Published online Jun 6, 2024. doi: 10.12998/wjcc.v12.i16.2713
Revised: April 18, 2024
Accepted: April 19, 2024
Published online: June 6, 2024
Processing time: 85 Days and 3.1 Hours
Photoaging, a result of chronic sun exposure, leads to skin damage and pigmen
To evaluate the efficacy and safety of intradermal type Ι collagen (Col Ι) injection for treating photoaging.
This prospective, self-controlled study investigated the impact of intradermal injections of Col Ι on skin photodamage in 20 patients from the Yunnan Province. Total six treatment sessions were conducted every 4 wk ± 3 d. Before and after each treatment, facial skin characteristics were quantified using a VISIA skin detector. Skin thickness data were assessed using the ultrasound probes of the Dermalab skin detector. The Face-Q scale was used for subjective evaluation of the treatment effect by the patients.
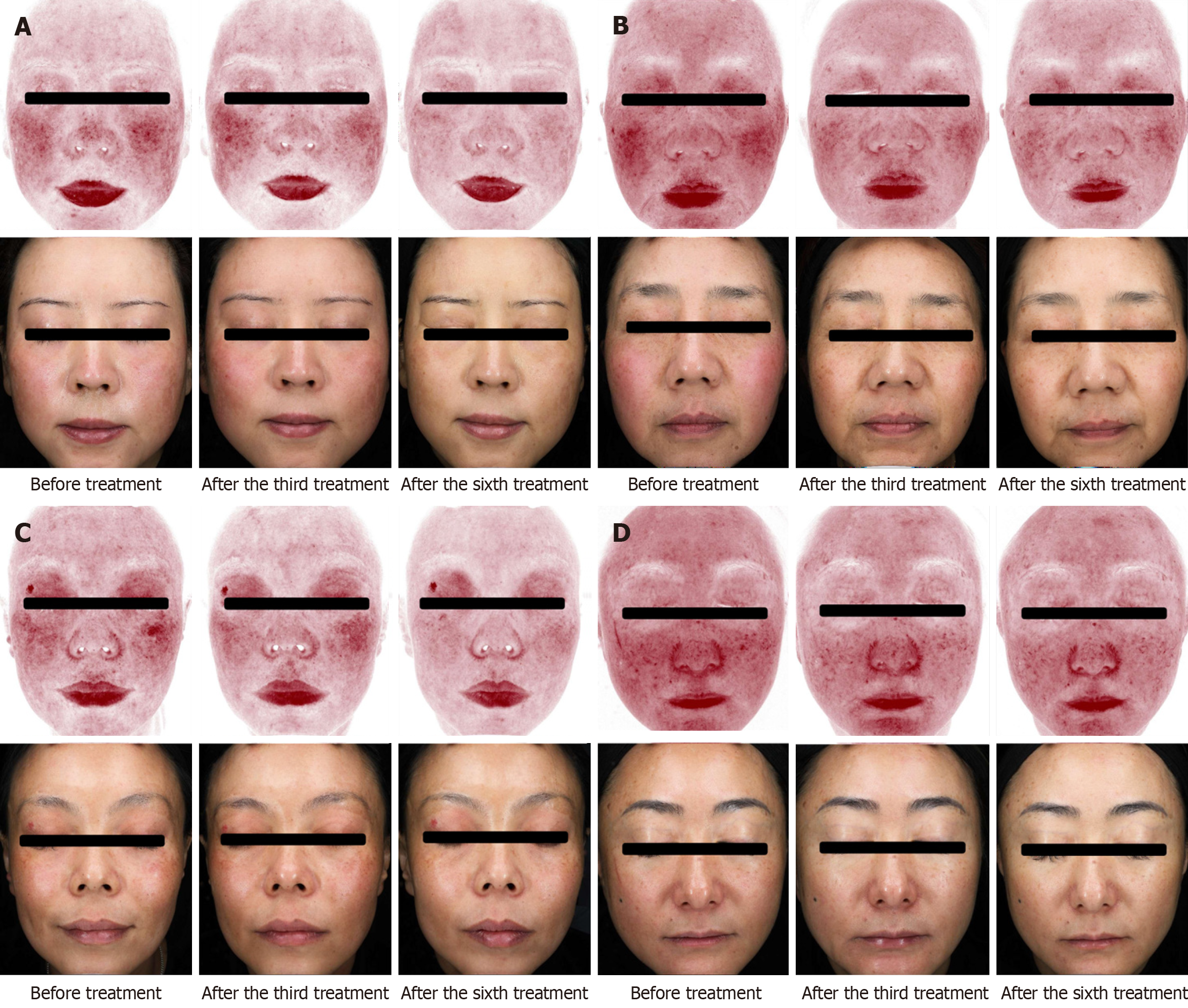
The skin thickness of the right cheek consistently increased after each treatment session compared with baseline. The skin thickness of the left cheek significantly increased after the third through sixth treatment sessions compared with baseline. The skin thickness of the right zygomatic region increased after the second to sixth treatment sessions, whereas that of the left zygomatic region showed a significant increase after the fourth through sixth treatment sessions. The skin thickness of both temporal regions significantly increased after the fifth and sixth treatment sessions compared with baseline (P < 0.05). These findings were also supported by skin ultrasound images. The feature count for the red areas and wrinkle feature count decreased following the treatment (P < 0.05). VISIA assessments also revealed a decrease in the red areas after treatment. The Face-Q-Satisfaction with Facial Appearance Overall and Face-Q-Satisfaction with Skin scores significantly increased after each treatment session. The overall appearance of the patients improved after treatment.
Intradermal Col Ι injection improves photoaging, with higher patient satisfaction and fewer adverse reactions, and could be an effective treatment method for populations residing in high-altitude areas.
Core Tip: Intradermal injections of type I collagen show promise in improving skin quality and treating photoaging in high-altitude regions like Yunnan. Using a negative pressure electronic injection device, the treatment enhances skin thickness, reduces redness and wrinkles, and leads to high patient satisfaction with minimal adverse effects. This approach addresses key manifestations of photoaging in high-altitude populations and could provide an effective, minimally invasive therapeutic option.
- Citation: Yang B, He A, Bu BB, Zhuo G, Zhou QZ, He JH, Liu L, Huang WL, Zhao X. Clinical efficacy of intradermal type I collagen injections in treating skin photoaging in patients from high-altitude areas. World J Clin Cases 2024; 12(16): 2713-2721
- URL: https://www.wjgnet.com/2307-8960/full/v12/i16/2713.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i16.2713
Skin photoaging, also known as exogenous aging, is mainly caused by ultraviolet (UV) radiation, which accounts for 80% of the skin-aging-inducing external factors[1]. UV radiations damage the structure and function of collagen (Col; I and III), the major component of the dermal layer, and accelerate skin aging through a series of complex molecular and cellular mechanisms[2-5]. Clinically, its manifestations include skin dryness and roughness, capillary dilation, increased wrinkles, reduced elasticity, and pigmentation. Notably, some patients may develop skin tumors, manifesting as thinning of all levels of the skin and changes in skin structure, accompanied by decreased synthesis and proliferation and changes in the Col I and III ratio in the extracellular matrix (ECM) of the dermis layer[6]. Currently, plant-derived anti-photoaging formulations[7-9], vitamin supplementation[10], chemical peel treatments, laser therapy[11,12], radiofrequency mi
The study has obtained ethical approval from the Ethics Committee of the First People's Hospital of Kunming City and signed informed consent forms. enrolled 20 patients who sought treatment for skin photoaging at our department between June 2021 and April 2022 and met the inclusion and exclusion criteria (Table 1). Among them, 19 were female (95%), and 1 was male (5%). The mean age was 46.4 years, ranging from 36 to 54 years.
| Inclusion criteria | Exclusion criteria |
| Age ≥ 18 yr, male or female | Pregnancy or lactation |
| Glogau photoaging score of II or III | Systemic diseases, such as diabetes, autoimmune diseases, and severe heart, liver, and kidney dysfunction |
| Residing in areas with an altitude of ≥ 1800 m for > 10 yr | Current treatment with glucocorticoids, estrogen, and other drugs that may cause facial pigmentation and affect the experimental results |
| Voluntarily receiving intradermal injection of Sunmax Col for facial rejuvenation and signing informed consent forms | Facial laser, freezing, and other treatments within the past 3 months |
| Facial filling or botulinum toxin injection within the past 6 months | |
| Known allergies to Col or other ingredients in Sunmax Col | |
| Failure to follow up as planned or data loss affecting the evaluation of efficacy |
Treatment procedure and precautions: In this study, we used a Derma Shine Generation I negative pressure electron injection device to administer a full-face Sunmax Col injection to all patients. The procedure was as follows: (1) After cleaning their skin, patients laid down in the supine position, and the operator applied topical anesthesia to their faces. After 30 min, the anesthesia was removed, and the faces of the patients were disinfected with an iodine III skin disinfectant; (2) A 2.5 mL of diluted Col mixture was prepared by the operator by mixing 1.5 mL of 0.9% normal saline (NS) with 1 mL of Sunmax Col (Furoumei) drawn using a disposable sterile syringe (2.5 mL), which was connected to a three-way tube for injection; (3) The operator wiped off the iodine from the faces of the patients using 0.9% NS; (4) The operator added a 2.5-mL Col mixture into the Derma Shine Generation I negative pressure electron injection device and set the parameters (dose per injection, 0.025 mL; injection speed, slow; negative pressure suction, 10%; and retraction force, 80%). Next, the operator injected the mixture evenly into the face of the patient in the following order: bilateral cheeks, forehead, periorbital area, and perioral area. The injection depth for the cheeks was generally 0.8-1.0 mm, and that for the forehead, periorbital area, and perioral area was generally 0.6-0.8 mm. Notably, the injection depth was adjusted according to the skin condition and pain tolerance of the patient; and (5) After the procedure, the medical cold com
Treatment effect evaluation (VISIA facial skin imaging analyzer and Dermalab SkinLab Combo ultrasound): For objective evaluation, we used a VISIA facial skin imaging analyzer and a Dermalab SkinLab Combo ultrasound probe to collect objective data from the patients[15,16]. Each patient received six treatments, with an interval of 4 wk ± 3 d between treatments. Before treatment and 2 wk ± 3 d after each treatment, eight indicators of facial skin quality—surface spots, wrinkles, texture, pores, UV spots, brown spots, red areas, and porphyrins—were analyzed using the VISIA facial skin imaging analyzer. The Dermalab SkinLab Combo ultrasound probe indirectly reflects the density of the subcutaneous Col, enabling the measurement of the skin thickness of the bilateral cheeks, temporal regions, and malar regions.
Face-Q scale self-evaluation: For the subjective evaluation, we selected seven Face-Q subscales: Face-Q-Satisfaction with Facial Appearance Overall, Face-Q-Satisfaction with Skin, Face-Q-Satisfaction with Outcome, Face-Q-Satisfaction with Decision, Face-Q-Social Function, Face-Q-Psychological Function, and Face-Q-Adverse Effects: Skin. The patients self-evaluated Face-Q-Satisfaction with Facial Appearance Overall and Face-Q-Satisfaction with Skin before treatment and 2 wk ± 3 d after each treatment. Further, the patients were followed up, and the evaluation of the Face-Q-Adverse Effects: Skin was also performed. Face-Q-Satisfaction with Outcome, Face-Q-Satisfaction with Decision, Face-Q-Social Function, and Face-Q-Psychological Function evaluations of all patients were completed after six treatments. Except for the Face-Q Adverse Effects: Skin scale, all other scales range from 0 to 100. A higher score indicates a better outcome (Table 2).
| Scale name | Content |
| Face-Q-Satisfaction with Facial Appearance Overall | Evaluates overall facial appearance |
| Face-Q-Satisfaction with Skin | Evaluates facial skin texture, color, and overall appearance |
| Face-Q-Satisfaction with Outcome | Evaluates the results of facial cosmetic treatments |
| Face-Q-Satisfaction with Decision | Evaluates decision-making regarding facial cosmetic treatments |
| Face-Q-Social Function | Evaluates the impact of facial appearance on social interactions |
| Face-Q-Psychological Function | Evaluates the psychological impact of facial appearance |
| Face-Q-Adverse Effects: Skin | Evaluates adverse reactions of the facial skin after treatment |
Pairwise comparisons between the post- and pre-treatment data after six treatments were performed using paired-sample t-tests to compare the VISIA feature counts, skin thickness, and FACE-Q scales. SPSS 27.0 software was used to analyze the data. P < 0.05 was considered statistically significant.
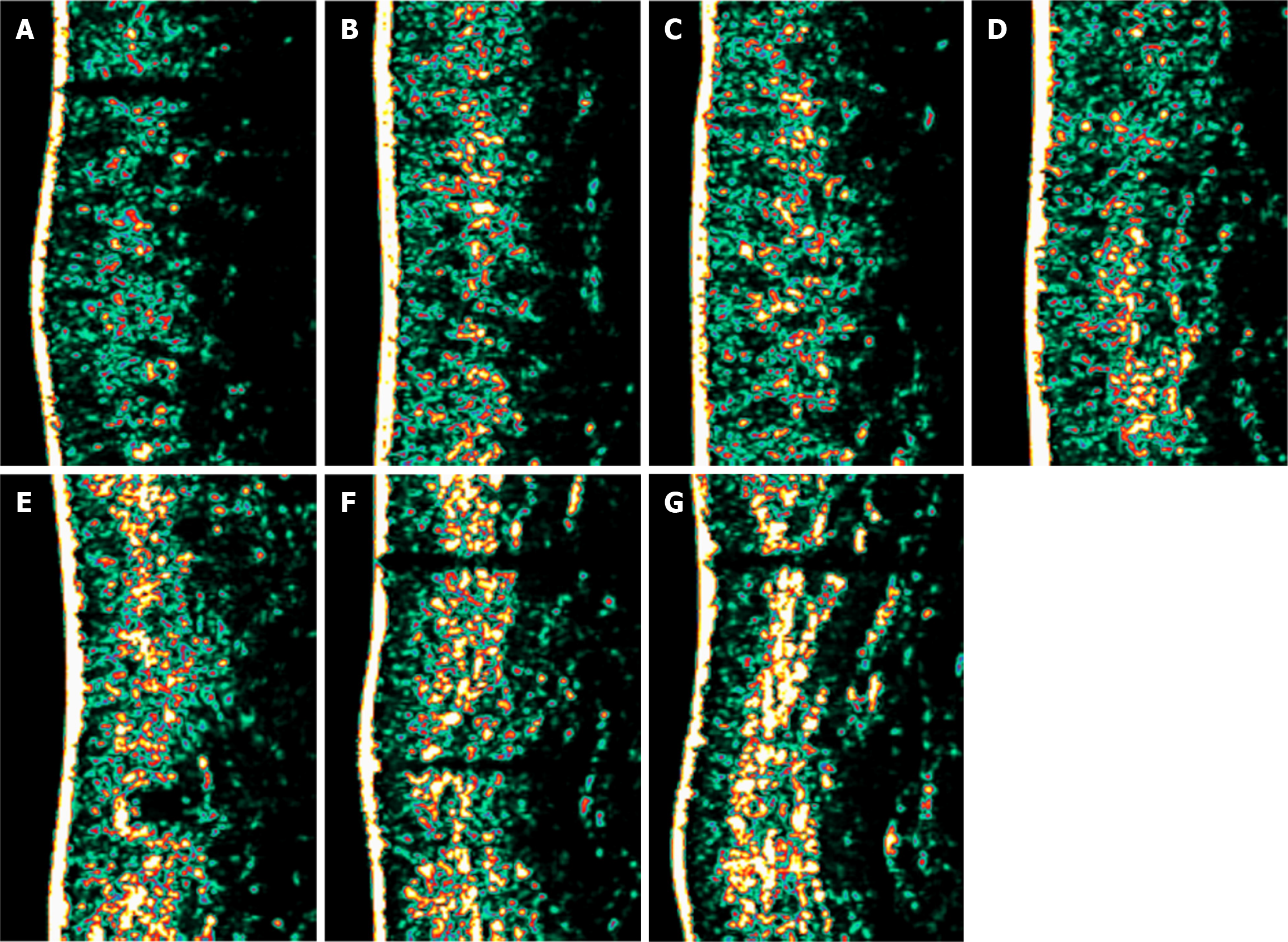
In this study, 20 patients received six treatments each. Notably, the skin thickness of the right cheek increased after each treatment compared with baseline, whereas the skin thickness of the left cheek increased after the third to sixth treatments compared with the previous treatments. The skin thickness of the right zygomatic area increased after the second to sixth treatments compared with the previous treatments, whereas the skin thickness of the left zygomatic area increased after the fourth to sixth treatments compared with the previous treatments. The skin thickness of both temporal areas increased after the fifth and sixth treatments compared with the previous treatments. All differences were statistically significant (P < 0.05). No statistically significant changes in skin thickness were observed for the rest of the treatments (P > 0.05). Skin ultrasound images showed that skin thickness increased after six treatments compared with baseline (Figure 1 and Table 3).
| Baseline | 1st | 2nd | 3rd | 4th | 5th | 6th | |
| Right cheek | 1.65 ± 0.19 | 1.76 ± 0.22a | 1.88 ± 0.19a | 1.98 ± 0.16a | 2.04 ± 0.15a | 2.11 ± 0.17a | 2.15 ± 0.20a |
| Left cheek | 1.62 ± 0.21 | 1.69 ± 0.21 | 1.78 ± 0.19 | 1.90 ± 0.17a | 1.99 ± 0.12a | 2.06 ± 0.13a | 2.12 ± 0.16a |
| Right zygomatic | 1.57 ± 0.18 | 1.67 ± 0.20 | 1.79 ± 0.18a | 1.89 ± 0.16a | 1.96 ± 0.15a | 2.02 ± 0.14a | 2.08 ± 0.17a |
| Left zygomatic | 1.56 ± 0.22 | 1.63 ± 0.24 | 1.75 ± 0.23 | 1.86 ± 0.20 | 1.94 ± 0.18a | 2.01 ± 0.16a | 2.05 ± 0.15a |
| Right temple | 1.48 ± 0.25 | 1.54 ± 0.26 | 1.63 ± 0.24 | 1.75 ± 0.21 | 1.86 ± 0.18 | 1.96 ± 0.17a | 2.00 ± 0.20a |
| Left temple | 1.44 ± 0.24 | 1.52 ± 0.25 | 1.62 ± 0.26 | 1.73 ± 0.23 | 1.83 ± 0.20 | 1.94 ± 0.18a | 1.99 ± 0.16a |
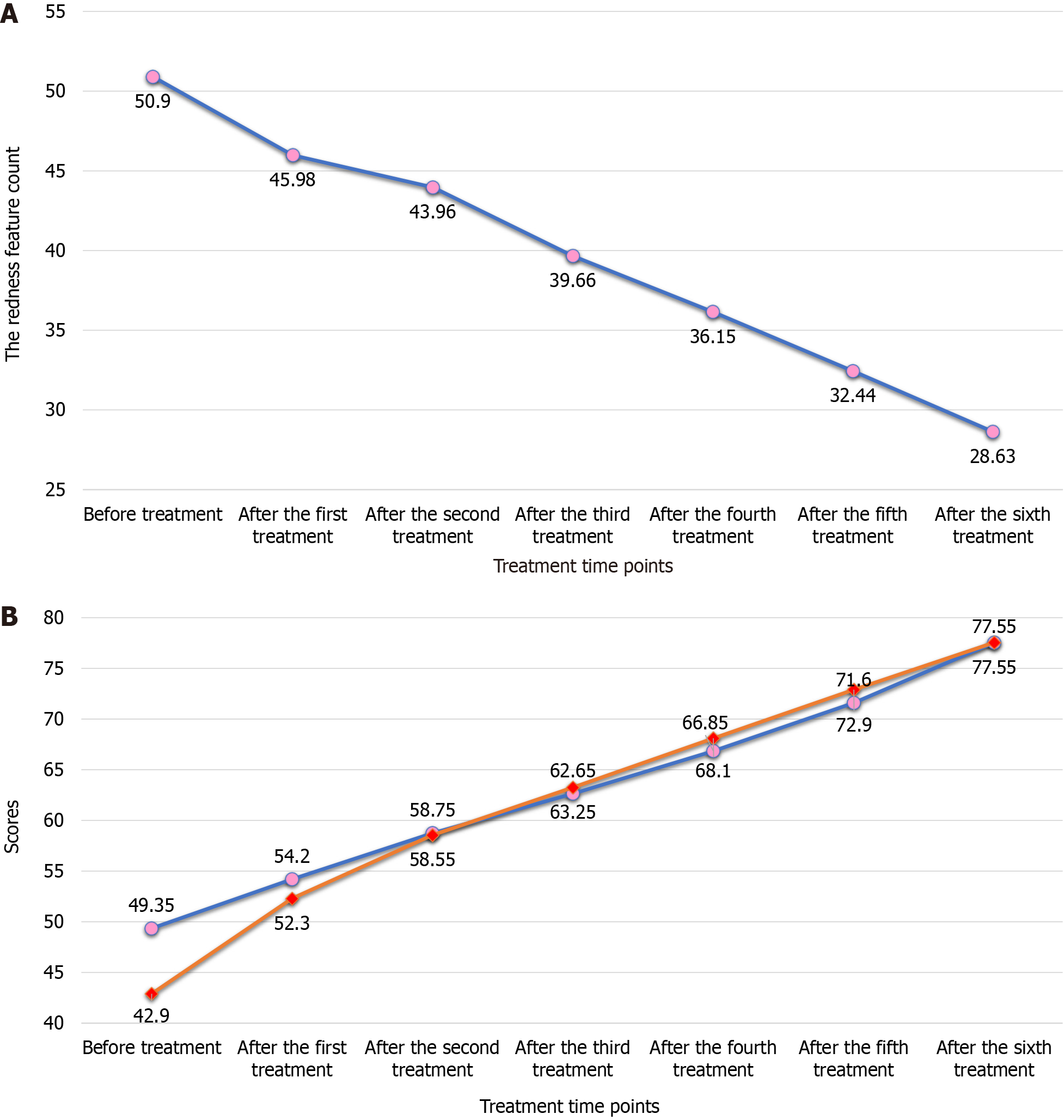
The redness feature count decreased after each of the six treatments compared with baseline (P < 0.05), and redness improved after each treatment compared with the previous treatment (Figure 2 and Table 4). The wrinkle feature count decreased after the sixth treatment compared with the previous treatment (P < 0.05), whereas the other parameter feature counts did not show any statistically significant changes (P > 0.05).
The scores of Face-Q-Satisfaction with Facial Appearance Overall and Face-Q-Satisfaction with Skin increased after each of the six treatments compared with baseline (P < 0.01; Figures 2 and 3, Table 5). The scores of Face-Q-Satisfaction with Outcome, Face-Q-Satisfaction with Decision, Face-Q-Social Function, Face-Q-Psychological Function, and Face-Q-Adverse Effects: Skin were 67.05 ± 15.50, 83.8 ± 14.50, 76.5 ± 8.7, 78.75 ± 7.5, and 13.85 ± 3.82, respectively (Table 6). The overall appearance of the patients improved after treatments.
| Scale | Score |
| Face-Q-Satisfaction with Outcome | 67.05 ± 15.50 |
| Face-Q-Satisfaction with Decision | 83.8 ± 14.50 |
| Face-Q-Social Function | 76.5 ± 8.7 |
| Face-Q-Psychological Function | 78.75 ± 7.5 |
| Face-Q-Adverse Effects: Skin | 13.85 ± 3.82 |
In this study, we enrolled 20 patients living in high-altitude areas for a long time, with Glogau scores of II or III. All the enrolled patients received six treatments and completed all follow-ups. We employed subjective and objective assessment methods to observe the efficacy and safety of the Derma Shine Generation I negative pressure electron injection device for Col I intradermal injections for treating facial cutaneous photoaging in people living in high-altitude regions[17]. We aimed to identify an efficient and cost-effective treatment approach for facial cutaneous photoaging in people living in high-altitude areas. Dermal fillers, such as hyaluronic acid, Col, and other filling materials, can replenish the lost soft tissue volume of the skin and are increasingly used in the treatment of skin photoaging to reduce wrinkles and fine lines of facial skin[18-20]. Currently, microneedle introduction and manual injection are effective methods of Col administration[21]; however, they require operators with operational expertise and clinical experience, and the treatment effects and adverse reaction rates may vary substantially across operators. Consequently, to overcome these problems, we used the Derma Shine Generation I negative pressure electron injection device for the pharmaceutical preparation of Col in this experiment. Using this method, the injection depth, dosage, and speed can be kept consistent, facilitating a more uniform and comfortable injection, ensuring that Col is injected into the dermis, and reducing the wastage of Col reagents. Based on the currently known mechanisms of skin photoaging, we speculated that the mechanisms underlying the impro
However, in our study, wrinkles improved only after the sixth treatment was completed. The reasons for this may be as follows: First, we diluted Col Ι with NS to meet the injection conditions of the negative pressure electronic injector. Second, the injection method involved a uniform injection of the entire face, and the injection was not specifically targeted at the wrinkled area. Notably, the generation of wrinkles also depends on the facial expression of the patient. Therefore, to improve wrinkles, diluting Col and using a negative pressure electronic injection device is not as effective as manual injection of Col. For patients with photoaged skin and notable wrinkles, manual injection combined with electronic injection may achieve better results. Notably, red skin areas and melanin indicators of people in high-altitude areas are higher than those in plain areas[25,26]. Intradermal injection of Col I using a negative pressure electronic injector can improve the symptoms of skin photoaging. Therefore, this treatment method is more suitable for improving skin pho
Intradermal type I collagen injections offer several potential advantages over other preventive and therapeutic measures for cutaneous photoaging. Compared to topical plant-derived or vitamin formulations, injectable collagen may more directly replenish lost dermal collagen. Chemical peels and laser therapy primarily affect the epidermis with less impact on dermal thickness and ectracellular matrix composition. Radiofrequency microneedling and surgical interventions are more invasive with increased risk of adverse effects and downtime. While other injectable treatments like hyaluronic acid fillers can improve wrinkles, they do not directly replace depleted collagen. Intradermal collagen injections may therefore provide a minimally invasive, targeted approach to restoring dermal collagen content and thickness. However, further studies directly comparing intradermal collagen to other treatment modalities are needed to establish its relative efficacy and advantages.
The intradermal injection of Col I improved skin photoaging in patients from high-altitude areas, showing significant enhancement in facial erythema and skin thickness. The patients exhibited high satisfaction levels and minimal adverse reactions, suggesting that this treatment could be an effective method for treating photoaging in populations residing in high-altitude areas.
| 1. | Fuji S, Tanaka K, Kishikawa S, Morita S, Doi M. Quartz crystal microbalance sensor for the detection of collagen model peptides based on the formation of triple helical structure. J Biosci Bioeng. 2022;133:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Flament F, Amar D, Forichon M, Caron J, Negre C. Distinct Habits Of Sun Exposures Lead To Different Impacts On Some Facial Signs Of Chinese Men Of Different Ages. Clin Cosmet Investig Dermatol. 2019;12:833-841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Yun MY, Bae EY, Lee SW, Yim SH, Ly SY, Choi HJ. Anti-photoaging effect of skin cream manufactured with ziyuglycoside I isolated from Sanguisorba officinalis on ultraviolet B-induced hairless mice. Biosci Biotechnol Biochem. 2019;83:1197-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Subedi L, Lee TH, Wahedi HM, Baek SH, Kim SY. Corrigendum to "Resveratrol-Enriched Rice Attenuates UVB-ROS-Induced Skin Aging via Downregulation of Inflammatory Cascades". Oxid Med Cell Longev. 2018;2018:6052623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Xiao J, Liu B, Zhuang Y. Effects of rambutan (Nephelium lappaceum) peel phenolics and Leu-Ser-Gly-Tyr-Gly-Pro on hairless mice skin photoaging induced by ultraviolet irradiation. Food Chem Toxicol. 2019;129:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | El-Sayed MH, Saleh HM, El Zawahry KMA, Mostafa AE. The dermoscopic features of facial aging among Egyptians: A comparative study between males and females. J Cosmet Dermatol. 2019;18:1803-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Kim YH, Cho A, Kwon SA, Kim M, Song M, Han HW, Shin EJ, Park E, Lee SM. Potential Photoprotective Effect of Dietary Corn Silk Extract on Ultraviolet B-Induced Skin Damage. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Nanni V, Canuti L, Gismondi A, Canini A. Hydroalcoholic extract of Spartium junceum L. flowers inhibits growth and melanogenesis in B16-F10 cells by inducing senescence. Phytomedicine. 2018;46:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Kim J, Cho SY, Kim SH, Cho D, Kim S, Park CW, Shimizu T, Cho JY, Seo DB, Shin SS. Effects of Korean ginseng berry on skin antipigmentation and antiaging via FoxO3a activation. J Ginseng Res. 2017;41:277-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Bi Y, Xia H, Li L, Lee RJ, Xie J, Liu Z, Qiu Z, Teng L. Liposomal Vitamin D(3) as an Anti-aging Agent for the Skin. Pharmaceutics. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Yim S, Lee YH, Choi YJ, Kim WS. Split-face comparison of the picosecond 1064-nm Nd:YAG laser using a microlens array and the quasi-long-pulsed 1064-nm Nd:YAG laser for treatment of photoaging facial wrinkles and pores in Asians. Lasers Med Sci. 2020;35:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Yu W, Han Y, Wu X, Shang Y, Ying H, Ma G, Liu Y, Lin X. A split-face randomized controlled trial of treatment with broadband light for enlarged facial pores. J Dermatolog Treat. 2021;32:766-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Hong JY, Kwon TR, Kim JH, Lee BC, Kim BJ. Prospective, preclinical comparison of the performance between radiofrequency microneedling and microneedling alone in reversing photoaged skin. J Cosmet Dermatol. 2020;19:1105-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Maisel-Campbell AL, Ismail A, Reynolds KA, Poon E, Serrano L, Grushchak S, Farid C, West DP, Alam M. A systematic review of the safety and effectiveness of platelet-rich plasma (PRP) for skin aging. Arch Dermatol Res. 2020;312:301-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 15. | Wang X, Shu X, Li Z, Huo W, Zou L, Tang Y, Li L. Comparison of two kinds of skin imaging analysis software: VISIA(®) from Canfield and IPP(®) from Media Cybernetics. Skin Res Technol. 2018;24:379-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Li Z, Koban KC, Schenck TL, Giunta RE, Li Q, Sun Y. Artificial Intelligence in Dermatology Image Analysis: Current Developments and Future Trends. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 77] [Reference Citation Analysis (0)] |
| 17. | Klassen AF, Cano SJ, Scott A, Snell L, Pusic AL. Measuring patient-reported outcomes in facial aesthetic patients: development of the FACE-Q. Facial Plast Surg. 2010;26:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 277] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 18. | Patrick DL, Burke LB, Powers JH, Scott JA, Rock EP, Dawisha S, O'Neill R, Kennedy DL. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health. 2007;10 Suppl 2:S125-S137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 543] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 19. | Aaronson N, Alonso J, Burnam A, Lohr KN, Patrick DL, Perrin E, Stein RE. Assessing health status and quality-of-life instruments: attributes and review criteria. Qual Life Res. 2002;11:193-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1656] [Cited by in RCA: 1763] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 20. | Chiang YZ, Pierone G, Al-Niaimi F. Dermal fillers: pathophysiology, prevention and treatment of complications. J Eur Acad Dermatol Venereol. 2017;31:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 21. | Jeong SY, Park JH, Lee YS, Kim YS, Park JY, Kim SY. The Current Status of Clinical Research Involving Microneedles: A Systematic Review. Pharmaceutics. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Shin D, Lee Y, Huang YH, Lim HW, Jang K, Kim DD, Lim CJ. Probiotic fermentation augments the skin anti-photoaging properties of Agastache rugosa through up-regulating antioxidant components in UV-B-irradiated HaCaT keratinocytes. BMC Complement Altern Med. 2018;18:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Shin D, Lee S, Huang YH, Lim HW, Lee Y, Jang K, Cho Y, Park SJ, Kim DD, Lim CJ. Protective properties of geniposide against UV-B-induced photooxidative stress in human dermal fibroblasts. Pharm Biol. 2018;56:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Shin JW, Kwon SH, Choi JY, Na JI, Huh CH, Choi HR, Park KC. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 476] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 25. | Yang J, Tu Y, Man MQ, Zhang Y, Cha Y, Fan X, Wang Z, Zeng Z, He L. Seasonal variations of epidermal biophysical properties in Kunming, China: A self-controlled cohort study. Skin Res Technol. 2020;26:702-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Zhao C, Wang X, Mao Y, Xu Z, Sun Y, Mei X, Shi W. Variation of biophysical parameters of the skin with age, gender, and lifestyles. J Cosmet Dermatol. 2021;20:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Hsieh CH, Taiwan S-Editor: Lin C L-Editor: A P-Editor: Zhao S