Published online Jun 16, 2024. doi: 10.12998/wjcc.v12.i17.2917
Revised: January 30, 2024
Accepted: May 14, 2024
Published online: June 16, 2024
Processing time: 208 Days and 2.2 Hours
Following the withdrawal of paraquat, diquat (DQ) has emerged as the predo
Core Tip: Based on our team's extensive experience and accumulated evidence in treating fulminant diquat (DQ) intoxication, we believe that endothelial cell injury plays a crucial role in the organ damage caused by DQ poisoning. However, there is currently a lack of reports on the role of endothelial cell injury in the development of multiple organ dysfunction syndrome induced by fulminant DQ poisoning. Some studies have observed vascular endothelium injury after DQ poisoning, but vascular endothelial cell is not the main focus of these studies.
- Citation: Cen XY, Chen Y, Xu YA, Zhong GY. Vascular endothelium, a promising target for effectively treating fulminant diquat intoxication? World J Clin Cases 2024; 12(17): 2917-2920
- URL: https://www.wjgnet.com/2307-8960/full/v12/i17/2917.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i17.2917
Fulminant diquat (DQ) poisoning is characterized by its rapid progression, leading to multiple organ failure, posing significant challenges in treatment, and resulting in a high mortality rate.
DQ is a non-selective, fast-acting herbicide belonging to the same class of compounds as paraquat. Its chemical structure name is 1, 1'-ethylene-2, 2'-bipyridine[1]. In China, the sale and use of paraquat have been strictly prohibited since 2016, leading to DQ occupying a significant market share in the herbicide industry and becoming a commonly used herbicide. DQ intoxication is characterized by a clear dose-response relationship, and the clinical condition can be categorized based on the amount of DQ ingested. Mild poisoning is defined as ingestion of less than 9.35 mL of 100 g DQ dibromide/500 mL, while moderate to severe poisoning is categorized as ingestion between 9.35-112.20 mL. Fulminant poisoning is classified as ingestion of more than 112.20 mL. Limited research has been conducted on DQ poisoning in China, with one literature review writen in Chinese reporting 708 cases of DQ poisoning in recent years. The analysis of this data revealed a mortality rate of 40.93% for oral DQ poisoning, and an even higher mortality rate of 84.44% is associated with DQ poisoning resulting from ingestion of over 100ml. Another study documented all the patients with fulminant DQ poisoning suffered to death[2]. Most victims were deceased within 48 h after contacting DQ, and their rapid deterioration made it challenging to reverse with clinical intervention such as gastric lavage, antioxidant therapy, free radical clearance and blood purification. DQ intoxication can cause severe damage to multiple organs and tissues throughout the body, particularly impacting the kidneys, brain, circulatory system, lungs, and digestive system[2]. Fulminant DQ poisoning often leads to brain, lung and circulatory failure, which are the main causes of death[2,3]. Clinicians currently face severe challenges in treating DQ poisoning, especially fulminant cases, due to its rapid onset, systemic damage and high mortality. The limited knowledge of the mechanism of tissue damage induced by fulminant DQ poisoning has resulted in a lack of precise diagnostic and treatment strategy. Therefore, there is a urgent request for more research focused on the mechanism of poisoning in fulminant DQ cases.
Reversing multiple organ dysfunction syndrome (MODS) induced by fulminant DQ poisoning is a challenging task despite receiving multimodality treatments with antioxidant therapy and blood purification.
DQ, when ingested, is primarily absorbed through the gastrointestinal system, with only a small percentage (5%-10%) being taken up. Once in the body, approximately half of the DQ is excreted through the kidneys, while about 5% of ingestion remains in circulation and tissues[1]. The half-life of DQ can be as long as 13.1 h[4], leading to a broad spectrum of toxic effects. Clinical observations have shown that fulminant DQ poisoning often leads to severe and irreversible MODS, with a specific sequence of organ failure: Kidney-brain-circulation-lung. Previous studies on DQ poisoning have revealed that its main toxic effect is oxidative stress damage. DQ generated oxidative reaction resulted in histological injury [1]. This has been observed and confirmed in various organs and tissues, including renal tubules[5], intestinal epithelium[6], alveolar [7], and plasma [8]. DQ promoted organism injury by initiating inflammation[9], inhibiting cellular mitochondrial respiratory[10], and other pathways. Based on this understanding, glucocorticoids had anti-inflammatory properties against DQ injury in the kidneys[11], while glycine[12], and vitamin D3[13] exhibit good antioxidant effects and help to maintain the integrity of intestinal barrier after DQ poisoning. Pterostilbene, on the other hand, protects the liver through its antioxidant action[14]. However, in the clinical treatment of moderate to severe DQ poisoning, especially in cases of fulminant poisoning, antioxidant therapy and blood purification strategies have shown limited effectiveness, particularly in the absence of specific antidotes.
The vascular endothelial cell (VEC) may play a crucial role in the development of MODS resulting from fulminant DQ poisoning.
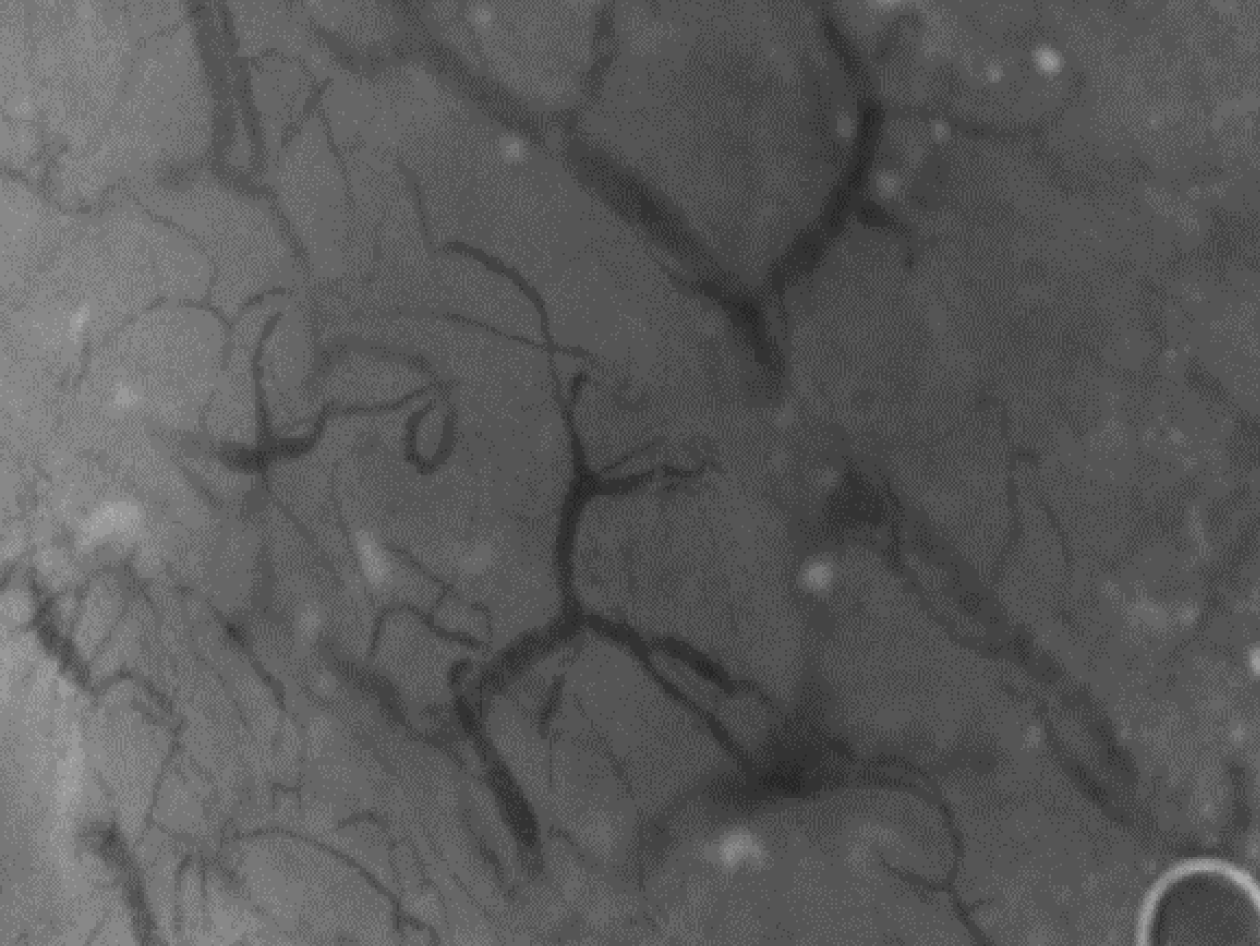
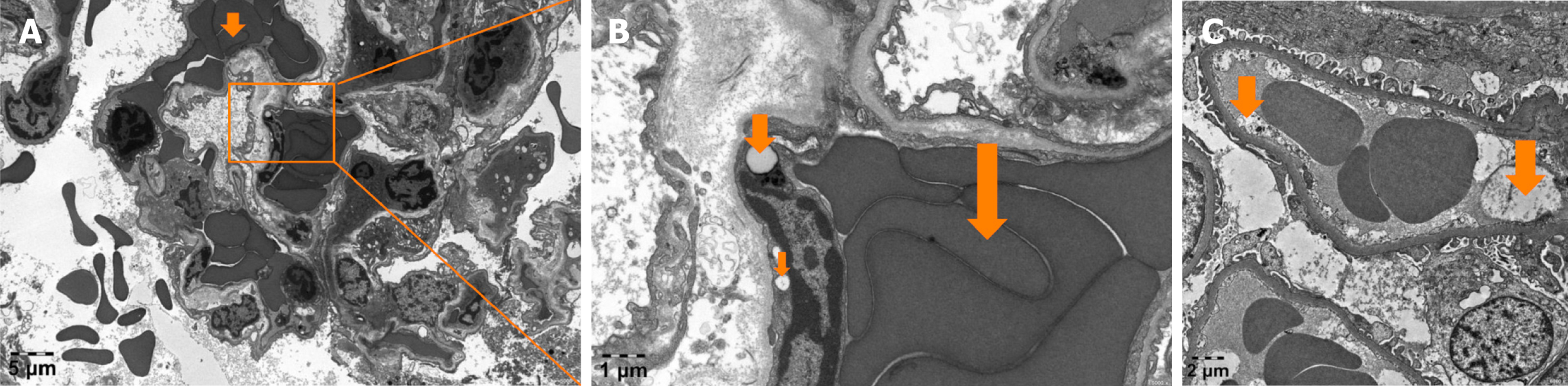
MODS induced by fulminant DQ poisoning is frequently observed in vital organs such as the kidneys, brain, circulatory system, and lungs. There seems to be consistent pattern of events, yet the underlying connections remain elusive due to a vacuum of research. However, based on our team's extensive experience and accumulated evidence in treating fulminant DQ intoxication, we believe that endothelial injury plays a crucial role in the organ damage. Here are the reasons supporting our belief: First, MODS caused by DQ poisoning tends to affect tissues with abundant microcirculation and endothelial glycocalyx, such as the kidneys, lungs, and brain. Second, we have observed severe microcirculatory dysfunction in the early stages of fulminant DQ poisoning using side-stream dark field technique (Figure 1). Third, post-mortem biopsies of fulminant DQ poisoning cases have revealed significant damage to endothelial cells (EC) in kidney and lung tissues (Figure 2). However, currently there is rare research on the role of VEC injury in the development of MODS induced by fulminant DQ poisoning. Some studies have observed and reported possible evidence of local EC injury after DQ poisoning, but systemic VEC is not the main focus of these studies[11,15].
In cases of fulminant DQ poisoning, the presence of DQ can lead to organ failure in the kidneys, brain, circulation and lungs. This may occur due to oxidative stress damage and apoptosis of VEC. It is essential to conduct scientific research on VEC to better understand their role in the development of MODS in fulminant DQ poisoning. This achivement can enhance our understanding of the mechanisms behind MODS, identify new targets for treatment, and improve clinical strategies for managing fulminant DQ poisoning. Additionally, it can offer improved protocols for standardized diagnosis and treatment, ultimately leading to higher treatment success rates and reduced mortality and disability rates associated with fulminant DQ poisoning.
| 1. | Magalhães N, Carvalho F, Dinis-Oliveira RJ. Human and experimental toxicology of diquat poisoning: Toxicokinetics, mechanisms of toxicity, clinical features, and treatment. Hum Exp Toxicol. 2018;37:1131-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (1)] |
| 2. | Meng N, Sun Y, Liu L, Yao D, Gao H, Ma Y, Jin Y, Dong Y, Zhu T, Tian Y. Clinical features of 86 cases of acute diquat poisoning. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2022;34:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (1)] |
| 3. | Zhou JN, Lu YQ. Lethal diquat poisoning manifests as acute central nervous system injury and circulatory failure: A retrospective cohort study of 50 cases. EClinicalMedicine. 2022;52:101609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 4. | Mao Z, Yu Y, Sun H, Wu C, Jiang Q, Chu C, Zhao C, Zhou Y, Zhang J, Cao Y, Chen F. Simultaneous determination of diquat and its two primary metabolites in rat plasma by ultraperformance liquid chromatography-tandem mass spectrometry and its application to the toxicokinetic study. Forensic Toxicol. 2022;40:332-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 5. | Rogers LK, Bates CM, Welty SE, Smith CV. Diquat induces renal proximal tubule injury in glutathione reductase-deficient mice. Toxicol Appl Pharmacol. 2006;217:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 6. | Jin Y, Zhai Z, Jia H, Lai J, Si X, Wu Z. Kaempferol attenuates diquat-induced oxidative damage and apoptosis in intestinal porcine epithelial cells. Food Funct. 2021;12:6889-6899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 7. | Coulombe P, Filion PR, Côté MG. Ready evaluation of lung alveolar toxic damage with histological sections morphometry. Toxicol Lett. 1984;20:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 8. | Liu Y, Yang Y, Dong H, Cutler RG, Strong R, Mattson MP. Thidoredxin-2 overexpression fails to rescue chronic high calorie diet induced hippocampal dysfunction. Exp Neurol. 2016;275 Pt 1:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 9. | Xu Q, Liu M, Chao X, Zhang C, Yang H, Chen J, Zhou B. Stevioside Improves Antioxidant Capacity and Intestinal Barrier Function while Attenuating Inflammation and Apoptosis by Regulating the NF-κB/MAPK Pathways in Diquat-Induced Oxidative Stress of IPEC-J2 Cells. Antioxidants (Basel). 2023;12:1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (1)] |
| 10. | Choi SE, Park YS, Koh HC. NF-κB/p53-activated inflammatory response involves in diquat-induced mitochondrial dysfunction and apoptosis. Environ Toxicol. 2018;33:1005-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 11. | Cui S, Zhang X, Wang C, Sun C, Shi L, Kan B, Li Q, Jian X. Study on the therapeutic effect of glucocorticoids on acute kidney injury in rats exposed to diquat. Biomed Pharmacother. 2023;166:115310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (1)] |
| 12. | Xu X, Wei Y, Hua H, Zhu H, Xiao K, Zhao J, Liu Y. Glycine Alleviated Intestinal Injury by Inhibiting Ferroptosis in Piglets Challenged with Diquat. Animals (Basel). 2022;12:3071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (1)] |
| 13. | Zhang H, Liu Y, Fang X, Gu L, Luo C, Chen L, Wang Q. Vitamin D(3) Protects Mice from Diquat-Induced Oxidative Stress through the NF-κB/Nrf2/HO-1 Signaling Pathway. Oxid Med Cell Longev. 2021;2021:6776956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 14. | Chen Y, Chen Y, Zhang H, Wang T. Pterostilbene as a protective antioxidant attenuates diquat-induced liver injury and oxidative stress in 21-day-old broiler chickens. Poult Sci. 2020;99:3158-3167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 15. | Atkinson JB, Hill KE, Burk RF. Centrilobular endothelial cell injury by diquat in the selenium-deficient rat liver. Lab Invest. 2001;81:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/