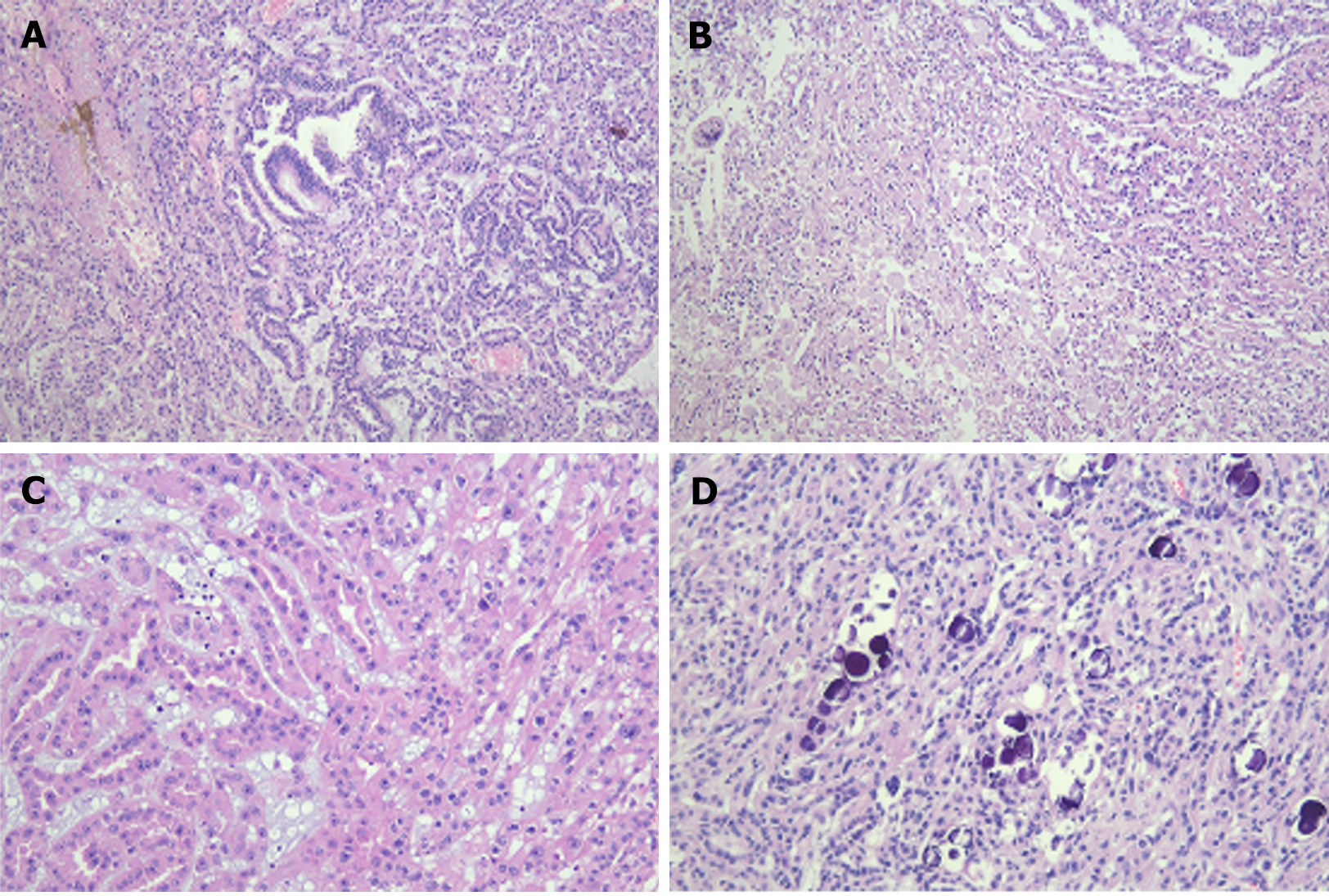
Immunohistochemical results showed diffuse cytoplasmic positivity of the tumor tissues for pan-cytokeratin, vimentin, epithelial membrane antigen, cytokeratin 7, and α-methylacyl coenzyme A racemase (Figure 3). Microscopic findings of hematoxylin and eosin (H&E) staining showed tubulopapillary structures lined by large eosinophilic epithelial cells with high-grade nuclear features and small basophilic cuboidal cells with low-grade nuclear features (Figure 4). The final pathological report was PRCC, according to the 2022 World Health Organization classification. The patient refused adjuvant treatment. She underwent follow-up evaluations every 3 months for the first year, then every 6 months for the next 5 years, and annually thereafter. During the initial 5-year period, abdominopelvic and chest CT scans, along with complete blood count examinations, liver and kidney function tests, and urine analysis were conducted every 6 months, supplemented by an annual bone scan. Subsequent to 6 years post-surgery, the same tests were conducted annually. She visited our hospital on February 13, 2024 and has remained disease-free for 11 years post-surgery.
To the best of our knowledge, no cases of RCC occurring in the ipsilateral retroperitoneum following SN for benign renal disease have been reported. There are three possible explanations for this unique occurrence. First, extrarenal RCC, which is believed to arise from mesonephric remnants, occurs after SN[4]. It is a rare disease with no anatomical connection to the native kidney[4]. Previous case reports have indicated that both kidneys were intact and that extrarenal RCC was present on one side of the body, located very close to a native kidney; however, this case presented with one intact kidney and three separate tumor masses in the contralateral retroperitoneum. We speculated that if there was no retroperitoneal mass at the time of SN, the mesonephric remnants may have slowly progressed to extrarenal RCC after SN.
The second possibility is tumor seeding from unrecognized RCC and indolent growth of tumor spills. While inflammatory renal disease has been reported alongside RCC in some cases, this association does not imply a general correlation between benign inflammatory renal disease and RCC. Rather, instances where inflammatory renal disease and RCC coexist within the same kidney have been documented on several occasions[8-10]. The association between renal malignancies and pyelonephritis can lead to misinterpretation, even in the modern era of imaging procedures, and it is sometimes difficult to preoperatively distinguish inflammatory renal disease from RCC. SN is performed safely and is uncomplicated in kidneys without renal anomalies or inflammation. However, inflammation associated with pyelonephritis can make SN more complicated and difficult to perform than radical nephrectomy[7]. Poor surgical conditions and a rough surgical technique that violates primary tumor boundaries can promote seeding. If our patient had a concomitant renal abscess and malignancy in the same kidney, tumor cell spillage or direct inoculation with neoplastic cells could have occurred.
Third, the tumors may have originated from the residual renal parenchyma due to incomplete resection of the left kidney, a scenario not previously reported. The dense and fibrotic tissue of the perinephric and perihilar areas can pose significant challenges in dissection and accidental organ or vascular injury due to obliteration and adhesion of regular tissue planes. In the medical history review, the patient in question had been unable to undergo a complete excision of the left kidney because of complications that occurred during surgery. Consequently, the remaining left renal tissue may not have regressed naturally; thus, the possibility of transformation into RCC cannot be ruled out.
PRCC is the second most common type of RCC, accounting for 15%–20% of RCC cases. PRCC manifests with a heterogeneous clinical course, including multifocal, indolent presentations and solitary tumors with a highly aggressive pattern[11]. Generally, PRCC has a lower potential for metastasis than clear-cell RCC and a better prognosis in patients with non-metastatic RCC[12]; however, PRCC is more aggressive than clear-cell RCC in patients with metastatic disease[13]. There have been no reports of tumor seeding from kidneys with severe inflammation and unrecognized PRCC or of transfor
The late occurrence of ipsilateral retroperitoneal RCC after SN for the treatment of inflammatory renal disease is remarkable. Clinicians should consider the potential late occurrence of RCC in the differential diagnosis of retroperitoneal masses in patients with a positive clinical history of SN related to inflammatory renal disease.
| 1. | Radin DR, Chandrasoma P. Coexistent xanthogranulomatous pyelonephritis and renal cell carcinoma. J Comput Tomogr. 1987;11:294-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Papadopoulos I, Wirth B, Wand H. Xanthogranulomatous pyelonephritis associated with renal cell carcinoma. Report on two cases and review of the literature. Eur Urol. 1990;18:74-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Smith RD, Khoubehi B, Chandra A, Glass JM, Mee AD. Xanthogranulomatous pyelonephritis and renal malignancy: unusual fellows in the renal bed. BJU Int. 2000;86:558-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Terada T. Extra-renal clear cell renal cell carcinoma probably arising from mesodermal embryonic remnants. Pathol Int. 2012;62:291-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Duarte RJ, Mitre AI, Chambô JL, Arap MA, Srougi M. Laparoscopic nephrectomy outside gerota fascia for management of inflammatory kidney. J Endourol. 2008;22:681-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Danilovic A, Ferreira TAC, Maia GVA, Torricelli FCM, Mazzucchi E, Nahas WC, Srougi M. Predictors of surgical complications of nephrectomy for urolithiasis. Int Braz J Urol. 2019;45:100-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Zelhof B, McIntyre IG, Fowler SM, Napier-Hemy RD, Burke DM, Grey BR; British Association of Urological Surgeons. Nephrectomy for benign disease in the UK: results from the British Association of Urological Surgeons nephrectomy database. BJU Int. 2016;117:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Rosevear HM, Meier MM, Gallagher BL, Joudi FN. Surgically discovered xanthogranulomatous pyelonephritis invading inferior vena cava with coexisting renal cell carcinoma. ScientificWorldJournal. 2009;9:5-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Arora P, Rao S, Khurana N, Ramteke VK. Renal replacement lipomatosis with coexistent papillary renal cell carcinoma, renal tubulopapillary adenomatosis, and xanthogranulomatous pyelonephritis: an extremely rare association and possible pathogenetic correlation. Urol J. 2013;10:906-908. [PubMed] |
| 10. | Tsai TH, Tang SH, Chuang FP, Wu ST, Sun GH, Yu DS, Chang SY, Cha TL. Ipsilateral synchronous neoplasms of kidney presenting as acute pyelonephritis and bladder metastasis. Urology. 2009;73:1163.e9-1163.11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Cancer Genome Atlas Research Network, Linehan WM, Spellman PT, Ricketts CJ, Creighton CJ, Fei SS, Davis C, Wheeler DA, Murray BA, Schmidt L, Vocke CD, Peto M, Al Mamun AA, Shinbrot E, Sethi A, Brooks S, Rathmell WK, Brooks AN, Hoadley KA, Robertson AG, Brooks D, Bowlby R, Sadeghi S, Shen H, Weisenberger DJ, Bootwalla M, Baylin SB, Laird PW, Cherniack AD, Saksena G, Haake S, Li J, Liang H, Lu Y, Mills GB, Akbani R, Leiserson MD, Raphael BJ, Anur P, Bottaro D, Albiges L, Barnabas N, Choueiri TK, Czerniak B, Godwin AK, Hakimi AA, Ho TH, Hsieh J, Ittmann M, Kim WY, Krishnan B, Merino MJ, Mills Shaw KR, Reuter VE, Reznik E, Shelley CS, Shuch B, Signoretti S, Srinivasan R, Tamboli P, Thomas G, Tickoo S, Burnett K, Crain D, Gardner J, Lau K, Mallery D, Morris S, Paulauskis JD, Penny RJ, Shelton C, Shelton WT, Sherman M, Thompson E, Yena P, Avedon MT, Bowen J, Gastier-Foster JM, Gerken M, Leraas KM, Lichtenberg TM, Ramirez NC, Santos T, Wise L, Zmuda E, Demchok JA, Felau I, Hutter CM, Sheth M, Sofia HJ, Tarnuzzer R, Wang Z, Yang L, Zenklusen JC, Zhang J, Ayala B, Baboud J, Chudamani S, Liu J, Lolla L, Naresh R, Pihl T, Sun Q, Wan Y, Wu Y, Ally A, Balasundaram M, Balu S, Beroukhim R, Bodenheimer T, Buhay C, Butterfield YS, Carlsen R, Carter SL, Chao H, Chuah E, Clarke A, Covington KR, Dahdouli M, Dewal N, Dhalla N, Doddapaneni HV, Drummond JA, Gabriel SB, Gibbs RA, Guin R, Hale W, Hawes A, Hayes DN, Holt RA, Hoyle AP, Jefferys SR, Jones SJ, Jones CD, Kalra D, Kovar C, Lewis L, Ma Y, Marra MA, Mayo M, Meng S, Meyerson M, Mieczkowski PA, Moore RA, Morton D, Mose LE, Mungall AJ, Muzny D, Parker JS, Perou CM, Roach J, Schein JE, Schumacher SE, Shi Y, Simons JV, Sipahimalani P, Skelly T, Soloway MG, Sougnez C, Tam A, Tan D, Thiessen N, Veluvolu U, Wang M, Wilkerson MD, Wong T, Wu J, Xi L, Zhou J, Bedford J, Chen F, Fu Y, Gerstein M, Haussler D, Kasaian K, Lai P, Ling S, Radenbaugh A, Van Den Berg D, Weinstein JN, Zhu J, Albert M, Alexopoulou I, Andersen JJ, Auman JT, Bartlett J, Bastacky S, Bergsten J, Blute ML, Boice L, Bollag RJ, Boyd J, Castle E, Chen YB, Cheville JC, Curley E, Davies B, DeVolk A, Dhir R, Dike L, Eckman J, Engel J, Harr J, Hrebinko R, Huang M, Huelsenbeck-Dill L, Iacocca M, Jacobs B, Lobis M, Maranchie JK, McMeekin S, Myers J, Nelson J, Parfitt J, Parwani A, Petrelli N, Rabeno B, Roy S, Salner AL, Slaton J, Stanton M, Thompson RH, Thorne L, Tucker K, Weinberger PM, Winemiller C, Zach LA, Zuna R. Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. N Engl J Med. 2016;374:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 849] [Cited by in RCA: 1032] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 12. | Zucchi A, Novara G, Costantini E, Antonelli A, Carini M, Carmignani G, Cosciani Cunico S, Fontana D, Longo N, Martignoni G, Minervini A, Mirone V, Porena M, Roscigno M, Schiavina R, Simeone C, Simonato A, Siracusano S, Terrone C, Ficarra V. Prognostic factors in a large multi-institutional series of papillary renal cell carcinoma. BJU Int. 2012;109:1140-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Steffens S, Janssen M, Roos FC, Becker F, Schumacher S, Seidel C, Wegener G, Thüroff JW, Hofmann R, Stöckle M, Siemer S, Schrader M, Hartmann A, Kuczyk MA, Junker K, Schrader AJ. Incidence and long-term prognosis of papillary compared to clear cell renal cell carcinoma--a multicentre study. Eur J Cancer. 2012;48:2347-2352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Li Y, Qiu X, Li W, Yang Y, Yang R, Zhao X, Guo H, Li X. Primary Extrarenal Type 2 Papillary Renal Cell Carcinoma: A Case Report. Urology. 2019;123:e1-e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Nunes G, Pinto-Marques P, Sequeira P, Mendonça E. Primary Extrarenal Renal Cell Carcinoma: A Unique Diagnosis Performed through Endoscopic Ultrasound. GE Port J Gastroenterol. 2019;26:378-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Srivishnu S, Bakshi G, Menon S. Primary extrarenal papillary renal cell carcinoma presenting as a neck mass. Indian J Urol. 2021;37:173-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/