Published online Jul 16, 2024. doi: 10.12998/wjcc.v12.i20.4357
Revised: May 1, 2024
Accepted: May 27, 2024
Published online: July 16, 2024
Processing time: 113 Days and 16.3 Hours
Lithium carbonate is used to manage various mood disorders, but it can cause thyroid abnormalities, including goiter, hypothyroidism, and hyperthyroidism. In rare cases, it can lead to giant goiter and subclinical hyperthyroidism, which may require surgical intervention in severe cases.
This case represents a rare development of giant goiter and subclinical hyper
It is essential to monitor thyroid function, test thyroid antibody levels, and perform thyroid ultrasounds con
Core Tip: This case uniquely highlighted an innovative approach to managing a rare occurrence of giant goiter and sub
- Citation: Chen XM, Jiang ZL, Wu X, Li XG. Lithium carbonate-induced giant goiter and subclinical hyperthyroidism in a patient with schizophrenia: A case report and review of literature. World J Clin Cases 2024; 12(20): 4357-4364
- URL: https://www.wjgnet.com/2307-8960/full/v12/i20/4357.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i20.4357
Lithium is a medication used to manage acute mania, unipolar and bipolar depression, and as a prophylactic measure against bipolar disorder. It has been extensively documented in the medical literature that it is associated with various thyroid abnormalities[1] including goiter, hypothyroidism, hyperthyroidism, and autoimmune thyroiditis.
Previous studies have shown that the prevalence of goiter caused by lithium treatment ranges from 30% to 55%, with giant goiter being a rare occurrence[2-6]. In a study of 330 patients receiving lithium therapy for recurrent manic depre
Lithium carbonate-induced hyperthyroidism is infrequent. Bocchetta and Loviselli[15] estimated an annual incidence of 0.1% through the observation of women equivalent to 680 patient-years. In a 2005 study, Kirov et al[8] prospectively followed 33 women with bipolar disorder who were treated with lithium. The study revealed only one case of hyper
The patient was a 41-year-old Chinese woman with a neck tumor that was discovered 3 years previously. She complained of worsened breathing of 1 d duration.
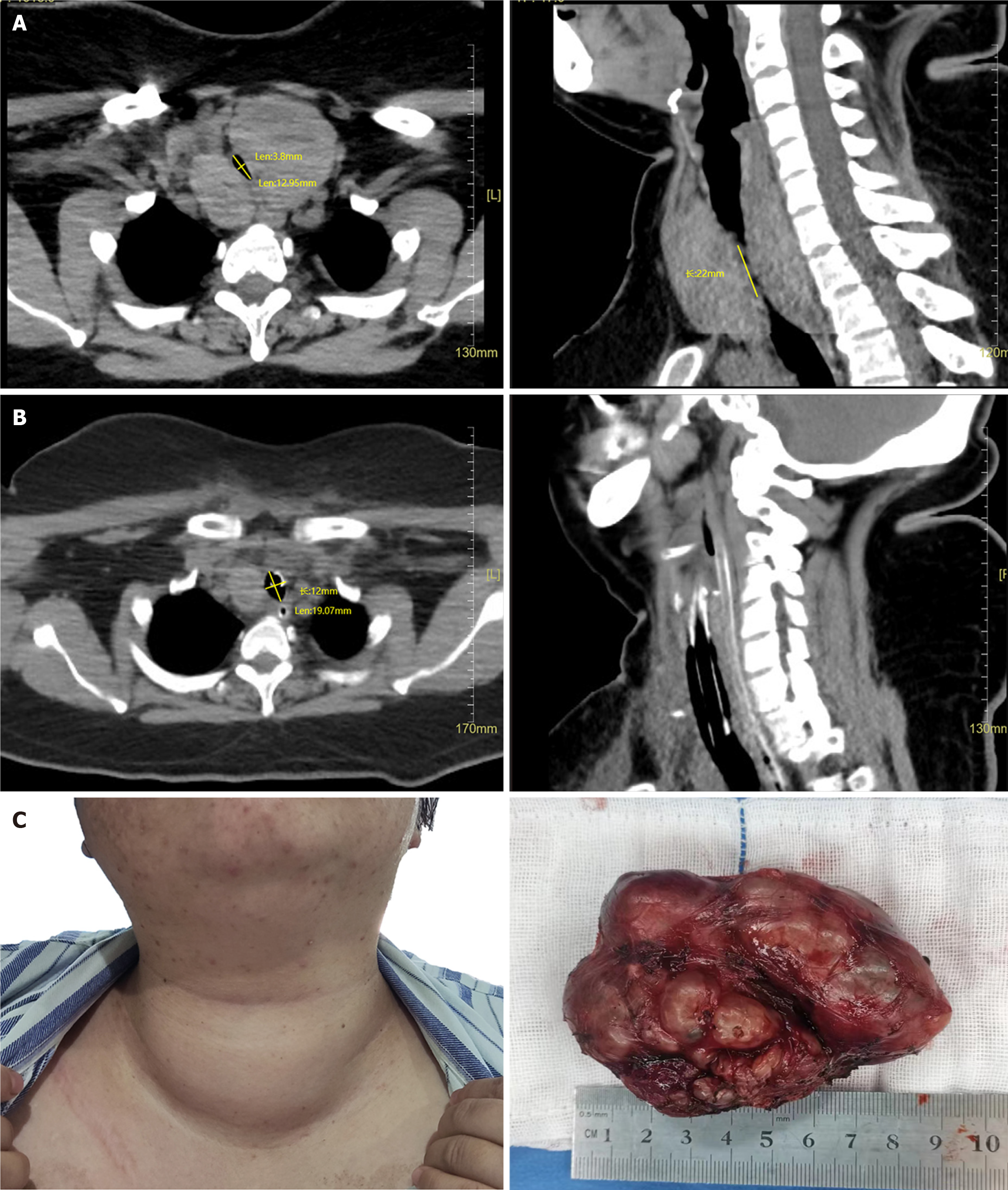
Three years ago, a neck lump was discovered, leading to hospital admission after a 1-d episode of breathing difficulty. At that time, thyroid ultrasonography showed no signs of airway compression or hyperthyroidism symptoms. The left lobe measured approximately 2.9 cm × 4.6 cm × 6.0 cm. The right lobe measured 3.1 cm × 3.1 cm × 5.5 cm, and a mixed mass measuring 0.7 cm × 1.5 cm was seen in the left lobe. In the previous year, the size of thyroid gland had significantly increased, causing occasional discomfort, such as shortness of breath, difficulty swallowing, and hoarseness. However, no diagnosis or treatment was pursued. The patient experienced worsening breathing difficulties and hoarseness 1 d before this evaluation. A recent thyroid ultrasonography revealed a left lobe size of 7.1 cm × 3.8 cm × 3.6 cm and a right lobe size of 7.6 cm × 5.0 cm × 4.4 cm (Figure 1A), prompting her to visit our hospital.
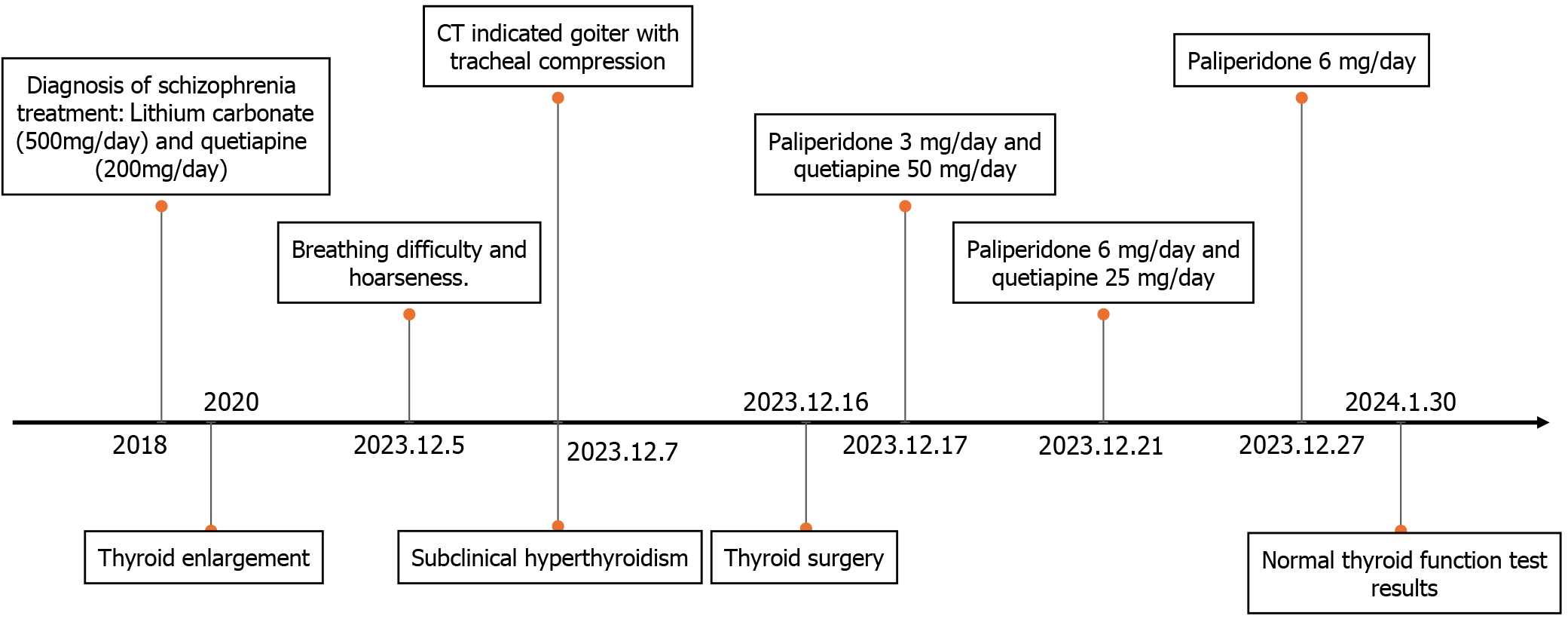
The woman had been diagnosed with schizophrenia 5 years ago owing to unusual behavior and emotional instability. The patient was unable to remember her hallucinations, delusions, and previous medical records. We were unable to obtain her detailed medical and medication history from family members. She vaguely acknowledged that she had experienced disorganized speech and behavior, suspicious hallucinations, and delusions during the onset of her illness, along with short-term emotional instability, irritability, and impulsive behaviors. For 5 years, she had been effectively managed with lithium carbonate (500 mg/d) and quetiapine (200 mg/d), which helped maintain stable symptom control. Her medical history included chronic gastritis, hepatitis B carrier status, and no congenital diseases.
She had a body mass index of 40.27 kg/m², irregular menstrual cycles (every 3 mo to 4 mo), and a 6-year-old daughter. The patient had a rare blood type, O RH (-). Her family history was unremarkable.
Upon admission, the patient presented with dyspnea, hoarseness, a heart rate of 103 beats/min, a blood pressure of 122/76 mmHg, and finger pulse oxygen saturation of 92%. The patient's neck lymph nodes were not palpably enlarged. Thyroid examination revealed Grade III enlargement, with a palpable mass on both sides and a tough texture. The patient’s mental condition was stable, without hallucinations or delusions. The next step in the plan was to perform thyroid surgery, followed by pathological biopsy to definitively determine the presence of thyroid cancer.
Laboratory results revealed elevated white blood cells (11.7 × 109/L), low hemoglobin (108 g/L), decreased TSH (0.03 mIU/L), and elevated thyroglobulin autoantibodies (TGAb) (7.06 IU/mL). Free triiodothyronine (FT3) and free thyroxine (FT4) were normal. The serum lithium-ion concentration was 0.3 mmol/L (Table 1).
| Blood test | WBC | Hb | ANC | NP | |
| Results | 11.7 × 109/L | 108 g/L | 7.7 × 109/L | 65.6% | |
| Thyroid hormone | TSH | FT3 | FT4 | TGAb | TPOAb |
| Results | 0.03 mIU/L | 3.9 pmol/L | 9.4 pmol/L | 7.06 IU/mL | 1.14 IU/mL |
Thyroid ultrasonography confirmed thyroid enlargement with solid and mixed lesions, and the patient was diagnosed with nodular goiter. Neck computed tomography (CT) revealed significant enlargement of the left and right lateral lobes of the thyroid gland. The dimensions of the left lobe were approximately 5.0 cm × 4.9 cm × 8.7 cm, and those of the right lobe were approximately 3.3 cm × 4.6 cm × 8.0 cm. Density heterogeneity was reduced, and nodular low-density shadows with unclear boundaries were observed. Additionally, the trachea exhibited narrowing and a shift to the right, with the minor diameter of the ellipse of the narrowest part measuring 3.8 mm and a stenosis length of approximately 22 mm. The neck and chest CT findings led to diagnoses of bilateral lower lobe pneumonia, nodular goiter, tracheal stenosis, and tracheomalacia. These results indicated that the patient had a substantial goiter that had compressed the airway.
The final diagnoses were nodular goiter (right lobe), follicular adenoma (left lobe), schizophrenia, tracheomalacia, tracheal stenosis, subclinical hyperthyroidism, chronic gastritis, bilateral lower lung pneumonia, and hepatitis B surface antigen carriers.
Because significant enlargement of the thyroid occurred without any associated bleeding, the patient maintained stable vital signs after oxygen inhalation, and her blood oxygen saturation remained at 99%. Elective surgery was considered, and to reduce the risk of intraoperative bleeding and shrink the thyroid mass, the patient started taking Lugol's iodine 1 wk before the operation. Simultaneously, her pneumonia was managed. To prevent respiratory depression during surgery, dexmedetomidine was administered, and tracheal intubation was performed. The procedure involved bilateral subtotal thyroidectomy and recurrent laryngeal nerve exploration under general anesthesia. Pathological evaluation of intraoperative frozen sections revealed follicular adenoma in the left lobe and a nodular goiter with adenomatous changes in the right lobe. The patient remained intubated until the 4th postoperative day, when a neck CT confirmed successful airway expansion without collapse. The narrowest ellipse minor diameter was 12 mm. The tracheal tube and the neck drainage tube were subsequently removed. On the 1st day after extubation, the patient received paliperidone 3 mg/d and quetiapine 50 mg/d, with an increase to paliperidone 6 mg/d and quetiapine 25 mg/d on the 5th day. No postoperative complications occurred, and the patient was discharged 11 d after the operation.
Upon discharge, the patient exhibited smooth breathing, clear speech, normal swallowing, excellent mental condition, and emotional stability. There were no hallucinations or delusions. Pathology revealed a nodular goiter (right lobe of the thyroid) and a follicular adenoma (left lobe of the thyroid). On January 2, 2024, a thyroid function test revealed a TSH level of 0.2 mIU/L, a TGAb level of 8.93 IU/mL, and normal FT3 and FT4 levels. On January 30, 2024, a thyroid function test revealed normal levels of TSH, FT3, and FT4, with no retesting of antibodies. Before hospital admission, the patient had visited doctors repeatedly, feeling disheartened by the perceived difficulty and hopelessness of treating her condition. However, following the operation, the patient expressed deep satisfaction, noting a significant improvement in her quality of life. See Figure 1B and C (Supplementary material) and Figure 2 for further details.
Lithium carbonate is used to treat and prevent bipolar disorder, especially mania, and it has specific effects on depression[17]. Prolonged use of lithium carbonate has been associated with abnormalities in thyroid function and morphology, including clinical or subclinical hyperthyroidism, hypothyroidism, and goiter[15].
This patient presented with decreased TSH levels, increased TGAb levels, normal FT3 and FT4 levels, and no clinical manifestations of hyperthyroidism, suggesting a diagnosis of subclinical hyperthyroidism. Further testing, including thyroid ultrasonography, chest CT, and pathological evaluation, revealed nodular goiter (right lobe of the thyroid) and follicular adenoma (left lobe of the thyroid). The long-term use of lithium carbonate has been implicated in goiter formation, inhibiting thyroid hormone synthesis, promoting thyroid follicle expansion and follicular cavity enlargement, and inducing glial hyperplasia[18]. Additionally, lithium may stimulate thyroid cell proliferation and differentiation, leading to the substantial goiter observed in this patient, which needed prompt surgical intervention[19,20].
Currently, lithium carbonate-induced hyperthyroidism is associated with sterile thyroiditis, including granulomatous, lymphocytic, and nonspecific thyroiditis[21]. According to the literature, lithium ions may cause autoimmune reactions that lead to the production of autoantibodies and contribute to hyperthyroidism [22]. Scanelli et al[23] proposed a hypothesis that lithium benefits the "escape" mechanism following the inhibition of hormone release, which may explain why hyperthyroidism occurs. Another possible explanation is that lithium has a direct toxic effect on the thyroid gland. This effect is similar to the non-inflammatory ultrastructural lysosomal and mitochondrial damage that is observed with amiodarone treatment[24]. Bandyopadhyay et al[6] suggest that the thyroid gland may be damaged by direct toxicity, which causes the release of thyroglobulin and thyroid hormones. In this patient, elevated TGAb levels indicate a potential autoimmune reaction that affected the thyroid gland, leading to subclinical hyperthyroidism.
The patient reported regular and consistent use of lithium carbonate for the previous 5 years. Noticeable thyroid enlargement occurred approximately 2 years after starting the medication. Unfortunately, this issue was not given the attention it deserved at the time. This was compounded by poor local healthcare conditions and a lack of awareness among primary care physicians regarding the side effects of lithium carbonate. As a result, the connection between lithium carbonate and thyroid enlargement was not immediately identified, which led to the eventual growth of a thyroid gland that was significantly enlarged and posed a danger to the patient's life. The published literature shows evidence of a clear pathological mechanism linking long-term lithium carbonate use to thyroid enlargement and thyroiditis. Therefore, we believe that the most likely cause of the patient’s thyroid enlargement is the lithium carbonate treatment.
Before starting lithium carbonate treatment, the patient did not show clinical manifestations of either hypothyroidism or hyperthyroidism, nor did she show signs of immune-mediated nephritis. Consequently, thyroid autoantibodies related to thyroid autoimmune inflammation were not assessed before initiating lithium carbonate therapy. Based on current evidence, after discontinuing lithium carbonate the patient's thyroid function returned to normal during follow-up. Additionally, postoperative pathological examination revealed benign lesions (nodular goiter and follicular adenoma), effectively eliminating the possibility of thyroid cancer. Considering the evidence, it appears that lithium carbonate-induced thyroid dysfunction was the most important factor.
Therefore, the long-term use of lithium carbonate requires regular monitoring of thyroid hormone and antibody levels and frequent thyroid ultrasound testing. If abnormalities arise, it is essential to promptly discontinue lithium carbonate and replace it with alternative mood stabilizers. It is recommended that patients receiving lithium therapy undergo baseline and annual thyroid function testing and clinical thyroid assessments. This is especially important for middle-aged women (≥ 50 years of age) and those with positive thyroid autoantibodies or a family history of thyroid disease[1].
The patient had a history of schizophrenia for 5 years and exhibited unusual behavior and emotional instability. Because of the potential for noncooperation, surgery was performed while the patient continued to receive psychiatric treatment. After the operation, given the patient's apparent emotional stability, we discontinued lithium carbonate and initiated a gradual transition from quetiapine to paliperidone 6 mg/d for antipsychotic treatment. Quetiapine has been associated with the development of hypothyroidism in patients with schizophrenia, so it was replaced with paliperidone after extubation[25]. Paliperidone, known for its minimal impact on thyroid function, has been shown to be effective and well tolerated for the treatment of schizophrenia[26]. Postoperatively, the patient exhibited controlled mental symptoms, without hallucinations, delusions, or apparent mood disturbances.
The patient had pre-existing tracheomalacia and airway stenosis, with the narrowest airway part measuring only 3.8 mm. Imaging revealed goiter compression of the trachea and recurrent laryngeal nerve, resulting in hoarseness, dyspnea, and decreased blood oxygen saturation to 92% in the absence of supplemental oxygen, warranting prompt surgery[27]. The risk associated with surgical anesthesia for this patient lies in the potential loss of spontaneous breathing postinduction with general anesthesia. Given the softened trachea, relying on respiratory airway pressure to open stenosis becomes ineffective, posing a risk of suffocation. Therefore, the choice of a sedative that does not inhibit breathing was crucial for a safely inducing anesthesia. Dexmedetomidine, through the activation of presynaptic and postsynaptic α2 receptors in the locus coeruleus center, induces a hypnotic effect, creating an unconscious state akin to natural sleep[28]. Notably, this unique property allowed the patient to remain in a state that was easily guided and cooperative. Airway protection, including measures such as intubation and tracheal sling, is imperative before surgery. In this patient, we opted for awake tracheal intubation guided by fiberoptic bronchoscopy. Dexmedetomidine was used for sedation to prevent respiratory depression, ensuring a safe process. Tracheal intubation was successfully achieved by navigating through the narrowest part of the airway.
The patient presented with a giant goiter and subclinical hyperthyroidism. The intraoperative frozen pathology results revealed a follicular adenoma in the left lobe of the thyroid and a nodular goiter with adenoma-like changes in the right lobe. Both were confirmed to be benign lesions. Consequently, the surgical approach involved bilateral subtotal thyroidectomy along with recurrent laryngeal nerve exploration. Subsequent bilateral subtotal thyroidectomy was performed, sparing approximately 2 g of the gland on both sides to minimize the risk of recurrent laryngeal nerve injury and hy
Typically, thyroidectomy surgery does not require perioperative blood management[27]. Given the patient's rare blood type (O RH−), Lugol's iodine was administered preoperatively to reduce the risk of bleeding. The probability of a giant goiter causing intraoperative bleeding is greater than that of smaller goiters[32]. Preoperative administration of Lugol's iodine solution has been shown to decrease blood flow velocity and reduce intraoperative blood loss during thyroidectomy[33]. The reduction in bleeding not only enhances visualization but also facilitates the preservation of surrounding nerves, blood vessels, and parathyroid glands[33]. Intraoperative bleeding was managed meticulously, and the patient, diagnosed postoperatively with tracheomalacia, was closely monitored in the intensive care unit (ICU). Extending the time for tracheal extubation proved successful, with subsequent CT imaging confirming airway expansion. As a result, intraoperative bleeding was limited to 50 mL, with hemoglobin levels remaining above 70 g/L, the hematocrit exceeding 30%, normal cardiac function, and no requirement for blood transfusion[34]. The patient experienced improved breathing and voice clarity after surgery.
Following surgery, the patient was identified as having tracheomalacia. To prevent airway collapse, she was transferred to the ICU for monitoring and treatment. For patients with mild symptoms, continuous positive airway pressure is an option but severe tracheomalacia may require stent implantation[35]. Extended endotracheal intubation has been considered a viable treatment for tracheomalacia after retrosternal goiter surgery[36]. In this case, we prolonged the time for tracheal extubation, and a neck CT scan on the 4th d after surgery revealed airway expansion. The patient was able to breathe spontaneously and had satisfactory swallowing function, meeting the criteria for extubation. Conse
In patients with schizophrenia receiving long-term lithium carbonate treatment, giant goiters and subclinical hy
We would like to express our sincere gratitude to all those who contributed to the completion of this case report. Firstly, we extend our appreciation to the patient, whose cooperation and consent were indispensable for the documentation of this case. We also thank the medical and support staff at the Affiliated Brain Hospital of Guangzhou Medical University for their assistance throughout the diagnostic and treatment process. Their oversight ensured that all procedures were conducted in compliance with established guidelines and regulations.
| 1. | Kibirige D, Luzinda K, Ssekitoleko R. Spectrum of lithium induced thyroid abnormalities: a current perspective. Thyroid Res. 2013;6:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Perrild H, Hegedüs L, Baastrup PC, Kayser L, Kastberg S. Thyroid function and ultrasonically determined thyroid size in patients receiving long-term lithium treatment. Am J Psychiatry. 1990;147:1518-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Bocchetta A, Bernardi F, Pedditzi M, Loviselli A, Velluzzi F, Martino E, Del Zompo M. Thyroid abnormalities during lithium treatment. Acta Psychiatr Scand. 1991;83:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Lazarus JH. Lithium and thyroid. Best Pract Res Clin Endocrinol Metab. 2009;23:723-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Lazarus JH, Richards AR, Addison GM, Owen GM. Treatment of thyrotoxicosis with lithium carbonate. Lancet. 1974;2:1160-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 56] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Bandyopadhyay D, Nielsen C. Lithium-induced hyperthyroidism, thyrotoxicosis and mania: a case report. QJM. 2012;105:83-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Schou M, Amdisen A, Eskjaer Jensen S, Olsen T. Occurrence of goitre during lithium treatment. Br Med J. 1968;3:710-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Kirov G, Tredget J, John R, Owen MJ, Lazarus JH. A cross-sectional and a prospective study of thyroid disorders in lithium-treated patients. J Affect Disord. 2005;87:313-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Bocchetta A, Mossa P, Velluzzi F, Mariotti S, Zompo MD, Loviselli A. Ten-year follow-up of thyroid function in lithium patients. J Clin Psychopharmacol. 2001;21:594-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Johnston AM, Eagles JM. Lithium-associated clinical hypothyroidism. Prevalence and risk factors. Br J Psychiatry. 1999;175:336-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 76] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Kirov G. Thyroid disorders in lithium-treated patients. J Affect Disord. 1998;50:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Lazarus JH, John R, Bennie EH, Chalmers RJ, Crockett G. Lithium therapy and thyroid function: a long-term study. Psychol Med. 1981;11:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 60] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Lazarus JH, McGregor AM, Ludgate M, Darke C, Creagh FM, Kingswood CJ. Effect of lithium carbonate therapy on thyroid immune status in manic depressive patients: a prospective study. J Affect Disord. 1986;11:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 39] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Sharma PP. Use of Lithium in Hyperthyroidism Secondary to Graves' Disease: A Case Report. Am J Case Rep. 2022;23:e935789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 15. | Bocchetta A, Loviselli A. Lithium treatment and thyroid abnormalities. Clin Pract Epidemiol Ment Health. 2006;2:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Siyam FF, Deshmukh S, Garcia-Touza M. Lithium-associated hyperthyroidism. Hosp Pract (1995). 2013;41:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | López-Jaramillo C, Lopera-Vásquez J, Ospina-Duque J, García J, Gallo A, Cortez V, Palacio C, Torrent C, Martínez-Arán A, Vieta E. Lithium treatment effects on the neuropsychological functioning of patients with bipolar I disorder. J Clin Psychiatry. 2010;71:1055-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Barbesino G. Drugs affecting thyroid function. Thyroid. 2010;20:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Bauer M, Blumentritt H, Finke R, Schlattmann P, Adli M, Baethge C, Bschor T, Müller-Oerlinghausen B, Berghöfer A. Using ultrasonography to determine thyroid size and prevalence of goiter in lithium-treated patients with affective disorders. J Affect Disord. 2007;104:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Rao AS, Kremenevskaja N, Resch J, Brabant G. Lithium stimulates proliferation in cultured thyrocytes by activating Wnt/beta-catenin signalling. Eur J Endocrinol. 2005;153:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Miller KK, Daniels GH. Association between lithium use and thyrotoxicosis caused by silent thyroiditis. Clin Endocrinol (Oxf). 2001;55:501-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Wilson R, McKillop JH, Crocket GT, Pearson C, Jenkins C, Burns F, Burnett AK, Thomson JA. The effect of lithium therapy on parameters thought to be involved in the development of autoimmune thyroid disease. Clin Endocrinol (Oxf). 1991;34:357-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Scanelli G. [Lithium thyrotoxicosis. Report of a case and review of the literature]. Recenti Prog Med. 2002;93:100-103. [PubMed] |
| 24. | Cappiello E, Boldorini R, Tosoni A, Piraneo S, Bernasconi R, Raggi U. Ultrastructural evidence of thyroid damage in amiodarone-induced thyrotoxicosis. J Endocrinol Invest. 1995;18:862-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Zhao Y, Wen SW, Li M, Sun Z, Yuan X, Retnakaran R, Zhang R, Zhai D. Dose-response association of acute-phase quetiapine treatment with risk of new-onset hypothyroidism in schizophrenia patients. Br J Clin Pharmacol. 2021;87:4823-4830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Zhang L, Li J, Zhao Y, Su Y, Si T. Critical evaluation of paliperidone in the treatment of schizophrenia in Chinese patients: a systematic literature review. Neuropsychiatr Dis Treat. 2016;12:113-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Del Rio P, Polistena A, Chiofalo MG, De Pasquale L, Dionigi G, Docimo G, Graceffa G, Iacobone M, Medas F, Pezzolla A, Sorrenti S, Spiezia S, Calò PG. Management of surgical diseases of thyroid gland indications of the United Italian Society of Endocrine Surgery (SIUEC). Updates Surg. 2023;75:1393-1417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Weerink MAS, Struys MMRF, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical Pharmacokinetics and Pharmacodynamics of Dexmedetomidine. Clin Pharmacokinet. 2017;56:893-913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 799] [Article Influence: 99.9] [Reference Citation Analysis (0)] |
| 29. | Hollander JM, Davies TF. Graves’ Disease. Clinical Management of Thyroid Disease. ELSEVIER. 2009;153-189. [DOI] [Full Text] |
| 30. | Shaw GY, Pierce E. Malpractice litigation involving iatrogenic surgical vocal fold paralysis: a closed-claims review with recommendations for prevention and management. Ann Otol Rhinol Laryngol. 2009;118:6-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Jiang Y, Gao B, Zhang X, Zhao J, Chen J, Zhang S, Luo D. Prevention and treatment of recurrent laryngeal nerve injury in thyroid surgery. Int J Clin Exp Med. 2014;7:101-107. [PubMed] |
| 32. | Yamanouchi K, Minami S, Hayashida N, Sakimura C, Kuroki T, Eguchi S. Predictive factors for intraoperative excessive bleeding in Graves' disease. Asian J Surg. 2015;38:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Yilmaz Y, Kamer KE, Ureyen O, Sari E, Acar T, Karahalli O. The effect of preoperative Lugol's iodine on intraoperative bleeding in patients with hyperthyroidism. Ann Med Surg (Lond). 2016;9:53-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Baker L, Park L, Gilbert R, Ahn H, Martel A, Lenet T, Davis A, McIsaac DI, Tinmouth A, Fergusson DA, Martel G. Intraoperative Red Blood Cell Transfusion Decision-making: A Systematic Review of Guidelines. Ann Surg. 2021;274:86-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 35. | Yang D, Cascella M. Tracheomalacia. StatPearls. Treasure Island (FL) ineligible companies: StatPearls Publishing; 2023. |
| 36. | Ren W, Shang X, Fu H, Peng Z. Prolonged endotracheal intubation: a feasible option for tracheomalacia after retrosternal goitre surgery. Ann Palliat Med. 2020;9:1764-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/