Published online Jul 6, 2024. doi: 10.12998/wjcc.v12.i19.4010
Revised: April 25, 2024
Accepted: May 10, 2024
Published online: July 6, 2024
Processing time: 113 Days and 1.6 Hours
Renal anastomosing hemangioma (AH) is a rare benign vascular tumor characterized by unique histopathological features.
We report a highly unusual case of renal AH. A male patient had undergone partial nephrectomy for clear cell carcinoma of the kidney four years prior. A follow-up computed tomography scan in the third postoperative year revealed a new mass near the surgical site on the same side of the kidney, raising suspicions of tumor recurrence. However, the characteristics on contrast-enhanced magnetic resonance imaging and ultrasonography were more consistent with those of a benign lesion. The patient strongly insisted on undergoing surgery due to concerns about the possibility of renal cancer recurrence. Postoperative pathology confirmed the diagnosis of renal AH.
This case report presents the imaging features of a patient with rare renal AH and a history of renal clear cell carcinoma, providing broader insights into the differential diagnosis of new lesions after surgery for renal cell carcinoma.
Core Tip: Renal anastomosing hemangioma (AH) is a rare benign vascular tumor characterized by unique histopathological features. We reported a highly unusual case of renal AH, where the AH occurred secondarily in the remaining kidney tissue following a partial nephrectomy for renal clear cell carcinoma. This case report describes the imaging characteristics of a patient with rare renal AH and a history of renal clear cell carcinoma, including ultrasound, computed tomography, and magnetic resonance imaging. We hope that our case contributes to a broader understanding of the differential diagnosis of new lesions after nephrectomy for renal cell carcinoma.
- Citation: Chen J, Cai DM. Renal anastomosing hemangioma following partial nephrectomy for renal cell carcinoma: A case report. World J Clin Cases 2024; 12(19): 4010-4015
- URL: https://www.wjgnet.com/2307-8960/full/v12/i19/4010.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i19.4010
Anastomosing hemangioma (AH) is an exceedingly rare benign vascular tumor that was first described in 2009 and officially recognized by the World Health Organization (WHO) in 2020[1,2]. Due to the rarity of AH, comprehensive imaging data are limited, leading to insufficient awareness of its imaging characteristics and making misdiagnosis as a malignant lesion more likely[3]. We present a unique case of renal AH following partial nephrectomy for renal cell car
A 59-year-old male was admitted to our hospital, and a left renal mass was discovered.
The patient was generally well and did not mention experiencing any discomfort.
The patient underwent left partial nephrectomy four years prior for a renal mass, with pathology revealing clear cell carcinoma with an International Association of Urological Pathology/WHO grade of 2. The patient recovered well postoperatively and underwent regular follow-up examinations. The two-years postoperative follow-up showed that there was no tumor recurrence.
Patient and family histories were negative.
The patient did not complain of any hematuria, lumbago or abdominal pain, or lower urinary tract symptoms.
The levels of creatinine (142 mmol/L; reference range: 57-97 mmol/L) and serum cystatin-C (1.14 mg/L; reference range: 0.51-1.09 mg/L) were mildly elevated. Furthermore, other blood test results were unremarkable.
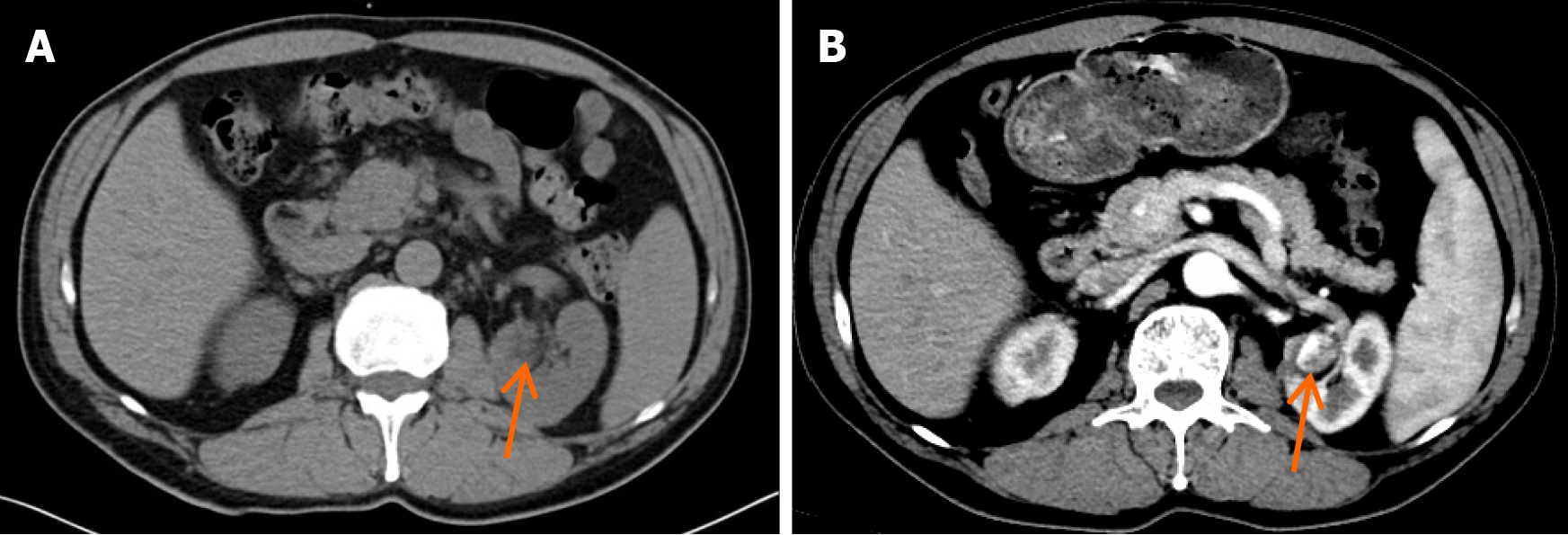
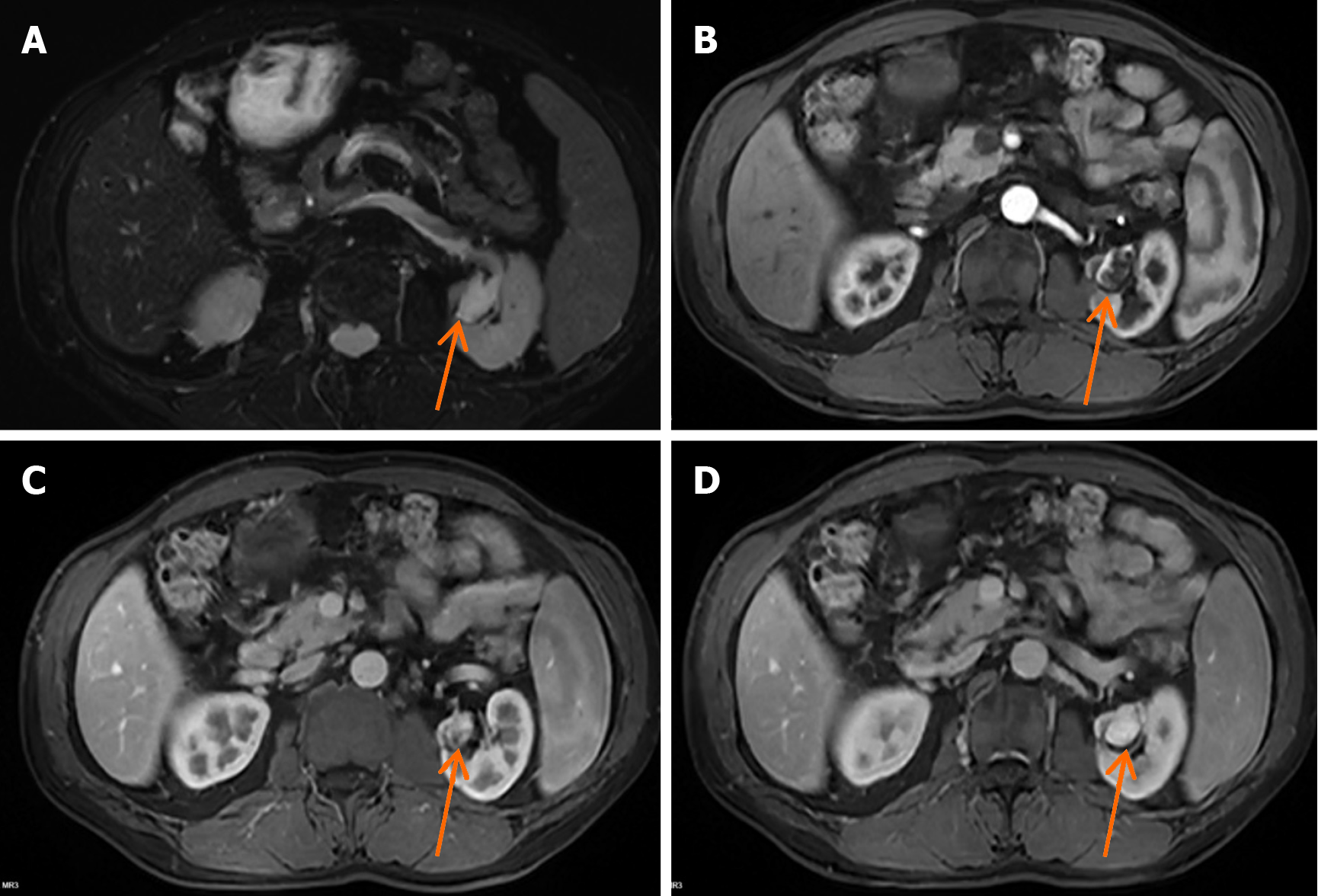
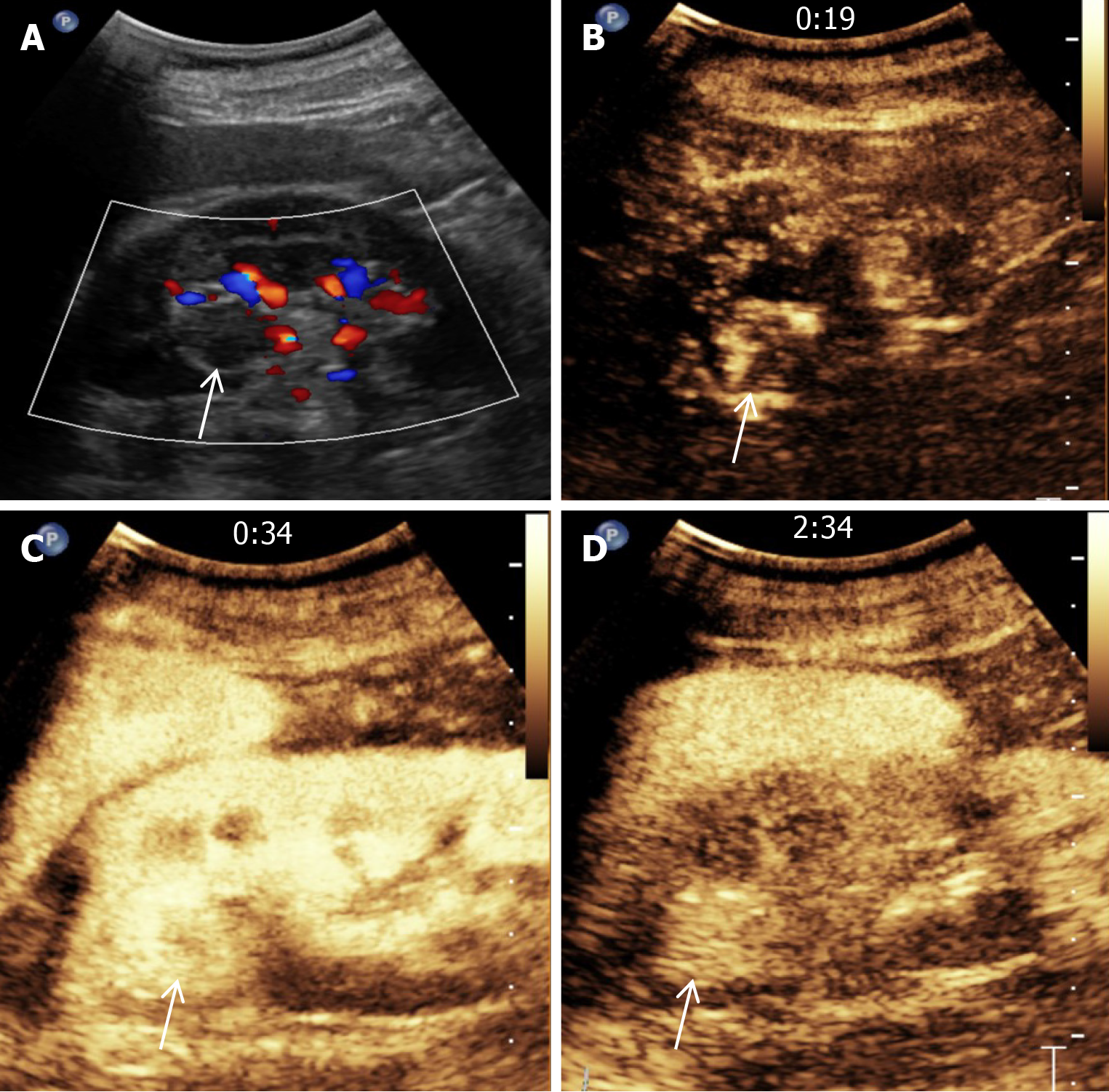
The patient had undergone regular follow-up examinations after left partial nephrectomy. The follow-up two years after surgery showed that the tumor had not recurred. However, the three-year follow-up conventional computed tomography (CT) (Figure 1A) and contrast-enhanced CT (Figure 1B) revealed a new nodule measuring approximately 22 mm × 17 mm, and exhibiting irregular enhancement that was located near the surgical site on the left kidney, raising suspicion of cancer recurrence. A renal lesion in the left kidney presented with high signal intensity on T2-weighted imaging (Figure 2A). Contrast-enhanced magnetic resonance imaging (MRI) (Figure 2B-D) revealed irregular enhancement during the arterial phase and uniform enhancement during the venous phase and delayed phase. Conventional ultrasound demonstrated a well-defined hypoechoic nodule with clear borders, and color doppler imaging (Figure 3A) indicated a lack of blood flow signals within the lesion. Moreover, contrast-enhanced ultrasound (CEUS) was performed. The enhancement pattern (Figure 3B-D) of the lesion was centripetal, extending from the periphery to the center, ultimately manifesting as sustained high enhancement. There was no atypical enhancement delineation or abnormal washout throughout the entire CEUS cycle. Both contrast-enhanced MRI (CE-MRI) and CEUS findings suggested a greater likelihood of a benign lesion.
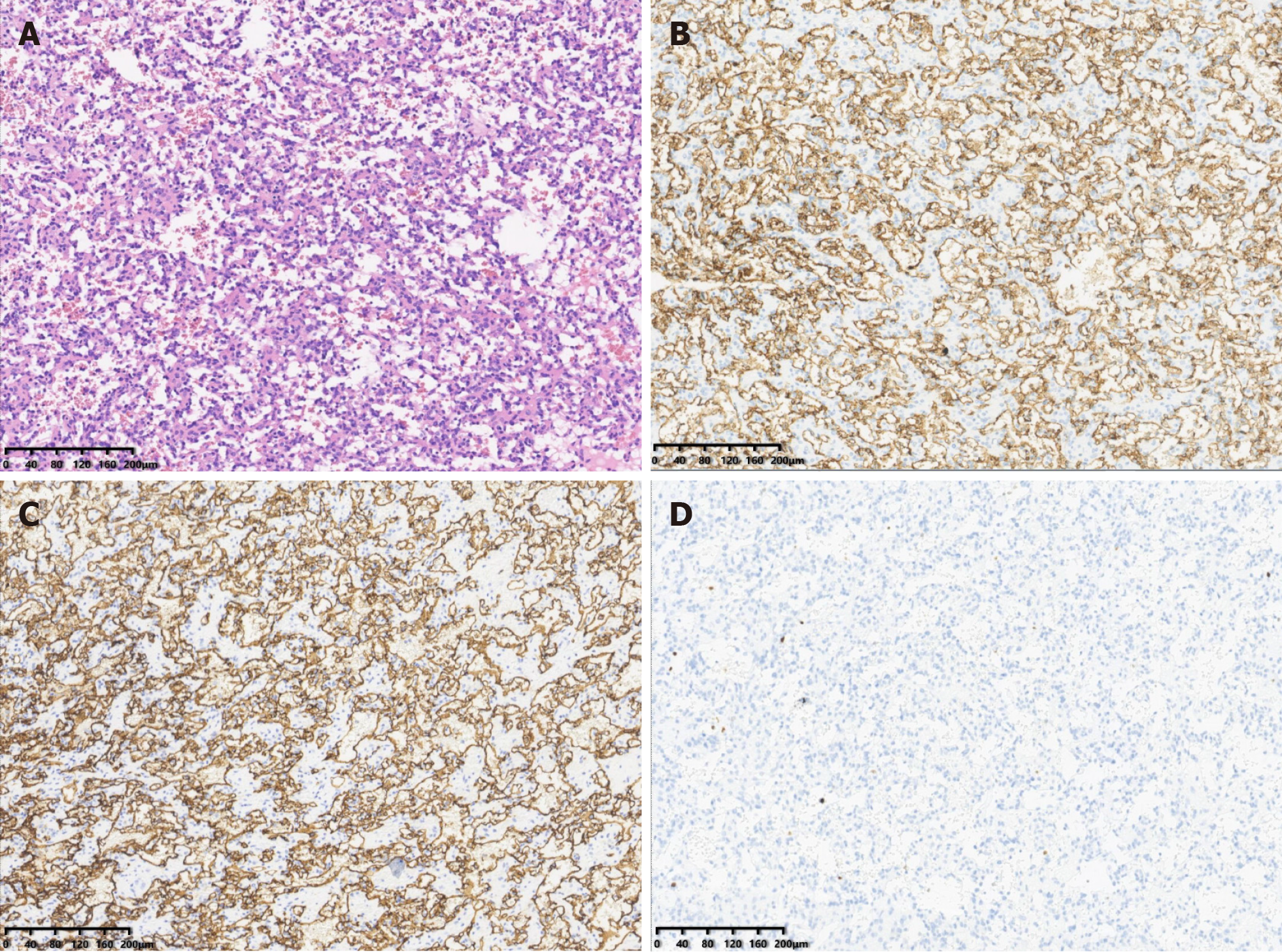
The tumor was ultimately surgically excised. Histologic examination (Figure 4A) of the tumor revealed that it was composed of anastomosing capillary-sized vessels. On immunohistochemistry, the tumor had strong CD31 (Figure 4B), CD34 (Figure 4C), and estrogen receptor expression. It was negative for smooth muscle actin, desmin, and P53. The Ki-67 (Figure 4D) positivity rate ranged between 2% and 5%.
Although the results of CEUS and CE-MRI suggested a tendency for a benign lesion in the left kidney, due to concerns about renal cancer recurrence, the patient ultimately insisted on undergoing left nephrectomy.
No postoperative complications were reported after the surgery. The patient is currently well and is undergoing semi
AH is an extremely rare vascular tumor that most commonly occurs in the genitourinary tract, particularly in the kidneys[4-6]. AH has also been reported to occur in the adrenal gland, liver, and gastrointestinal tract[1,7]. Our case is unique in that AH developed on the same side as prior renal cell carcinoma, leading to strong suspicion of renal cancer recurrence at the initial diagnosis. To our knowledge, this is the first reported case of AH following surgery for renal cell carcinoma. Although the association between the occurrence of AH and surgical history remains unclear, our case offers insights into the differential diagnosis of new lesions following surgery for renal cell carcinoma. Previous studies have reported that almost two-thirds of renal AH cases occur in patients with end-stage renal disease[8]. Although our patient does not have end-stage renal disease, our patient had mild renal damage, which may be due to a previous surgical history of partial renal resection.
In general, renal AH occurs unilaterally, typically at a mean patient age of 51 years (range: 21-83 years), with sizes ranging from 0.6 cm to 5 cm (median 2 cm)[9,10]. Additionally, there is a higher reported incidence of renal AH among male patients[11]. The previous research findings are consistent with our case. Studies indicate that common clinical features of renal AH include hematuria, flank or abdominal pain, or lower urinary tract symptoms[12,13]. However, our patient did not exhibit the aforementioned clinical manifestations. In this patient, renal AH was identified through regular imaging follow-up after partial nephrectomy. As reported, a distinctive sieve-like arrangement resembling the anastomosing pattern of splenic sinusoids is a common and distinctive feature of renal AH[6,14]. This was also a crucial criterion for the diagnosis of renal AH in our patient.
Imaging data for renal AH are limited[12,15]. Previous studies have predominantly reported CT findings. In most cases, AH appears as a well-defined nodular enhancement with a characteristic peripheral and nodular enhancement pattern in the arterial phase, followed by uniform enhancement in the venous phase and persistent homogeneous enhancement in the delayed phase[7,13]. However, our patient's contrast-enhanced CT findings were atypical, showing overall uneven enhancement. Previously, renal AH characteristics on contrast-enhanced MRI were described as T2 high-signal lesions resembling cysts, with the arterial phase showing peripheral, discontinuous, and uneven enhancement and the venous phase and delayed phase exhibiting uniform enhancement[16-18]. Our patient's MRI findings were consistent with those in previous studies. The CEUS characteristics of renal AH have not been reported in the previous literature. In this study, CEUS imaging revealed that the lesion exhibited an initial centripetal strengthening followed by sustained high enhancement, without the typical abnormal washout and ring enhancement observed in renal cell carcinoma. This enhancement pattern is reminiscent of the common characteristics observed in cavernous hemangiomas. Therefore, both MRI and CEUS played crucial roles in the diagnosis of our case. Notably, compared with CE-MRI, the contrast agent used for CEUS is particularly suitable for patients with end-stage renal disease because it is not nephrotoxic[19,20]. Since AH commonly occurs in patients with end-stage renal disease, CEUS has broad potential for the diagnosis of AH.
The prognosis after surgical resection of renal AH is favorable, and conservative treatments such as partial ne
Renal AH occurring following partial nephrectomy for renal cell carcinoma is extremely rare and can be easily misdiagnosed as recurring renal cell carcinoma. Contrast-enhanced MRI and CEUS may play effective roles in distinguishing between the two conditions.
We wish to acknowledge Zhang MN (Department of Pathology, West China Hospital, Sichuan University, Chengdu) for his support in this pathology study.
| 1. | Zhou J, Yang X, Zhou L, Zhao M, Wang C. Anastomosing Hemangioma Incidentally Found in Kidney or Adrenal Gland: Study of 10 Cases and Review of Literature. Urol J. 2020;17:650-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 2. | Shanbhogue K, Khandelwal A, Hajdu C, Cao W, Surabhi VR, Prasad SR. Anastomosing hemangioma: a current update on clinical, pathological and imaging features. Abdom Radiol (NY). 2022;47:2335-2346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 3. | Merritt B, Behr S, Umetsu SE, Roberts J, Kolli KP. Anastomosing hemangioma of liver. J Radiol Case Rep. 2019;13:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Chen B, Guo R, Erickson LA. Anastomosing Hemangioma. Mayo Clin Proc. 2022;97:1756-1757. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Perdiki M, Datseri G, Liapis G, Chondros N, Anastasiou I, Tzardi M, Delladetsima JK, Drakos E. Anastomosing hemangioma: report of two renal cases and analysis of the literature. Diagn Pathol. 2017;12:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Brown JG, Folpe AL, Rao P, Lazar AJ, Paner GP, Gupta R, Parakh R, Cheville JC, Amin MB. Primary vascular tumors and tumor-like lesions of the kidney: a clinicopathologic analysis of 25 cases. Am J Surg Pathol. 2010;34:942-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Tao LL, Dai Y, Yin W, Chen J. A case report of a renal anastomosing hemangioma and a literature review: an unusual variant histologically mimicking angiosarcoma. Diagn Pathol. 2014;9:159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Lappa E, Drakos E. Anastomosing Hemangioma: Short Review of a Benign Mimicker of Angiosarcoma. Arch Pathol Lab Med. 2020;144:240-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Zhao M, Li C, Zheng J, Sun K. Anastomosing hemangioma of the kidney: a case report of a rare subtype of hemangioma mimicking angiosarcoma and review of the literature. Int J Clin Exp Pathol. 2013;6:757-765. [PubMed] |
| 10. | Büttner M, Kufer V, Brunner K, Hartmann A, Amann K, Agaimy A. Benign mesenchymal tumours and tumour-like lesions in end-stage renal disease. Histopathology. 2013;62:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Heidegger I, Pichler R, Schäfer G, Zelger B, Aigner F, Bektic J, Horninger W. Long-term follow up of renal anastomosing hemangioma mimicking renal angiosarcoma. Int J Urol. 2014;21:836-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Montgomery E, Epstein JI. Anastomosing hemangioma of the genitourinary tract: a lesion mimicking angiosarcoma. Am J Surg Pathol. 2009;33:1364-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Kryvenko ON, Gupta NS, Meier FA, Lee MW, Epstein JI. Anastomosing hemangioma of the genitourinary system: eight cases in the kidney and ovary with immunohistochemical and ultrastructural analysis. Am J Clin Pathol. 2011;136:450-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Cheon PM, Rebello R, Naqvi A, Popovic S, Bonert M, Kapoor A. Anastomosing hemangioma of the kidney: radiologic and pathologic distinctions of a kidney cancer mimic. Curr Oncol. 2018;25:e220-e223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Katabathina VS, Vikram R, Nagar AM, Tamboli P, Menias CO, Prasad SR. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics. 2010;30:1525-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Patel SR, Abimbola O, Bhamber T, Weida C, Roy O. Incidental finding of bilateral renal and adrenal anastomosing hemangiomas: A rare case report. Urol Case Rep. 2019;27:100912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Silva MA, Fonseca EKUN, Yamauchi FI, Baroni RH. Anastomosing hemangioma simulating renal cell carcinoma. Int Braz J Urol. 2017;43:987-989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Zheng LP, Shen WA, Wang CH, Hu CD, Chen XJ, Shen YY, Wang J. Anastomosing hemangioma arising from the left renal vein: A case report. World J Clin Cases. 2020;8:4986-4992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Xu Y, Li H, Wang C, Zhang M, Wang Q, Xie Y, Shao X, Tian L, Yuan Y, Yan W, Feng T, Li F, Ni Z, Mou S. Improving Prognostic and Chronicity Evaluation of Chronic Kidney Disease with Contrast-Enhanced Ultrasound Index-Derived Peak Intensity. Ultrasound Med Biol. 2020;46:2945-2955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Wilson SR, Greenbaum LD, Goldberg BB. Contrast-enhanced ultrasound: what is the evidence and what are the obstacles? AJR Am J Roentgenol. 2009;193:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | Zhang W, Wang Q, Liu YL, Yu WJ, Liu Y, Zhao H, Zhuang J, Jiang YX, Li YJ. Anastomosing hemangioma arising from the kidney: a case of slow progression in four years and review of literature. Int J Clin Exp Pathol. 2015;8:2208-2213. [PubMed] |