Published online Jul 6, 2024. doi: 10.12998/wjcc.v12.i19.3815
Revised: April 10, 2024
Accepted: May 24, 2024
Published online: July 6, 2024
Processing time: 122 Days and 8 Hours
Intracerebral hemorrhage mainly occurs in middle-aged and elderly patients with hypertension, and surgery is currently the main treatment for hypertensive cerebral hemorrhage, but the bleeding caused by surgery will cause damage to the patient's nerve cells, resulting in cognitive and motor dysfunction, resulting in a decline in the patient's quality of life.
To investigate associations between cerebral arterial blood flow and executive and cognitive functions in depressed patients after acute hypertensive cerebral hemorrhage.
Eighty-nine patients with depression after acute hypertensive cerebral hemorrhage who were admitted to our hospital between January 2019 and July 2021 were selected as the observation group, while 100 patients without depre
The MoCA score, net scores I, II, III, IV, and the total net score of the scratch test in the observation group were significantly lower than those in the control group (P < 0.05). Concurrently, the total number of responses, number of incorrect responses, number of persistent errors, and number of completed responses of the first classification in the WCST test were significantly higher in the observation group than those in the control group (P < 0.05). Blood flow in the basilar artery, left middle cerebral artery, right middle cerebral artery, left anterior cerebral artery, and right anterior cerebral artery was significantly lower in the observation group than in the control group (P < 0.05). The basilar artery, left middle cerebral artery, right middle cerebral artery, left anterior cerebral artery, and right anterior cerebral artery were positively correlated with the net and total net scores of each part of the Paddle Pin test and the MoCA score (P < 0.05), and negatively correlated with each part of the WCST test (P < 0.05). In the observation group, the post-treatment improvement was more prominent in the Paddle Pin test, WCST test, HAMD-24 score, and MoCA score compared with those in the pre-treatment period (P < 0.05). Blood flow in the basilar artery, left middle cerebral artery, right middle cerebral artery, left anterior cerebral artery, and right anterior cerebral artery significantly improved in the observation group after treatment (P < 0.05).
Impaired attention, and executive and cognitive functions are correlated with cerebral artery blood flow in patients with depression after acute hypertensive cerebral hemorrhage and warrant further study.
Core Tip: Through a cohort of studies of visiting patients, we have concluded that impaired attention, executive and cognitive function in depressed patients after acute hypertensive intracerebral hemorrhage are associated with cerebral arterial blood flow, and these results require more research.
- Citation: Zhang YZ, Zhang CY, Tian YN, Xiang Y, Wei JH. Cerebral arterial blood flow, attention, and executive and cognitive functions in depressed patients after acute hypertensive cerebral hemorrhage. World J Clin Cases 2024; 12(19): 3815-3823
- URL: https://www.wjgnet.com/2307-8960/full/v12/i19/3815.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i19.3815
In patients with hypertension, cerebral hemorrhage is induced by vascular retention due to long-term blood pressure and impaired blood circulation in the cerebral arteries of the body. Clinical data[1] show that cerebral hemorrhage mostly occurs in middle-aged and elderly patients with hypertension and has become a common complication in these patients. Surgery is the main treatment for hypertensive cerebral hemorrhage, but the hemorrhage caused by surgery can cause damage to the patient's nerve cells, leaving cognitive and motor dysfunction and reducing the patient's quality of life.
Foreign studies[2] have found that the velocity of cerebral blood flow slows and the vascular reactivity significantly reduces in patients with cognitive decline, while changes in velocity reflect the overall changes in cerebral blood flow indicating a direct relationship. A recent study[3] revealed significant cerebral blood flow hypoperfusion in brain regions such as the frontal lobe in patients with cognitive impairment and suggested that reduced local cerebral artery blood flow velocity and abnormal cerebral blood flow perfusion were associated. However, further studies are still needed. Therefore, the relationship between cerebral artery blood flow and executive and cognitive functions, such as attention, in depressed patients with acute hypertensive cerebral hemorrhage was investigated with an aim to provide data reference for the assessment of postoperative cognitive function and improvement of prognosis in clinical hypertensive cerebral hemorrhage.
Eighty-nine patients with depression after acute hypertensive cerebral hemorrhage who were admitted in our hospital between January 2019 and July 2021 were selected as the observation group, while 100 patients without depression who had acute hypertensive cerebral hemorrhage were selected as the control group. Comparisons on clinical general information between the observation and control groups are shown in Table 1. The results are comparable to current studies. This study was approved by the hospital ethics committee.
| Clinical Information | Observation group (n = 89) | Control group (n = 100) | t/χ2 | P |
| Gender | ||||
| Male | 54 (60.67) | 58 (58.00) | 0.139 | 0.709 |
| Female | 35 (39.33) | 42 (42.00) | ||
| Age (yr) | 55.79 ± 9.87 | 53.36 ± 10.15 | 1.664 | 0.098 |
| Body mass index (kg/m2) | 22.41 ± 2.36 | 22.18 ± 2.09 | 0.711 | 0.478 |
| Academic qualifications | ||||
| Below high school | 57 (64.04) | 70 (70.00) | 0.758 | 0.384 |
| High School and above | 32 (35.96) | 30 (30.00) | ||
| Brain hemorrhage lesions | ||||
| Basal joint | 39 (43.82) | 45 (45.00) | 0.716 | 0.949 |
| Thalamus | 23 (25.84) | 23 (23.00) | ||
| Lobe of the brain | 13 (14.61) | 17 (17.00) | ||
| Cerebellum | 9 (10.11) | 8 (8.00) | ||
| Brainstem | 5 (5.62) | 7 (7.00) | ||
| Amount of cerebral hemorrhage | ||||
| < 30 mL | 26 (29.21) | 30 (30.00) | 0.023 | 0.989 |
| 30-60 mL | 40 (44.94) | 45 (45.00) | ||
| > 60 mL | 23 (25.84) | 25 (25.00) | ||
| Cerebral hemorrhage side | ||||
| Right side | 29 (32.58) | 31 (31.00) | 0.055 | 0.815 |
| Left side | 60 (67.42) | 69 (69.00) | ||
| Surgical method | ||||
| Boneless flap | 55 (61.80) | 64 (64.00) | 0.098 | 0.754 |
| Small Bone Window | 34 (38.20) | 36 (36.00) |
Inclusion criteria: (1) Diagnosis of acute hypertensive cerebral hemorrhage that met the criteria in the Chinese Guidelines for the Diagnosis and Treatment of Cerebral Hemorrhage[4], and the diagnosis of depression met the criteria in the Diagnostic and Statistical Manual of Mental Progress IV with a score of ≥ 20 on the 24-item Hamilton Depression Inventory (HAMD-24); (2) debulking craniotomy or small bone window craniotomy for hematoma removal performed in our hospital; and (3) informed consent from the patient and family.
Exclusion criteria: (1) Preoperative history of mental illness; (2) other serious diseases such as malignant tumors and hematologic diseases; and (3) drug abuse, alcoholism, and other bad habits.
Cerebral artery blood flow examination: A transcranial color Doppler ultrasound diagnostic instrument (model TC7-NB, manufactured by Neusoft Xikang, with a probe frequency of 2 MHz) was used to perform the cerebral artery blood flow examination three months after patient surgery. Blood flow velocities of the left posterior cerebral artery and right posterior cerebral artery were measured via temporal and occipital windows.
All patients were examined after three months postoperatively for recovery of relevant functions. (1) Scratch test[5] was used to assess patient attention. The test was divided into 5 parts (I to V), each part conducted in 3 min. The 5-part test was done in succession, according to the formula: Fine score = coarse score (number of scratch errors + 1/2 missed scratch). The net score for each part and the total score were calculated; the higher the net score, the better the patient's attention; (2) the Wisconsin Card Sorting Test (WCST)[6] was used to assess the executive function of the patients. Patients were instructed to sort the color, shape, and number of four cards that appeared on the screen at each time. A total of 128 cards was shown. The total number of responses, number of incorrect responses, number of persistent errors, and number of completed responses for the first round were calculated; (3) patients were evaluated for depression using the HAMD-24[7], a 7-factor scale that includes anxiety/somatization, weight change, cognitive impairment, day-night change, blockage, sleep disturbance, and feelings of hopelessness. Scores ≥ 20 confirmed depression and a higher score indicated greater severity of the depressive symptoms; and (4) the Montreal Cognitive Assessment Scale (MoCA)[8] was used to assess the cognitive function of the patients. The scale includes eight domains, namely, attention and concentration, executive function, memory, language, visual-structural skills, abstract thinking, computation, and orientation, with a total score of 30 and ≥ 26 being normal.
Statistical analysis was performed using SPSS (version 22.0), normally distributed measures were expressed as mean ± SD, and t test was used to analyze the differences between groups. Count data were expressed as n (%), and χ2 test was used to analyze the differences between groups. Correlations were analyzed using Pearson correlation. Significance was set at P = 0.05.
The MoCA score, net scores I, II, III, IV, and the total net score of the scratch test were significantly lower in the observation group than in the control group (P < 0.05). Concurrently, the total number of responses, number of incorrect responses, number of persistent errors, and number of completed responses of the first classification were significantly higher in the WCST test of the observation group than those in the control group (P < 0.05) (Table 2).
| Indicators | Observation group (n = 89) | Control group (n = 100) | t | P value |
| Paddle pin quiz (points) | ||||
| Net score I | 32.20 ± 8.89 | 57.65 ± 9.90 | -18.504 | < 0.001 |
| Net score II | 36.60 ± 9.17 | 61.50 ± 9.24 | -18.558 | < 0.001 |
| Net score III | 17.20 ± 5.51 | 32.20 ± 5.58 | -18.556 | < 0.001 |
| Net score IV | 21.84 ± 8.05 | 28.80 ± 7.10 | -6.316 | < 0.001 |
| Net score V | 7.70 ± 2.40 | 15.50 ± 4.43 | -14.788 | < 0.001 |
| Total net score | 115.80 ± 12.26 | 195.76 ± 20.03 | -32.609 | < 0.001 |
| Wisconsin Card Sorting Test | ||||
| Total number of responses | 122.45 ± 14.48 | 92.52 ± 16.60 | 13.134 | < 0.001 |
| Number of error responses | 52.50 ± 13.37 | 19.54 ± 5.54 | 22.576 | < 0.001 |
| Number of persistent errors | 26.20 ± 8.89 | 8.90 ± 1.15 | 19.286 | < 0.001 |
| Number of completed answers for the 1st classification | 26.10 ± 6.86 | 15.70 ± 4.12 | 12.791 | < 0.001 |
| MoCA score (points) | 24.40 ± 2.21 | 27.80 ± 2.13 | -10.762 | < 0.001 |
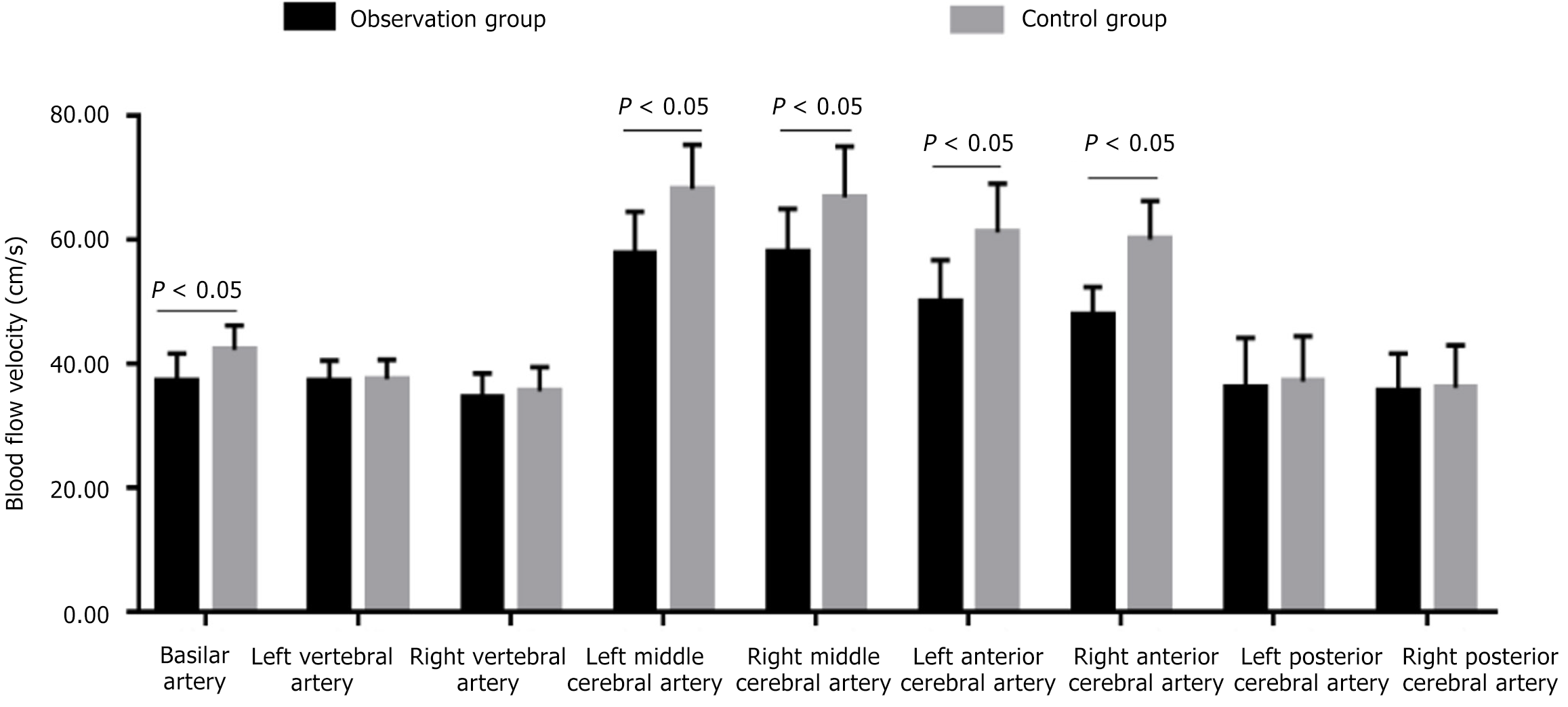
Blood flow in the basilar artery, left middle cerebral artery, right middle cerebral artery, left anterior cerebral artery, and right anterior cerebral artery was significantly lower in the observation group than in the control group (P < 0.05) (Figure 1; Table 3).
| Cerebral arteries | Observation group (n = 89) | Control group (n = 100) | t | P value |
| Basilar artery (cm/s) | 37.20 ± 4.43 | 42.20 ± 3.95 | -8.203 | < 0.001 |
| Left vertebral artery (cm/s) | 37.15 ± 3.34 | 37.50 ± 3.15 | -0.741 | 0.460 |
| Right vertebral artery (cm/s) | 34.41 ± 4.03 | 35.54 ± 3.88 | -1.962 | 0.051 |
| Left middle cerebral artery (cm/s) | 57.65 ± 6.82 | 68.15 ± 7.15 | -10.298 | < 0.001 |
| Right middle cerebral artery (cm/s) | 57.80 ± 7.15 | 66.80 ± 8.20 | -7.996 | < 0.001 |
| Left anterior cerebral artery (cm/s) | 50.03 ± 6.68 | 61.15 ± 7.90 | -10.380 | < 0.001 |
| Right anterior cerebral artery (cm/s) | 47.80 ± 4.55 | 60.03 ± 6.22 | -15.266 | < 0.001 |
| Left posterior cerebral artery (cm/s) | 36.12 ± 8.03 | 37.09 ± 7.33 | -0.868 | 0.386 |
| Right posterior cerebral artery (cm/s) | 35.51 ± 6.11 | 36.12 ± 6.84 | -0.643 | 0.521 |
Cerebral artery blood flow was correlated with attention, executive ability, and MoCA scores in the observation group. The basilar artery, left middle cerebral artery, right middle cerebral artery, left anterior cerebral artery, and right anterior cerebral artery were positively correlated with the net and total net scores of each part of the Paddle Pin test and the MoCA score (P < 0.05), and negatively correlated with each part of the WCST test (P < 0.05) (Tables 4 and 5).
| Cerebral arteries | Paddle pin quiz (points) | |||||
| Net score I | Net score II | Net score III | Net score IV | Net score V | Total net score | |
| Basilar artery (cm/s) | 0.322a | 0.335a | 0.104 | 0.142 | 0.402a | 0.422a |
| Left vertebral artery (cm/s) | 0.187 | 0.141 | 0.203 | 0.098 | 0.104 | 0.117 |
| Right vertebral artery (cm/s) | 0.105 | 0.112 | 0.114 | -0.058 | -0.087 | 0.184 |
| Left middle cerebral artery (cm/s) | 0.503a | 0.487a | 0.505a | 0.427a | 0.112 | 0.503a |
| Right middle cerebral artery (cm/s) | 0.488a | 0.502a | 0.412a | 0.403a | 0.109 | 0.477a |
| Left anterior cerebral artery (cm/s) | -0.098 | 0.415a | 0.309a | 0.378a | 0.415a | 0.432a |
| Right anterior cerebral artery (cm/s) | -0.102 | 0.433a | 0.318a | 0.390a | 0.470a | 0.387a |
| Left posterior cerebral artery (cm/s) | 0.234 | -0.054 | 0.087 | 0.099 | -0.107 | 0.108 |
| Right posterior cerebral artery (cm/s) | 0.105 | -0.101 | 0.112 | 0.100 | -0.087 | 0.102 |
| Cerebral arteries | Wisconsin Card Sorting Test | MoCA score (points) | |||
| Total number of responses | Number of error responses | Number of persistent errors | Number of completed answers for the 1st classification | ||
| Basilar artery (cm/s) | -0.385a | -0.098 | -0.112 | -0.324a | 0.312a |
| Left vertebral artery (cm/s) | -0.065 | -0.106 | -0.082 | -0.114 | 0.018 |
| Right vertebral artery (cm/s) | -0.097 | -0.114 | -0.109 | -0.078 | 0.095 |
| Left middle cerebral artery (cm/s) | -0.398a | -0.423a | -0.115 | -0.447a | 0.387a |
| Right middle cerebral artery (cm/s) | -0.503a | -0.406a | -0.124 | -0.435a | 0.406a |
| Left anterior cerebral artery (cm/s) | -0.411a | -0.378a | -0.411a | -0.347a | 0.355a |
| Right anterior cerebral artery (cm/s) | -0.365a | -0.109 | -0.362a | -0.303a | 0.378a |
| Left posterior cerebral artery (cm/s) | 0.065 | -0.087 | -0.098 | 0.012 | 0.046 |
| Right posterior cerebral artery (cm/s) | -0.098 | 0.045 | -0.077 | 0.087 | 0.112 |
The Paddle Pin test, WCST test, HAMD-24 score, and MoCA score improved in the observation group after treatment from the pre-treatment period (P < 0.05) (Table 6).
| Indicators | Before treatment (n = 89) | After treatment (n = 89) | t | P value |
| Paddle pin quiz (points) | ||||
| Net score I | 32.20 ± 8.89 | 40.54 ± 9.12 | 6.178 | < 0.001 |
| Net score II | 36.60 ± 9.17 | 42.21 ± 8.43 | 4.249 | < 0.001 |
| Net score III | 17.20 ± 5.51 | 22.25 ± 4.80 | 6.520 | < 0.001 |
| Net score IV | 21.84 ± 8.05 | 24.63 ± 7.12 | 2.449 | 0.015 |
| Net score V | 7.70 ± 2.40 | 9.12 ± 1.87 | 4.403 | < 0.001 |
| Total net score | 115.80 ± 12.26 | 135.58 ± 20.43 | 7.832 | < 0.001 |
| Wisconsin Card Sorting Test | ||||
| Total number of responses | 122.45 ± 14.48 | 75.50 ± 15.03 | 21.223 | < 0.001 |
| Number of error responses | 52.50 ± 13.37 | 38.65 ± 11.20 | 7.491 | < 0.001 |
| Number of persistent errors | 26.20 ± 8.89 | 22.40 ± 9.02 | 2.831 | 0.005 |
| Number of completed answers for the 1st classification | 26.10 ± 6.86 | 20.03 ± 5.74 | 6.402 | < 0.001 |
| HAMD-24 score (points) | 27.20 ± 2.03 | 21.02 ± 2.11 | 19.912 | < 0.001 |
| MoCA score (points) | 24.40 ± 2.21 | 26.12 ± 2.32 | 5.064 | < 0.001 |
Blood flow in the basilar artery, left middle cerebral artery, right middle cerebral artery, left anterior cerebral artery, and right anterior cerebral artery improved in the observation group after treatment compared with that before treatment (P < 0.05) (Table 7).
| Cerebral arteries | Before treatment (n = 89) | 3 months after treatment (n = 89) | t | P value |
| Basilar artery (cm/s) | 37.20 ± 4.43 | 40.03 ± 4.10 | 4.423 | 0.000 |
| Left vertebral artery (cm/s) | 37.15 ± 3.34 | 37.25 ± 3.80 | 0.186 | 0.852 |
| Right vertebral artery (cm/s) | 34.41 ± 4.03 | 34.84 ± 3.95 | 0.719 | 0.473 |
| Left middle cerebral artery (cm/s) | 57.65 ± 6.82 | 62.28 ± 7.03 | 4.460 | 0.000 |
| Right middle cerebral artery (cm/s) | 57.80 ± 7.15 | 63.15 ± 6.87 | 5.090 | 0.000 |
| Left anterior cerebral artery (cm/s) | 50.03 ± 6.68 | 55.59 ± 6.92 | 5.454 | 0.000 |
| Right anterior cerebral artery (cm/s) | 47.80 ± 4.55 | 54.49 ± 5.03 | 9.305 | 0.000 |
| Left posterior cerebral artery (cm/s) | 36.12 ± 8.03 | 36.03 ± 7.78 | 0.076 | 0.940 |
| Right posterior cerebral artery (cm/s) | 35.51 ± 6.11 | 35.80 ± 5.22 | 0.340 | 0.734 |
Relevant data[9] showed that the incidence of hypertensive cerebral hemorrhage accounted for about 30% of acute cerebrovascular diseases in China and was a common critical cerebrovascular disease among the elderly. Cerebral parenchymal hemorrhage leading to cerebral hematoma, which in turn induces primary and secondary damage to the central nervous system, is the main pathological manifestation of this disease, while cognitive dysfunction and decreased attentional executive ability are both significant secondary damage outcomes[7,10]. The results of this study show that the MoCA score, net score I, II, III, n IV, and total net score of the scratch test in the observation group were significantly lower than those in the control group. Conversely, the number of total responses, incorrect responses, persistent errors, and number of completed first categorized responses on the WCST were significantly higher in the observation group than those in the control group. These results suggest that there is impairment of attention and executive power in depressed patients after acute hypertensive cerebral hemorrhage. This indicates that changes in attention and executive power of patients should be observed after hypertensive cerebral hemorrhage surgery and timely preventive and restorative treatment should be given to improve the quality of life of patients.
Color Doppler ultrasound can clearly show the vascular alignment, avoid important vessels, find the best surgical access, minimize damage to normal brain tissue, provide real-time intraoperative ultrasound which can accurately measure the size of intracranial hematoma, locate the intracranial hematoma, and gradually aid the removal of the hematoma under a microscope, helping to reduce brain tissue damage and better protect the cerebral vessels[11-13]. The comparison of cerebral artery blood flow between the two groups revealed that the blood flow in the basilar artery, left middle cerebral artery, right middle cerebral artery, left anterior cerebral artery, and right anterior cerebral artery in the observation group was significantly lower than that in the control group, which may be mainly due to the fact that patients with cognitive impairment mostly have damage in the frontal, parietal, temporal lobes and basal ganglia, and the cerebral hemodynamics in the internal carotid artery system is not perfused enough, which in turn leads to their cognitive impairment.
The present study continued to correlate cerebral artery blood flow with attention, executive ability, and MoCA scores in the observation group and found that the basilar artery, left middle cerebral artery, right middle cerebral artery, left anterior cerebral artery, and right anterior cerebral artery were positively correlated with the net and total net scores of each part of the Paddle Pin test and negatively correlated with each part of the WCST test. This suggests that there may be a relationship between cognitive impairment and slow cerebral artery blood flow velocity in depression, further confirming the relationship between cerebral artery blood flow velocity and cognitive function in depression. Longitudinal studies and analyses of cerebral artery hemodynamics and cognitive function-related indicators in depressed patients may be considered in the future to provide data references for the prevention and improvement of cognitive dysfunction in patients.
The WCST is a neuropsychological test commonly used to examine attention. Clinical practice has shown that WCST scores are closely related to frontal lobe function and can specifically reflect frontal lobe function[14-17]. The WCST is a commonly used neuropsychological test to examine attention. Furthermore, this study found that Paddle Pin test, WCST test, HAMD-24 score, and MoCA score improved in the observation group after treatment compared to the pre-treatment period. This result also further suggests that cerebral blood flow velocity is closely related to attention and executive function impairment, confirming the hypothesis that the relationship between cerebral blood flow velocity and partial cognitive impairment in depression, and that the regulation of disturbed cerebral arterial hemodynamics may contribute to the improvement of cognitive function, which deserves further investigation[18-20]. However, there are some limitations in this study, as only the changes in mean arterial blood flow velocity were analyzed but not in combination with cerebral perfusion imaging, which needs to be studied in more depth and detail in the future. Additionally, the sample size of this study was small and the observation time was not long enough; thus, it was not possible to study the factors that may affect cerebral blood flow velocity and cognitive function according to the different age of onset, duration, and refractory nature of depression.
The impaired attention, executive, cognitive functions in depressed patients after acute hypertensive cerebral hemorrhage are correlated with cerebral arterial blood flow in patients and are worthy of further study.
| 1. | Reznik ME, Fakhri N, Moody S, Murray K, Costa S, Yaghi S, Schrag M, Madsen TE, Burton TM, Cutting S, Mahta A, Wendell LC, Thompson BB, Rao SS, Stretz C, Furie KL, Mac Grory B. Arrival blood pressure in hypertensive and non-hypertensive spontaneous intracerebral hemorrhage. J Neurol Sci. 2020;416:117000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Gong M, Zhang H, Shi Z, Yuan Q, Su X. Application of intraoperative ultrasound in neurosurgery for hypertensive intracerebral hemorrhage. J Clin Neurosci. 2021;90:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Renard D, Castelnovo G, Ion I, Guillamo JS, Thouvenot E. Single and simultaneous multiple intracerebral hemorrhages: a radiological review. Acta Neurol Belg. 2020;120:819-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Shao X, Wang Q, Shen J, Liu J, Chen S, Jiang X. Comparative Study of Micro-Bone Window and Conventional Bone Window Microsurgery for Hypertensive Intracerebral Hemorrhage. J Craniofac Surg. 2020;31:1030-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Fakan B, Reisz Z, Zadori D, Vecsei L, Klivenyi P, Szalardy L. Predictors of localization, outcome, and etiology of spontaneous intracerebral hemorrhages: focus on cerebral amyloid angiopathy. J Neural Transm (Vienna). 2020;127:963-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Huang H, Huang G, Gu J, Chen K, Huang Y, Xu H. Relationship of Serum Uric Acid to Hematoma Volume and Prognosis in Patients with Acute Supratentorial Intracerebral Hemorrhage. World Neurosurg. 2020;143:e604-e612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Liu J, Cheng J, Zhou H, Deng C, Wang Z. Efficacy of minimally invasive surgery for the treatment of hypertensive intracerebral hemorrhage: A protocol of randomized controlled trial. Medicine (Baltimore). 2021;100:e24213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Nelson SE. Commentary on Associations of Radiographic Small Vessel Disease with Acute Intracerebral Hemorrhage Volume, Hematoma Expansion, and Intraventricular Hemorrhage. Neurocrit Care. 2020;32:361-362. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Onose G, Anghelescu A, Blendea D, Ciobanu V, Daia C, Firan FC, Oprea M, Spinu A, Popescu C, Ionescu A, Busnatu Ș, Munteanu C. Cellular and Molecular Targets for Non-Invasive, Non-Pharmacological Therapeutic/Rehabilitative Interventions in Acute Ischemic Stroke. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 10. | Zhang J, Lu S, Wang S, Zhou N, Li G. Comparison and analysis of the efficacy and safety of minimally invasive surgery and craniotomy in the treatment of hypertensive intracerebral hemorrhage. Pak J Med Sci. 2018;34:578-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Katsumata M, Ota T, Tsuruta W, Akiyama T, Sakai Y, Shigeta K, Kaneko J, Nogawa S, Ichijo M, Shiokawa Y, Hirano T. Comparisons of Characteristics and Outcomes after Mechanical Thrombectomy for Vertebrobasilar Occlusion with Cardioembolism or Atherosclerotic Brain Infarction: Data from the Tokyo-Tama-Registry of Acute Endovascular Thrombectomy (TREAT). World Neurosurg. 2021;148:e680-e688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 12. | Lee EJ, Lee MY, Hung YC. The application of transcranial Doppler sonography in patients with chronic subdural haematoma. Acta Neurochir (Wien). 1999;141:835-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Song GF, Li X, Feng Y, Yu CH, Lian XY. Acupuncture combined Bobath approach for limbs paralysis after hypertensive intracerebral hemorrhage: A protocol for a systematic review. Medicine (Baltimore). 2019;98:e14750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Lehmann N, Villringer A, Taubert M. Colocalized White Matter Plasticity and Increased Cerebral Blood Flow Mediate the Beneficial Effect of Cardiovascular Exercise on Long-Term Motor Learning. J Neurosci. 2020;40:2416-2429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Marnat G, Delvoye F, Finitsis S, Lapergue B, Gariel F, Consoli A, Desilles JP, Mazighi M, Dargazanli C, Bourcier R, Darcourt J, Chalumeau V, Elhorany M, Clarençon F, Richard S, Gory B, Sibon I; ETIS Investigators. A Multicenter Preliminary Study of Cangrelor following Thrombectomy Failure for Refractory Proximal Intracranial Occlusions. AJNR Am J Neuroradiol. 2021;42:1452-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Kato Y, Hayashi T, Suzuki K, Maruyama H, Kikkawa Y, Kurita H, Takao M. Resumption of Direct Oral Anticoagulants in Patients with Acute Spontaneous Intracerebral Hemorrhage. J Stroke Cerebrovasc Dis. 2019;28:104292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Lattanzi S, Di Napoli M, Ricci S, Divani AA. Matrix Metalloproteinases in Acute Intracerebral Hemorrhage. Neurotherapeutics. 2020;17:484-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 18. | Lou XH, Cai YY, Yang XQ, Zheng HJ, Yu YJ, Wang CH, Huang LN. Serum netrin-1 concentrations are associated with clinical outcome in acute intracerebral hemorrhage. Clin Chim Acta. 2020;508:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Puig J, Blasco G, Terceño M, Daunis-I-Estadella P, Schlaug G, Hernandez-Perez M, Cuba V, Carbó G, Serena J, Essig M, Figley CR, Nael K, Leiva-Salinas C, Pedraza S, Silva Y. Predicting Motor Outcome in Acute Intracerebral Hemorrhage. AJNR Am J Neuroradiol. 2019;40:769-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Liu Y, Yang S, Cai E, Lin L, Zeng P, Nie B, Xu F, Tian Q, Wang J. Functions of lactate in the brain of rat with intracerebral hemorrhage evaluated with MRI/MRS and in vitro approaches. CNS Neurosci Ther. 2020;26:1031-1044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/