Published online Jun 26, 2024. doi: 10.12998/wjcc.v12.i18.3298
Revised: April 16, 2024
Accepted: April 26, 2024
Published online: June 26, 2024
Processing time: 103 Days and 21.1 Hours
Intestinal lymphangiectasia (IL) is characterized by the dilation of intestinal lymphatic vessels, which can rupture and cause loss of lymph into the intestine. Due to the high content of proteins, lipoproteins, and lymphocytes in the intestinal lymph, loss of lymph might result in hypoproteinemia, hypoalbuminemia, hypogammaglobulinemia, and lymphocytopenia. In addition, there may be a depletion of minerals, lipids, and fat-soluble vitamins. IL can be primary due to inherent malfunctioning of the lymphatic system, or secondly, a result of various factors that may hinder lymphatic drainage either directly or indirectly. This condition has emerged as a subject of significant clinical interest. Given that the intestinal lymphatic system plays an important role in the body’s fluid homeostasis, adaptive immunity, nutrient and drug absorption, intestinal transport, and systemic metabolism, its dysfunction may have wider implications. Although primary IL is rare, with varied clinical features, complications, treat
Core Tip: Exploring the intricate relationship between the intestinal lymphatic system and clinical outcomes, particularly in the context of intestinal lymphangiectasia, reveals crucial knowledge gaps that require attention. There is a broader implication of intestinal lymphatic dysfunction than is typically talked about. This article delves into the existing voids in our understanding, emphasizing the significance of future research to refine diagnostic approaches, establishing treatment guidelines, and uncovering novel therapeutic interventions for individuals affected by this complex condition. Bridging these knowledge gaps is essential to advancing patient care and improving outcomes in the realm of intestinal lymphatic disorders.
- Citation: Marrapu S, Kumar R. Intestinal lymphangiectasia: Understanding the bigger picture. World J Clin Cases 2024; 12(18): 3298-3303
- URL: https://www.wjgnet.com/2307-8960/full/v12/i18/3298.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i18.3298
The lymphatic system is a unidirectional transport network that returns unabsorbed fluid to the blood and serves as an additional circulatory system for the body. The lymphatics found in the centre of intestinal villi are called lacteals. They share structural similarities with other lymphatic capillaries. The integrity of lacteals is crucial for villous structure and function. Lymphangiogenesis is primarily regulated by vascular endothelial growth factor-C (VEGF-C) during embryonic development. The essential functions of the gut’s lymphatic system include the transport of dietary lipids via chylomicrons, immunosurveillance through the housing of B and T lymphocytes, and the removal of interstitial fluid to maintain intestinal homeostasis[1].
Of great interest is a recent article by Na et al[2] on adult primary intestinal lymphangiectasia (PIL) that provides insight into its clinical features, varied complications, treatment response, and prognosis. PIL is characterised by dilatation of intestinal lymphatics and eventual rupture, which can have multiple consequences. The study aimed to formulate diagnostic and treatment protocols, highlighting the knowledge gaps and the need for personalized and proactive treatment approaches. This has prompted us to write an editorial in which we broaden the scope to include the clinical implications of intestinal lymphatic dysfunction, provide a comprehensive list of its causes, and highlight the gaps in our knowledge on this condition that may require a great deal of research.
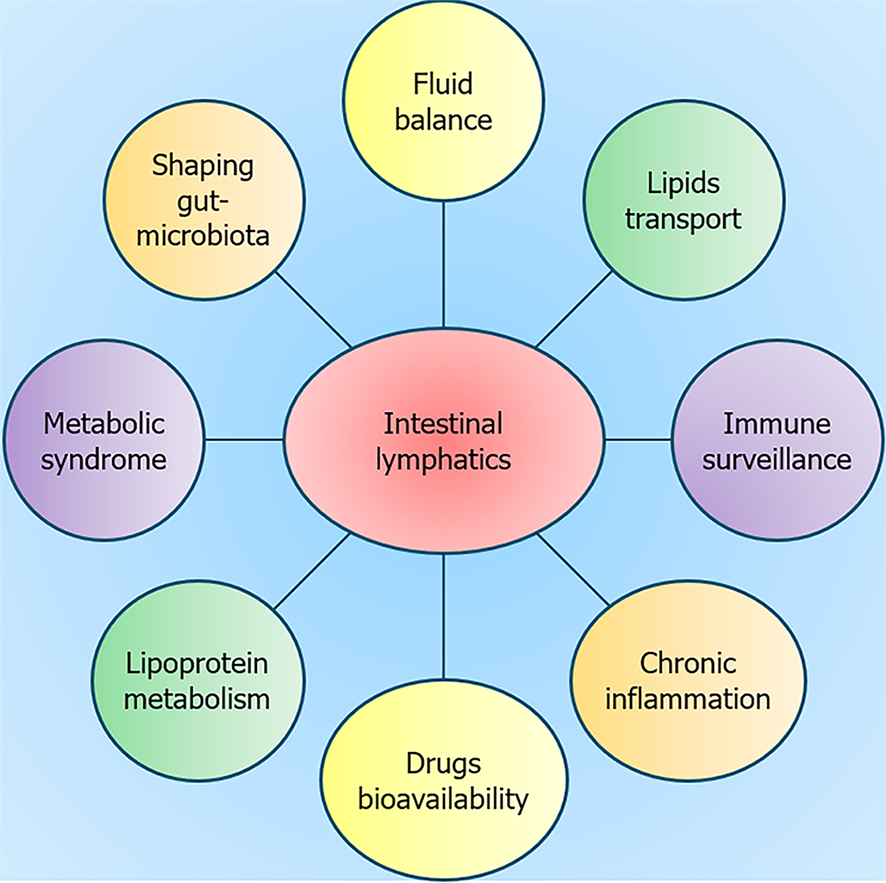
The gut lymphatic system plays a very important role in body fluid homeostasis, acquired immunity, chronic inflammation, shaping of gut microbiota, pharmacokinetic of drugs, and the transportation of lipid and waste materials (Figure 1). It is fast gaining recognition for its role in the development of insulin resistance and metabolic syndrome[3-5]. The enteroendocrine cells of the small intestine release incretin hormones like gastric inhibitory polypeptide and glucagon-like peptide-1, promoting insulin secretion in response to the absorption of fat and carbohydrates. The changes in the number of intestinal lymphatics and lacteal permeability may redirect these hormones to the bloodstream, where they could degrade rapidly. This alteration may possibly influence adiposity and suggests a potential link between the lymphatic system, incretin hormones, and metabolism[5]. Genetic and diet-induced obesity mouse models demonstrate that obesity-related inflammation has a detrimental impact on lymphatic function, resulting in increased permeability and diminished vessel density. Insulin sensitivity is increased when lacteal lymphangiogenesis is inhibited or lacteal permeability is altered, which acts as a defense against dietary-induced obesity[6]. The intestine and its lymphatics are involved in whole-body lipid and cholesterol homeostasis. Reverse cholesterol transport (RCT) is vital for clearance of peripheral tissue cholesterol via lymphatic vasculature[7,8]. The intestine and liver appear to be the main source of high-density lipoprotein (HDL) in lymph. The remodelling of HDL during transport through the lymphatics has implications for cardiometabolic diseases[9]. The association of insulin resistance with altered intestinal lymphatic HDL-apolipoprotein A-1 and microRNA-223 metabolism provides insight into the gut-mediated regulation of the RCT[10]. Thus, augmenting RCT in addition to a thorough understanding of lymphatic transport of lipids holds potential for mitigating atherosclerotic vascular diseases.
The interplay between the intestinal lymphatics and the gut microbiota is vital for maintaining gut health[11,12]. The gut lymphatics, with their immunomodulatory role and involvement in immune cell transport, significantly influence the composition and function of the gut microbiota. By facilitating the drainage of interstitial fluid and the distribution of antimicrobial peptides, the lymphatic system helps to maintain a delicate balance, preventing the translocation of microbial products and fostering immunological tolerance. Moreover, the way in which the intestinal lymphatic system reacts to intestinal infections influences the dynamics of gut microbiota[13]. Increased lymphatic flow during infection prevents tissue edema, whereas lymphostasis can result in chronic inflammation. Multiple sclerosis, neuromyelitis optica, and Alzheimer's disease are among the neuroinflammatory illnesses whose immunopathology is influenced by the lymphatic system and gut microbiota[13]. Lipid-based nano-drug delivery systems have emerged as a promising solution to overcome limitations in oral drug delivery, especially for drugs with poor water solubility and high hepatic first pass metabolism. These systems offer benefits like greater bioavailability, tailored administration, more efficient delivery, and protection against enzymatic degradation[14,15].
The intestinal lymphatic system is crucial to the complex interplay of the gut-liver axis, contributing significantly to the pathogenesis of cirrhosis and portal hypertension. In cirrhosis patients, portal hypertension disrupts gut lymphatic function, leading to lymph imbalance and the consequent formation of ascites. These lymphatics are essential for maintaining the integrity of the gut barrier, and dysfunction can result in bacterial translocation, fostering infections such as spontaneous bacterial peritonitis. Serving as a conduit for endotoxins, intestinal lymphatics expose the systemic circulation to inflammatory triggers, thereby inducing substantial systemic inflammation and influencing the trajectory of liver diseases. Additionally, intestinal lymphatic endothelial cells actively contribute to gut and liver immunity by influencing cell trafficking, activation, and antigen presentation during inflammation. Strategies targeting lymphatic function such as endothelial nitric oxide synthase inhibition, offer promising avenues for mitigating complications associated with cirrhosis[16]. In a study conducted by Ribera et al[17], inhibition of endothelial nitric oxide synthase in mesenteric lymphatic vessels resulted in enhanced lymphatic drainage with a reduction in ascites.
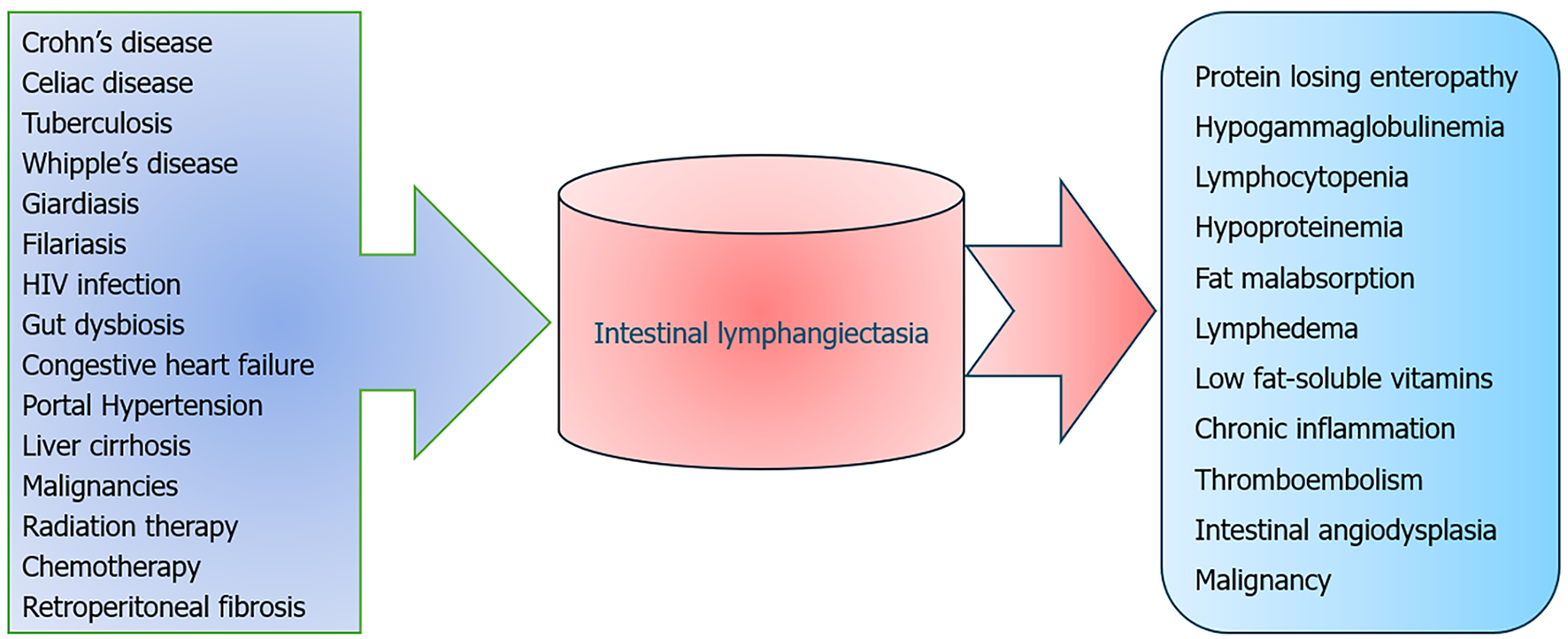
IL refers to the pathological dilatation of intestinal lacteals, leading to the accumulation and seepage of lymph into the lumen of the small intestine. Due to the high concentration of proteins, lipoproteins, and lymphocytes in intestinal lymph, its loss results in hypoproteinemia, hypoalbuminemia, hypogammaglobulinemia, and lymphocytopenia. Additionally, there may be a depletion of iron, calcium, lipids, and fat-soluble vitamins. PIL, due to inherent malfunctions in the lymphatic system, or secondary, resulting from various factors that hinder lymphatic drainage, either directly or indirectly[18].
PIL, also known as Waldman's disease, is a rare condition that primarily affects children but can be diagnosed in adults ranging in age from their twenties to their seventies[19]. The exact cause of PIL remains uncertain but appears to be due to altered expressions of regulatory molecules associated with lymphangiogenesis in the duodenal mucosa. Duodenal biopsy samples from individuals with IL showed elevated expression levels of VEGF receptor-3 and lymphatic vessel endothelial receptor 1, while mRNA expression of VEGF-C and VEGF-D was significantly suppressed compared to controls[20]. There are few reports on familial cases of Waldmann's disease, and many syndromes, including von Recklinghausen, Noonan, Turner, and Klippel-Trenaunay syndromes, are also associated with IL[19]. The prevalence and global distribution of this condition disease has not received sufficient attention due to its rarity.
The most prevalent clinical manifestation of IL is peripheral edema which is observed in 95% of cases[21]. Initially this edema is due to hypoalbuminemia which is typically pitting type and symmetrically distributed. Rarely, lymphedema may occur which is usually localized to the lower limbs. Stemmer's sign aids in distinguishing between lymphedema and hypoalbuminemia-induced edema. Ascites, including chylous ascites, and pleuro-pericardial effusion may also occur in this condition. Chronic diarrhea resulting from fat malabsorption is another common symptom that is frequently copious and results in a considerable loss of fat and fat-soluble vitamins. This may lead to weight loss, muscle loss, and weakness. Some cases may present with an abdominal mass with lymphangiomas in the duodenum, while others may experience abdominal pain, fatigue, and delayed growth[22]. PIL has also been found to be associated with intestinal angiodysplasia, which can result in occult, less frequently, or significant bleeding[23]. There are sporadic associations of PIL with celiac disease, yellow nail syndrome, and autoimmune polyglandular disease type 1[24,25].
The paramount consideration in diagnosing PIL in adults lies in the meticulous exclusion of entities that may precipitate lymphatic obstruction, resulting in secondary IL (Figure 2). The secondary causes may include one or more of the following: Chronic inflammatory diseases (such as Crohn's disease and celiac enteropathy), enteric infections (such as intestinal tuberculosis, Whipple’s disease, human immunodeficiency virus-enteropathy, giardiasis, lymphatic filariasis, and small intestinal bacterial overgrowth), cardiovascular conditions (such as congestive heart failure and constrictive pericarditis), portal hypertension caused by liver cirrhosis and Budd Chiari syndrome, malignancies (especially B-cell lymphomas), and iatrogenic factors such as radiation, chemotherapy or prior surgical interventions[18,26-28]. Intestinal tuberculosis, still a prevalent condition in developing countries, may lead to lymphatic obstruction, leading to IL. Moreover, lymphopenia and malabsorption caused by IL may have detrimental effects on tuberculosis patients[29]. In a recent study conducted by Arya et al[30], it was observed that 44.5% of cirrhosis patients experiencing refractory ascites exhibited lymphatic dysfunction. IL was noted in 27% of such patients. Interestingly, 22% of patients had IL found only upon histological inspection of duodenal biopsy specimens, while the characteristic features on endoscopy were absent. This lymphatic dysfunction might have contributed to the refractoriness of ascites and edema, which would require different strategies for fluid mobilization[30].
In clinical practice, patients exhibiting a combination of findings such as lymphocytopenia, hypogammaglobulinemia, hypoproteinemia, and hypoalbuminemia may be suspected of having IL. The definitive diagnosis of IL requires endoscopic evaluation and histopathological examination of small intestinal mucosa. Endoscopic findings include edematous and whitish villi which often resemble snowflakes. Diagnosis is confirmed through histopathological examination of mucosa showing dilated lacteals. It is crucial to highlight the absence of parasites or villous atrophy in biopsies[2]. It is important to consider that sporadic detection of IL during endoscopy does not always indicate a sinister condition. In a study, 1.9% of upper gastrointestinal endoscopies revealed the unintentional discovery of IL with histological confirmation[31]. Most incidentally detected IL in adult subjects show little to no clinical symptoms of malabsorption. Thus, many of the clinical features of IL may possibly signify the severe end of the disease spectrum. The substantial variability in the extent and location of affected lymph vessels likely contributes to this observed disparity. A lymphangiography or lymphoscintigraphy is indicated in patients with suspected rupture of subserosal lymphatics leading to chylothorax or chylous ascites[32]. Nevertheless, these techniques are cumbersome, lack precision, and have limited availability[33].
The mainstay of IL treatment includes dietary changes, medication, and, in some instances, surgical intervention. By avoiding gut lymphatic absorption, a low-fat, high-protein diet that includes medium-chain triglycerides (MCT) may lower lymphatic pressure. By directly accessing the portal venous circulation, MCT prevents the lymphatic system from becoming overloaded[34]. When it comes to long-term effectiveness, sirolimus and octreotide have shown the most promise. Octreotide reduces the lymphatic flow in the gut, while Sirolimus prevents lymphatic growth by blocking mammalian rapamycin signalling[35,36]. Surgery may be an option in case of segmental bowel involvement with symptoms refractory to other measures. Tailoring treatment approaches to individual patients remains a challenge, with a need for a better understanding of factors influencing the treatment response variability. Finally, addressing secondary IL would require dedicated efforts to establish effective therapeutic approaches, apart from treatment of underlying disease.
The prognosis of IL can vary significantly depending on cause, severity, location, and response to treatment. PIL patients are at risk of thromboembolism which may be related to a deficient anticoagulant property of albumin[37,38]. Lymphedema associated with IL is prone to cellulitis which may lead to sepsis[39]. About 5% of PIL cases might develop malignant lymphomas usually 3 years–25 years after the diagnosis[40].
IL presents as a rare and challenging disease with a spectrum of manifestations ranging from mild to severe. PIL is usually diagnosed in children. Diagnosing this condition in adults mandates ruling out various secondary causes. Given that the intestinal lymphatic system plays an important role in the body’s fluid homeostasis, immunity, nutrient and drug absorption, transport, and metabolism, its dysfunction may have wider implications; most of which remain unexplored. Current knowledge gaps in the diagnosis of IL include the challenge of accurately detecting segmental involvement when routine exams appear normal, the need for standardized measurements to quantify lymphatic dysfunction, and difficulty in distinguishing between primary and secondary causes. There is a need to address the limited availability of sophisticated imaging techniques and to find early biomarkers to predict the development of IL before it manifests clinically. There is also a need to comprehend the natural history and progression of lymphatic dysfunction over time. Identifying the variables influencing clinical heterogeneity in patients with IL and creating personalised diagnostic and therapeutic strategies could be key areas for future research. The significant influence that the lymphatic system and its regulatory mechanisms have on the metabolic consequences linked to obesity and insulin resistance provides possible therapeutic routes for treating obesity-related metabolic dysfunction and chronic inflammation.
| 1. | Cifarelli V, Eichmann A. The Intestinal Lymphatic System: Functions and Metabolic Implications. Cell Mol Gastroenterol Hepatol. 2019;7:503-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (35)] |
| 2. | Na JE, Kim JE, Park S, Kim ER, Hong SN, Kim YH, Chang DK. Experience of primary intestinal lymphangiectasia in adults: Twelve case series from a tertiary referral hospital. World J Clin Cases. 2024;12:746-757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (14)] |
| 3. | Kohan A, Yoder S, Tso P. Lymphatics in intestinal transport of nutrients and gastrointestinal hormones. Ann N Y Acad Sci. 2010;1207 Suppl 1:E44-E51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Festi D, Schiumerini R, Eusebi LH, Marasco G, Taddia M, Colecchia A. Gut microbiota and metabolic syndrome. World J Gastroenterol. 2014;20:16079-16094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 342] [Cited by in RCA: 373] [Article Influence: 31.1] [Reference Citation Analysis (6)] |
| 5. | Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5683] [Cited by in RCA: 6664] [Article Influence: 350.7] [Reference Citation Analysis (2)] |
| 6. | Norden PR, Kume T. The Role of Lymphatic Vascular Function in Metabolic Disorders. Front Physiol. 2020;11:404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Huang LH, Elvington A, Randolph GJ. The role of the lymphatic system in cholesterol transport. Front Pharmacol. 2015;6:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Lim HY, Thiam CH, Yeo KP, Bisoendial R, Hii CS, McGrath KC, Tan KW, Heather A, Alexander JS, Angeli V. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-mediated transport of HDL. Cell Metab. 2013;17:671-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 239] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 9. | Randolph GJ, Miller NE. Lymphatic transport of high-density lipoproteins and chylomicrons. J Clin Invest. 2014;124:929-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 180] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 10. | Mangat R, Borthwick F, Haase T, Jacome M, Nelson R, Kontush A, Vine DF, Proctor SD. Intestinal lymphatic HDL miR-223 and ApoA-I are reduced during insulin resistance and restored with niacin. FASEB J. 2018;32:1602-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Solari E, Marcozzi C, Negrini D, Moriondo A. Interplay between Gut Lymphatic Vessels and Microbiota. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Hokari R, Tomioka A. The role of lymphatics in intestinal inflammation. Inflamm Regen. 2021;41:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (9)] |
| 13. | Tsunoda I. Lymphatic system and gut microbiota affect immunopathology of neuroinflammatory diseases, including multiple sclerosis, neuromyelitis optica and Alzheimer's disease. Clin Exp Neuroimmunol. 2017;8:177-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Singh I, Swami R, Khan W, Sistla R. Lymphatic system: a prospective area for advanced targeting of particulate drug carriers. Expert Opin Drug Deliv. 2014;11:211-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Vishwakarma N, Jain A, Sharma R, Mody N, Vyas S, Vyas SP. Lipid-Based Nanocarriers for Lymphatic Transportation. AAPS PharmSciTech. 2019;20:83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Juneja P, Tripathi DM, Kaur S. Revisiting the gut-liver axis: gut lymphatic system in liver cirrhosis and portal hypertension. Am J Physiol Gastrointest Liver Physiol. 2022;322:G473-G479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Ribera J, Pauta M, Melgar-Lesmes P, Tugues S, Fernández-Varo G, Held KF, Soria G, Tudela R, Planas AM, Fernández-Hernando C, Arroyo V, Jiménez W, Morales-Ruiz M. Increased nitric oxide production in lymphatic endothelial cells causes impairment of lymphatic drainage in cirrhotic rats. Gut. 2013;62:138-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Alshikho MJ, Talas JM, Noureldine SI, Zazou S, Addas A, Kurabi H, Nasser M. Intestinal Lymphangiectasia: Insights on Management and Literature Review. Am J Case Rep. 2016;17:512-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Vignes S, Bellanger J. Primary intestinal lymphangiectasia (Waldmann's disease). Orphanet J Rare Dis. 2008;3:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (37)] |
| 20. | Hokari R, Kitagawa N, Watanabe C, Komoto S, Kurihara C, Okada Y, Kawaguchi A, Nagao S, Hibi T, Miura S. Changes in regulatory molecules for lymphangiogenesis in intestinal lymphangiectasia with enteric protein loss. J Gastroenterol Hepatol. 2008;23:e88-e95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (3)] |
| 21. | Le Bougeant P, Delbrel X, Grenouillet M, Leou S, Djossou F, Beylot J, Lebras M, Longy-Boursier M. [Familial Waldmann's disease]. Ann Med Interne (Paris). 2000;151:511-512. [PubMed] |
| 22. | Rao R, Shashidhar H. Intestinal lymphangiectasia presenting as abdominal mass. Gastrointest Endosc. 2007;65:522-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Macdonald J, Porter V, Scott NW, McNamara D. Small bowel lymphangiectasia and angiodysplasia: a positive association; novel clinical marker or shared pathophysiology? J Clin Gastroenterol. 2010;44:610-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Perisic VN, Kokai G. Coeliac disease and lymphangiectasia. Arch Dis Child. 1992;67:134-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Makharia GK, Tandon N, Stephen Nde J, Gupta SD, Tandon RK. Primary intestinal lymphangiectasia as a component of autoimmune polyglandular syndrome type I: a report of 2 cases. Indian J Gastroenterol. 2007;26:293-295. [PubMed] [DOI] [Full Text] |
| 26. | Freeman HJ, Nimmo M. Intestinal lymphangiectasia in adults. World J Gastrointest Oncol. 2011;3:19-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (39)] |
| 27. | Freeman HJ. Tropheryma whipplei infection. World J Gastroenterol. 2009;15:2078-2080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Laharie D, Degenne V, Laharie H, Cazorla S, Belleannee G, Couzigou P, Amouretti M. Remission of protein-losing enteropathy after nodal lymphoma treatment in a patient with primary intestinal lymphangiectasia. Eur J Gastroenterol Hepatol. 2005;17:1417-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Lerner TR, de Souza Carvalho-Wodarz C, Repnik U, Russell MR, Borel S, Diedrich CR, Rohde M, Wainwright H, Collinson LM, Wilkinson RJ, Griffiths G, Gutierrez MG. Lymphatic endothelial cells are a replicative niche for Mycobacterium tuberculosis. J Clin Invest. 2016;126:1093-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 30. | Arya R, Kumar R, Kumar T, Kumar S, Anand U, Priyadarshi RN, Maji T. Prevalence and risk factors of lymphatic dysfunction in cirrhosis patients with refractory ascites: An often unconsidered mechanism. World J Hepatol. 2023;15:1140-1152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Kim JH, Bak YT, Kim JS, Seol SY, Shin BK, Kim HK. Clinical significance of duodenal lymphangiectasia incidentally found during routine upper gastrointestinal endoscopy. Endoscopy. 2009;41:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Cholet C, Delalandre C, Monnier-Cholley L, Le Pimpec-Barthes F, El Mouhadi S, Arrivé L. Nontraumatic Chylothorax: Nonenhanced MR Lymphography. Radiographics. 2020;40:1554-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 33. | Kumar R, Anand U, Priyadarshi RN. Lymphatic dysfunction in advanced cirrhosis: Contextual perspective and clinical implications. World J Hepatol. 2021;13:300-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Alfano V, Tritto G, Alfonsi L, Cella A, Pasanisi F, Contaldo F. Stable reversal of pathologic signs of primitive intestinal lymphangiectasia with a hypolipidic, MCT-enriched diet. Nutrition. 2000;16:303-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Kuroiwa G, Takayama T, Sato Y, Takahashi Y, Fujita T, Nobuoka A, Kukitsu T, Kato J, Sakamaki S, Niitsu Y. Primary intestinal lymphangiectasia successfully treated with octreotide. J Gastroenterol. 2001;36:129-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Hu S, Wu X, Xu W, Tian X, Yang Y, Wang ST, Liu S, Xu X, Xu KF. Long-term efficacy and safety of sirolimus therapy in patients with lymphangioleiomyomatosis. Orphanet J Rare Dis. 2019;14:206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 37. | Jøorgensen KA, Stoffersen E. Heparin like activity of albumin. Thromb Res. 1979;16:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Castell JV, Gómez-Lechón MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990;12:1179-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 539] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 39. | Hallevy C, Sperber AD, Almog Y. Group G streptococcal empyema complicating primary intestinal lymphangiectasia. J Clin Gastroenterol. 2003;37:270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Waldman T, Strober W, Blaese R. Immunodeficiency Disease and Malignancy: Various Immunologic Deficiencies of Man and the Role of Immune Processes in the Control of Malignant Disease. Ann Intern Med. 1972;77:605-628. [DOI] [Full Text] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/