Published online Jul 6, 2024. doi: 10.12998/wjcc.v12.i19.4022
Revised: April 30, 2024
Accepted: May 22, 2024
Published online: July 6, 2024
Processing time: 106 Days and 21.5 Hours
Indeterminate dendritic cell tumor (IDCT) is a rare tumor of immune cells, and IDCT patients without skin lesions are rarely reported. Therefore, the clinical course in this type of patient is unclear, and further research on the underlying pathological mechanisms and appropriate treatments is needed.
This study describes a female IDCT patient with bile duct lesions. The strong mimicry of IDCT lesions confused doctors, and consequently, this patient, who had no skin lesions, was first diagnosed with cholangiocarcinoma. Then, she presented with persistent abdominal distension without jaundice. Enlarged mesenteric lymph nodes along with massive ascites were observed in the sub
For IDCT patients without skin lesions, early biopsy is the key to obtaining a correct diagnosis. Moreover, the collective management of IDCT patients is important. Further histological and molecular biology studies based on human specimens are critical for understanding the pathological mechanism of dendritic cell tumors in the future.
Core Tip: The clinical course in indeterminate dendritic cell tumor (IDCT) patients without skin lesions was about 2 years. The strong mimicry of IDCT lesions confused doctors, and consequently, this patient, who had no skin lesions, was first diagnosed with cholangiocarcinoma. No tumor cells or pathogens were found in the three subsequent ascites analyses. The correct diagnosis was eventually obtained by performing surgery for biopsy of the patient’s abdominal lymph nodes. IDCT is fairly rare, and the surgery for biopsy is important for the IDCT. Therefore, the collective management of IDCT patients in a hospital specializing in rare diseases is interesting and cost-effective.
- Citation: Liang H, Zhao YF, Zhang LP, Wu YK. Overall clinical course of indeterminate dendritic cell tumor patients without skin lesions: A rare case report. World J Clin Cases 2024; 12(19): 4022-4028
- URL: https://www.wjgnet.com/2307-8960/full/v12/i19/4022.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i19.4022
Indeterminate dendritic cell tumors (IDCTs) originate from the stromal-derived dendritic cells, a special type of cell involved in the process of antitumor immunity. The incidence of IDCT is very low. Fewer than 1% of neoplasms originating from lymph tissues are diagnosed as IDCT[1]. A recent study[2] showed that only one patient was diagnosed with IDCT (0.4%, 1/274) among the histiocytic and dendritic neoplasm cases recorded from 1995 to 2018. Although the number of IDCT cases is rare, some commonalities and differences have been analyzed in the current literature.
Skin lesions are found in almost all IDCT patients. Most patients are diagnosed with IDCT by biopsy of the skin lesions[3] or mucosa lesions[4]. However, a series of special IDCT cases have been reported recently because of the rarity of patients without skin lesions. A study reported that a patient had spontaneous splenic rupture due to the excess proliferation of indeterminate dendritic tumor cells in the spleen[5]. Indeterminate dendritic tumor cells can arise in the pancreas[6]. When the IDCT invades the abdominal organs, the difficulty of early biopsy increases greatly, and consequently, the diagnosis and treatment of this type of IDCT patient are delayed.
Although crucial for developing optimal treatment plans and predicting patient prognosis, the overall clinical development of IDCT involving the abdominal organs has rarely been reported. Additionally, the diverse types of lesions identified via IDCT may lead to misdiagnosis, especially in the absence of typical skin lesions. However, IDCT, a type of immune cell-related cancer, is worthy of mention[7]. The specific antitumor immune effects of dendritic cells disappear in IDCT.
This study reports the overall clinical course of a patient diagnosed with IDCT although typical skin lesions were absent.
A 59-year-old woman was admitted to the Department of Digestive Diseases because of the main complaint of persistent abdominal distension
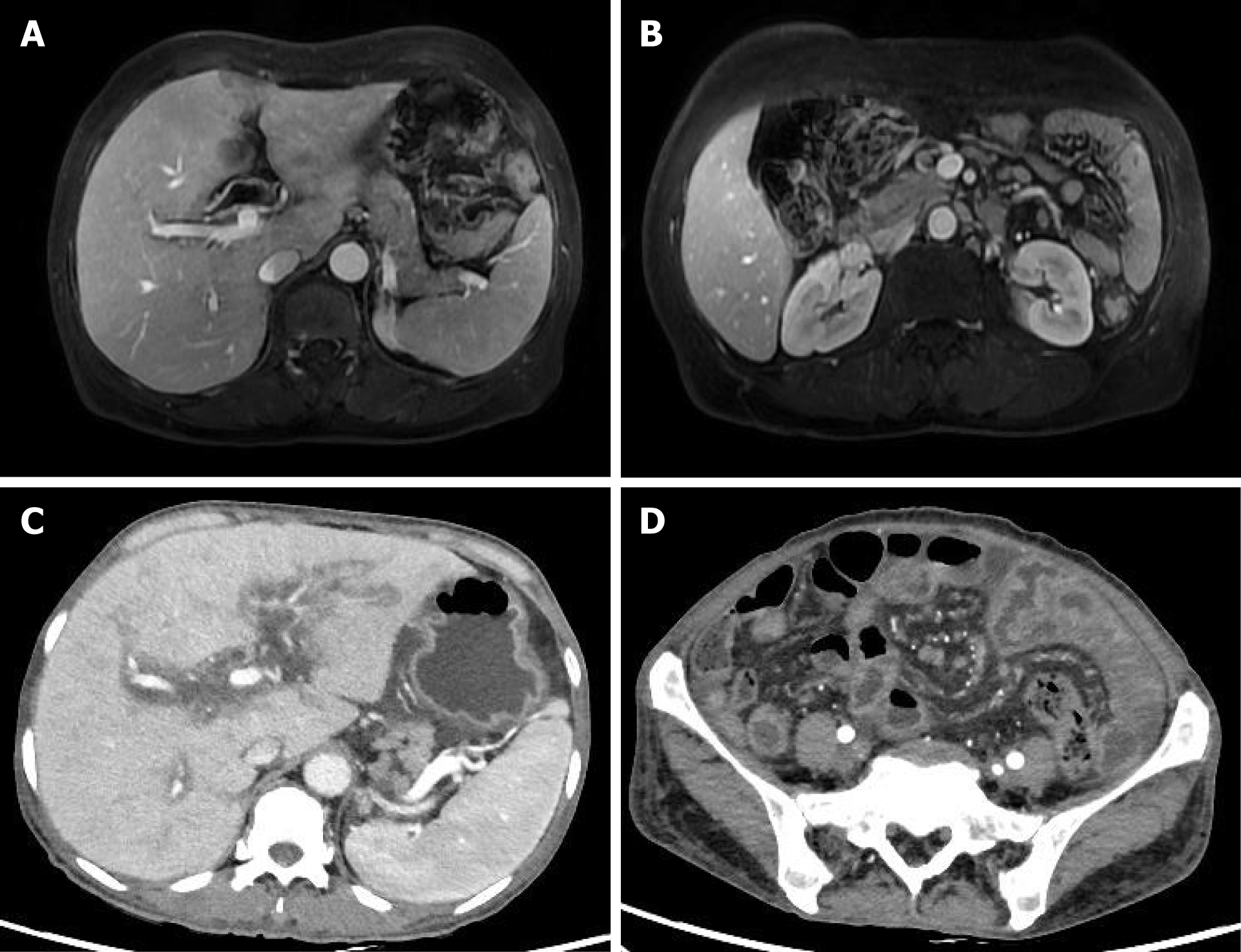
Three years prior, the patient had visited a urologist and was diagnosed with a ureteric calculus. Her liver, bile duct and mesenteric lymph nodes were in good condition at the time of the imaging examination. She was discharged from the hospital after receiving surgical treatments. Two years later, this patient presented with abdominal pain but not jaundice. Based on the lasting contrast-enhanced bile duct lesions on magnetic resonance imaging (MRI) (Figure 1A), cholangiocarcinoma was suspected in this patient. Importantly, enlarged mesenteric lymph nodes were found on MRI (Figure 1B). The patient refused surgical treatment for the bile duct lesions and asked to leave the hospital soon thereafter.
Six months later, this patient visited the hospital again because of mild abdominal distension. The tumor-like lesions located in the bile duct and liver were still present, but the mesenteric lymph nodes were enlarged on computed tomography (CT) (Figure 1C and D). After the patient refused to receive chemotherapy, she asked to leave the hospital after the alleviation of her abdominal distension. In the last three years, no rash on the skin or mucosa was observed in this patient.
The patient had no significant medical history and no tobacco or alcohol use.
There was no history of hepatitis, other infectious diseases or recent trauma and no family history of rare diseases.
The patient’s body temperature was 36.9 °C, and her respiratory rate was 17 breaths/min. Her pulse rate was 83 beats/min, and her blood pressure was 101/69 mmHg. She was cooperative with the examination, reporting mild tenderness upon palpation of her upper abdomen. No enlarged lymph nodes were palpated, and no skin lesions were observed. The body mass index of this patient was 19.1 cm (150 cm in height and 43 kg in weight).
The results of several abnormal blood examinations were recorded. The carbohydrate antigen 125 (CA-125) level was as high as 223.71 units/mL (reference range, 0–35). The level of gamma-glutamyl transferase (γ-GGT) was increased to 337 units per liter (reference range, 7–45), and the concentration of albumin was very low, at 22 g per liter (reference range, 40–55). Moreover, the patient’s hemoglobin level was decreased, at 90 g per liter (reference range, 110-150). No tumor cells were found in the three histological analyses of ascites. Both microbial examination for Mycobacterium tuberculosis and gene sequencing for M. tuberculosis DNA in the ascites were negative. Furthermore, the result of the bone marrow test for tumor cells was negative.
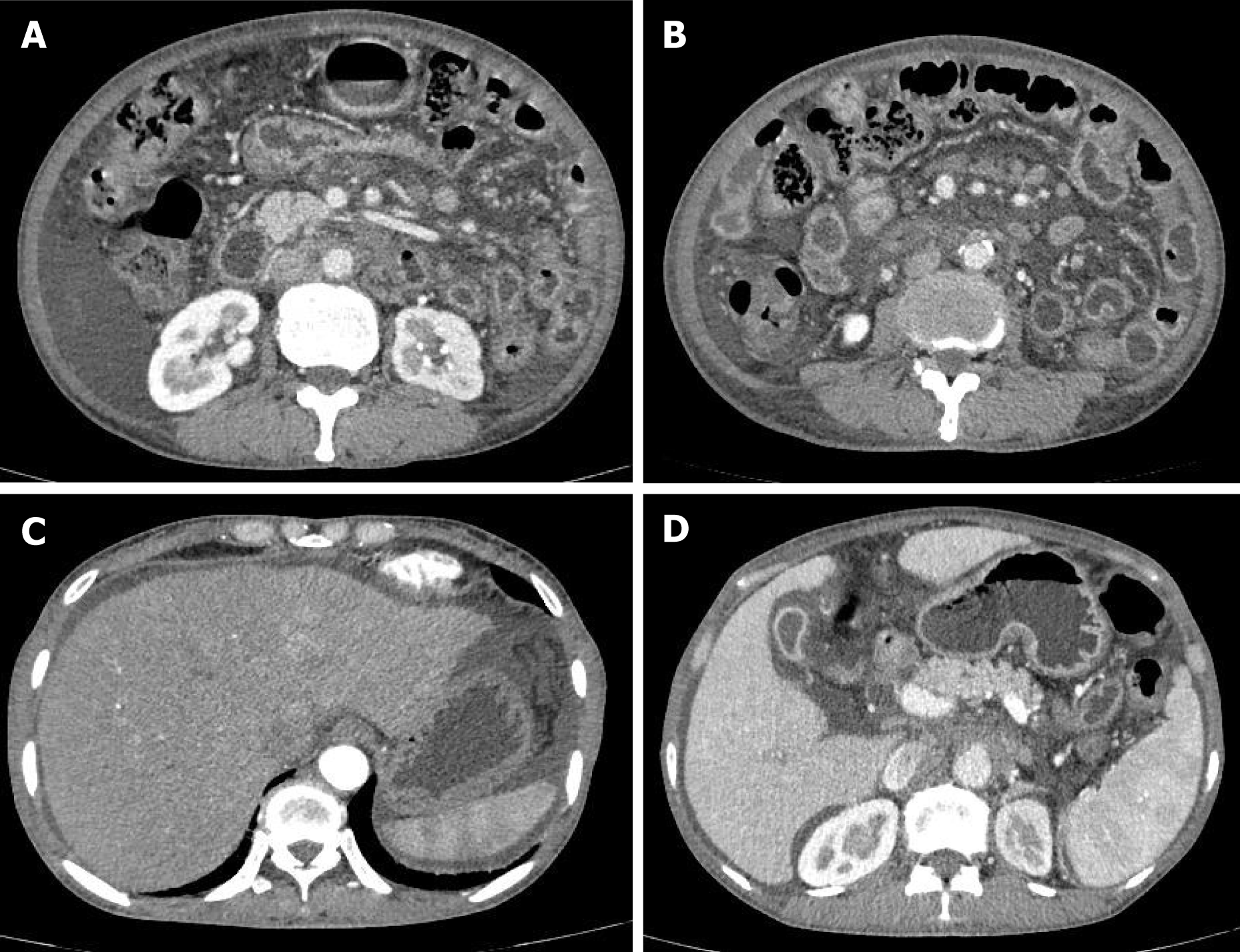
CT imaging revealed general dilatation of the intrahepatic bile ducts as well as many enlarged lymph nodes in the small bowel mesentery. The size of the largest lymph nodes was approximately 2 cm (Figure 2A and B). Compared to the previous imaging data, a fairly swollen omentum and mesentery, along with massive ascites, were observed in this CT examination (Figure 2C and D).
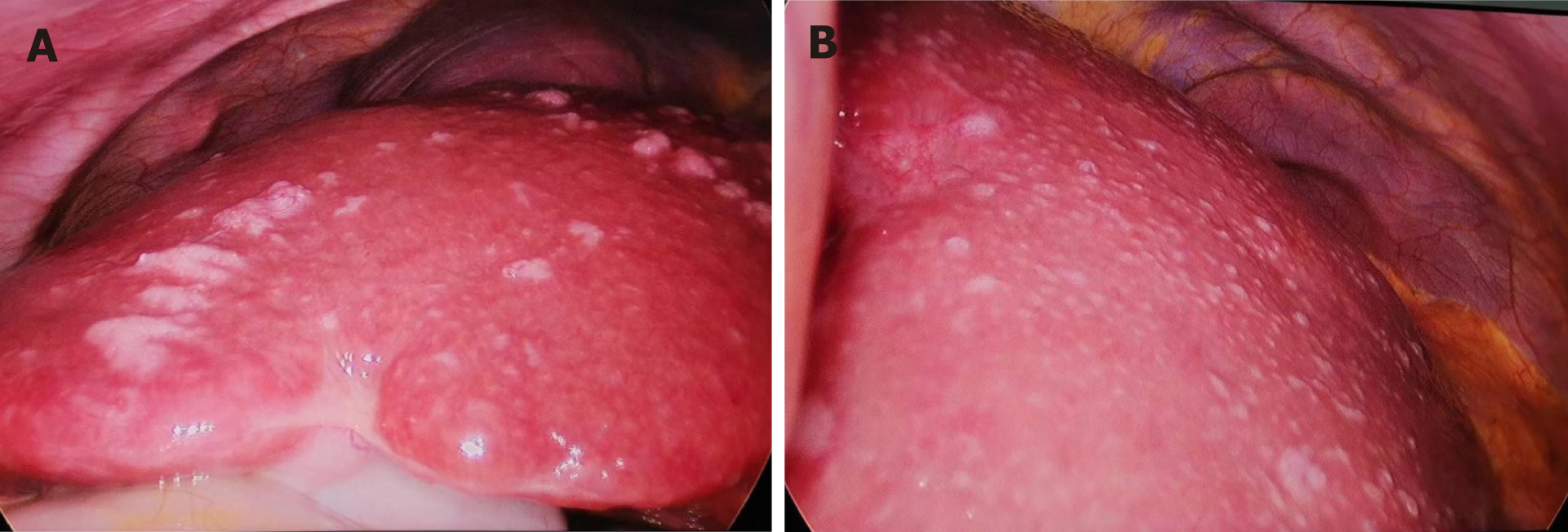
Consequently, cholangiocarcinoma or a rare malignant disease was first suspected. The worsened abdominal distension and massive ascites made the patient feel concerned. Finally, she underwent surgical treatment for biopsy of the abnormal liver lesions (Figure 3A and B) and enlarged lymph nodes (Figure 4A). During surgery, general foamy-like lesions were observed throughout the whole liver, and the ascites was faint yellow in color. The duration of this laparoscopic operation was 50 minutes. After completing this surgical treatment, the patient was discharged.
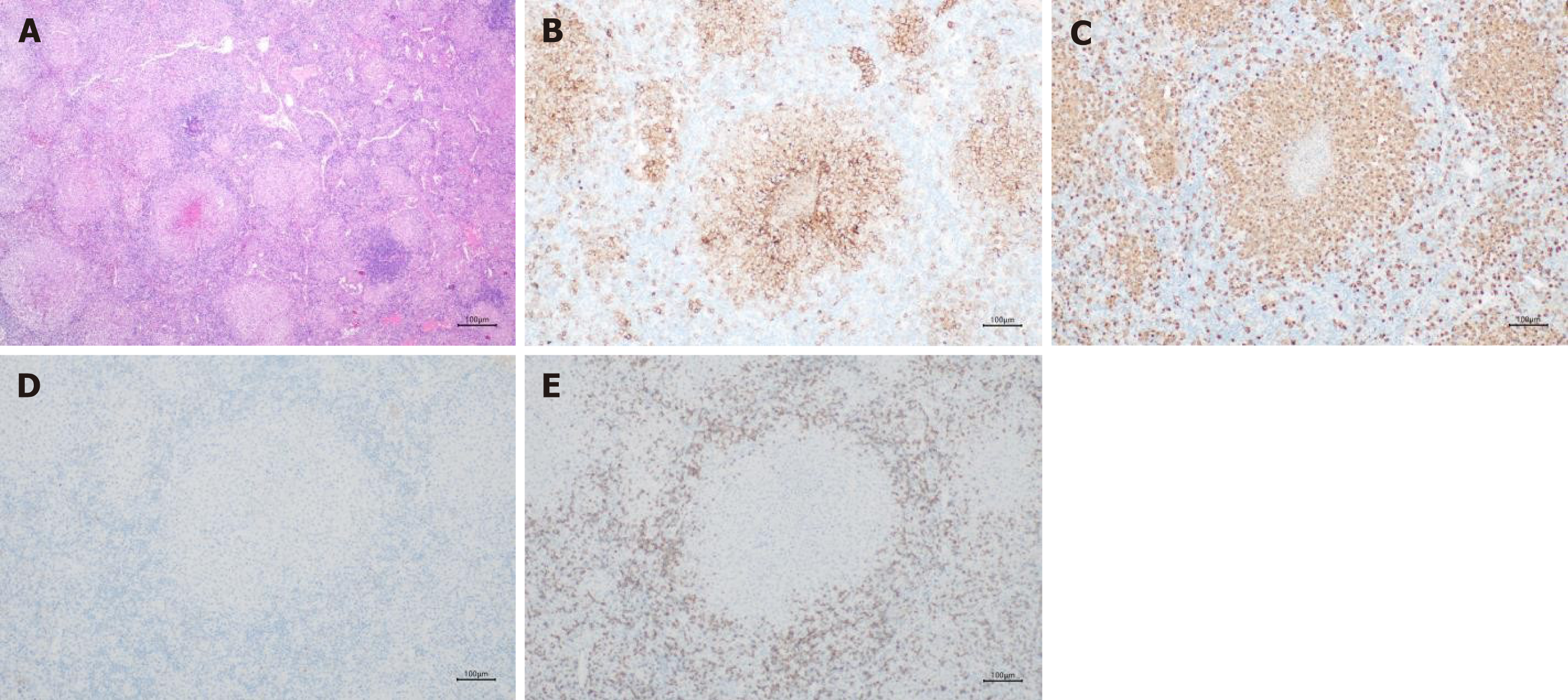
Histologic examinations revealed no tumor cells in the hepatic lesions. Some dendritic cells were found in the mesenteric lymph nodes. Immunohistochemical analysis revealed positive expression of CD1a and S100 (Figure 4B and C) in these dendritic cells. Moreover, there was no expression of Langerin or CD3 markers (Figure 4D and E). Finally, an indeterminate dendritic cell tumor was diagnosed in this patient.
The patient exhibited cachexia after receiving a cycle of cyclophosphamide therapy.
At the last follow-up, she was then referred to a large medical center specializing in the management of rare diseases.
A rare case involving a female patient with IDCT who lacked skin lesions is reported. Importantly, the overall clinical development of this type of IDCT was recorded. The patient presented some lesions resembling cholangiocarcinoma, but the biopsy results confirmed the correct diagnosis-IDCT. Finally, this patient was sent to a medical center specializing in rare diseases.
A recent review reported that most patients with IDCT exhibit various papules or nodules in the skin[8]. IDCT can occur directly in other organs, including the spleen[5], pancreas[6], thoracic spine[9], muscle[10] and bile duct. IDCT can imitate other diseases, and therefore, the diagnosis of IDCT patients with abdominal lesions may be delayed. Performing a biopsy on potential IDCT lesions is the optimal means for early diagnosis. It took 2 years to reach the correct diagnosis by performing surgery for biopsy of the abdominal lymph nodes in this patient. The strong mimicry of IDCT was the key factor leading to the initial misdiagnosis of cholangiocarcinoma.
The mimicry of IDCT should be noted. In this patient, the IDCT lesions resembling hepatocarcinoma and bile duct cancer lesions had perplexed the physicians. Additionally, the negative results of examinations for hematological tumors in the bone marrow had puzzled the doctors, not to mention the lack of evidence for Mycobacterium tuberculosis. The increasing levels of CA-125 and γ-GGT suggested that the tumor originated from biliary system tissue. Importantly, histologic examinations of the patient’s ascites failed to provide useful evidence for the diagnosis of IDCT. Therefore, surgery was conducted for biopsy of the abdominal lymph nodes, and the patient was ultimately diagnosed with IDCT. Surprisingly, it took nearly two years until the biopsy was performed to make the correct diagnosis. Timely surgery for biopsy may lead to an earlier diagnosis of IDCT, as has been reported for the rapid diagnosis of IDCT lesions in the pancreas[6].
The clinical traits of IDCT are unclear. According to the results of previous studies[11], patients of any age, ranging from 12 to 90 years, can present with IDCT. Additionally, differences in sex distributions have rarely been reported. Therefore, early biopsy is even more important for early diagnosis. Early diagnosis is beneficial for timely treatment, although there is no standard treatment protocol for IDCT. Cases of IDCT patients without skin lesions are very rare, so treatment regimen data are lacking. Potential chemotherapeutic drugs include cyclophosphamide, prednisone, vincristine and methotrexate[8]. Some patients experience remission and relapse of lesions, although receiving chemotherapy[12]. However, the optimal treatment plan is still unknown. The differences in prognosis between IDCT patients with skin lesions and those without skin lesions are unclear. Our study revealed that the overall duration of the clinical course of this type of IDCT is approximately 3 years.
Therefore, conducting more collective and in-depth studies is critical for clarifying the pathological mechanism of IDCT. First, patients with IDCT should be sent to large specialized medical centers, such as the Peking Union Medical College Hospital[13]. One reason is that it will be more convenient to conduct related clinical trials. For IDCT, blood or tissue specimen information should be recorded and managed in detail in cohort studies. Therefore, full tissue biopsy is the first step. Some studies have reported that BRAF gene mutation analysis in indeterminate dendritic tumors might provide guidelines for targeted therapeutic approaches[14]. Alterations in ETV3-NCOA2 translocation could also be detected in IDCT patients[11]. Gene sequencing is a good tool for conducting further research on IDCT. However, a review showed that only six of all reported cases were tested for BRAF V600E gene mutations[11]. Therefore, collective management of IDCT patients may facilitate further studies.
In addition, the special biological effects of dendritic cells have received increasing attention, especially their role in transmitting information about tumor antigens to lymphocytes[7]. Usually, gene analysis for this tumor is the first step. Further histological and molecular biology studies of tumor cells from IDCT patients may reveal more interesting and key changes for tumor treatment. Additionally, sensitivity tests of some drugs for IDCT can be conducted based on the tumor cells[15]. Unfortunately, the ability to conduct related experiments is limited at our medical center, but we want to participate in more in-depth research on IDCT. Therefore, we have referred this patient to a large specialized medical center for better management.
It is very rare for patients without skin lesions to be diagnosed with IDCT. The strong mimicry of IDCT led to our patient being first suspected to have cholangiocarcinoma. Timely surgical biopsy can prevent misdiagnosis. The overall clinical course of patients with this type of IDCT is short, at only a few years. Therefore, cohort studies of this disease conducted at specialized medical centers might be interesting and cost-effective.
| 1. | Dalia S, Shao H, Sagatys E, Cualing H, Sokol L. Dendritic cell and histiocytic neoplasms: biology, diagnosis, and treatment. Cancer Control. 2014;21:290-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Kim HM, Yang WI, Lyu CJ, Hahn SM, Yoon SO. Descriptive Analysis of Histiocytic and Dendritic Cell Neoplasms: A Single-Institution Experience. Yonsei Med J. 2020;61:774-779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 3. | Li Y, Wang TT, Zhang ZH, Wang L. Aggressive Indeterminate Dendritic Cell Tumor Mimicking Scalp Angiosarcoma. Ann Dermatol. 2017;29:614-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Ferran M, Toll A, Gilaberte M, Barranco C, Lloreta J, Pujol RM. Acquired mucosal indeterminate cell histiocytoma. Pediatr Dermatol. 2007;24:253-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Iqbal SB, Chittal AR, Rao SJ, Lakra P, Bhansali D, Pyrgos G. Spontaneous splenic rupture from indeterminate dendritic cell proliferation: a case report. J Surg Case Rep. 2022;2022:rjac211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Sortino R, Schmid M, El Baz Y, Loosen A, Tarantino I, Steffen T, Schmied BM, Abbassi F. Indeterminate dendritic cell tumor in the pancreas. J Surg Case Rep. 2020;2020:rjaa208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 1593] [Article Influence: 113.8] [Reference Citation Analysis (0)] |
| 8. | Li Y, Zhang C, Xiong J. Recurrent Chemotherapy Treated Indeterminate Dendritic Cell Tumor: Case Report and Literature Review. Clin Cosmet Investig Dermatol. 2023;16:2985-2993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 9. | Tan SK, Chieng LO, Madhavan K, Rosenberg A, Cote I. Indeterminate Dendritic Cell Tumor in Thoracic Spine: A Case Report. World Neurosurg. 2017;108:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Kiyohara T, Nakamaru S, Miyamoto M, Shijimaya T, Nagano N, Makimura K, Takama H, Akiyama M, Tanimura H. Indeterminate dendritic cell neoplasm accompanied by eosinophilic pneumonia successfully treated by systemic steroid therapy: Report of the first case with muscular and parotid involvement and review of published work. J Dermatol. 2018;45:1444-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Davick JJ, Kim J, Wick MR, Gru AA. Indeterminate Dendritic Cell Tumor: A Report of Two New Cases Lacking the ETV3-NCOA2 Translocation and a Literature Review. Am J Dermatopathol. 2018;40:736-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Tóth B, Katona M, Hársing J, Szepesi A, Kárpáti S. Indeterminate cell histiocytosis in a pediatric patient: successful treatment with thalidomide. Pathol Oncol Res. 2012;18:535-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Zhou N, Ge Y, Fang K, Liu J, Yu S, Zhong D, Wang Y, Bai C. BRAF wild-type recurrent indeterminate dendritic cell tumour presenting with leonine facies. J Eur Acad Dermatol Venereol. 2020;34:e230-e231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Thurner L, Bewarder M, Rosar F, Orth P, Meuter RB, Rixecker T, Lesan V, Kohn DM, Schneider G, Baumhoer D, Bohle RM, Veith C, Bittenbring JT. Indeterminate Dendritic Cell Tumor With Persistent Complete Metabolic Response to BRAF/MEK Inhibition. Hemasphere. 2021;5:e511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 15. | Yoshida GJ. Applications of patient-derived tumor xenograft models and tumor organoids. J Hematol Oncol. 2020;13:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 343] [Article Influence: 57.2] [Reference Citation Analysis (0)] |