Published online May 26, 2024. doi: 10.12998/wjcc.v12.i15.2627
Revised: March 10, 2024
Accepted: April 9, 2024
Published online: May 26, 2024
Processing time: 109 Days and 17.8 Hours
Multiple endocrine neoplasia type 2 (MEN2) is a rare, autosomal dominant endocrine disease. Currently, the RET proto-oncogene is the only gene implicated in MEN2A pathogenesis. Once an RET carrier is detected, family members should be screened to enable early detection of medullary thyroid carcinoma, pheochro
Herein, we present RET proto-oncogene mutations, clinical characteristics, and treatment strategies in a family with MEN2A. A family study was conducted on patients diagnosed with MEN2A. DNA was extracted from the peripheral blood of family members, and first-generation exon sequencing of the RET proto-oncogene was conducted. The C634Y mutation was identified in three family members spanning three generations. Two patients were sequentially diagnosed with pheochromocytomas and bilateral medullary thyroid carcinomas. A 9-year-old child harboring the gene mutation was diagnosed with medullary thyroid carcinoma. Surgical resection of the tumors was performed. All family members were advised to undergo complete genetic testing related to the C634Y mutation, and the corresponding treatments administered based on test results and associated clinical guidelines.
Advancements in MEN2A research are important for familial management, assessment of medullary thyroid cancer invasive risk, and deciding surgical timing.
Core Tip: Gene sequencing was performed on an adult Asian female patient with bilateral pheochromocytoma and thyroid carcinoma. A mutation, characterized by a nucleotide (c.T1901A) and amino acid (p.Cys634Tyr) change, was identified, supporting multiple endocrine neoplasia type 2A development. Genetic screening of family members revealed the patient’s father and son are carriers of the mutation. Clinical follow-up direction and treatment plan were guided by genetic testing. The patient and her son were diagnosed with orthotopic medullary thyroid carcinoma, facilitating timely and early detection in the early stage of cancer and potentially improving the survival rate of patients.
- Citation: Zhang HF, Huang SL, Wang WL, Zhou YQ, Jiang J, Dai ZJ. C634Y mutation in RET-induced multiple endocrine neoplasia type 2A: A case report. World J Clin Cases 2024; 12(15): 2627-2635
- URL: https://www.wjgnet.com/2307-8960/full/v12/i15/2627.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i15.2627
Multiple endocrine neoplasia type 2 (MEN2) is a rare, autosomal dominant endocrine disease. It has two distinct subtypes, MEN2A (95%) and MEN2B (5%)[1,2], with an incidence rate of MEN2A 13–24 per 1000000[1,2]. Currently, the RET proto-oncogene is the only gene implicated in the pathogenesis of MEN2A. With a clear correlation between the genotype and phenotype, the RET proto-oncogene serves as an essential diagnostic tool for MEN2A. Managing patients through RET proto-oncogene testing and genetic counseling can not only optimize the prognosis associated with the genotype but also improve the quality of life of the patient's family members.
In this study, we retrospectively analyzed the clinical characteristics and gene mutation status of three generations of a Han Chinese family diagnosed with MEN2A to explore the significance of RET mutations and their relevance for clinical diagnosis and treatment and determine their pathological characteristics.
A 30-year-old woman presented to the hospital with recurring headaches, palpitations, and hyperhidrosis.
The patient presented with paroxysmal headache, palpitation, hyperhidrosis, and a blood pressure of 180-165/95-105 mmHg, without obvious inducement 3 months ago. While these symptoms were slightly relieved after rest, they recurred.
No known drug allergy history was noted. The patient had no surgical history.
The patient experienced menstrual cycles lasting approximately 6 d every 28-30 d. No notable medical conditions in her family history existed, and she had no history of sexually transmitted diseases.
The vital signs were as follows: Temperature of 36.2 °C; respiration of 19 breaths/min; pulse rate of 110 beats/min; and blood pressure of 180/105 mmHg. The patient was alert and oriented, and her vital signs were stable.
Mesonephrinephrine substance (MNS) levels were as follows: 3-methoxytyramine (3-MT) 0.12 nmol/L (< 0.18), methoxyadrenaline (MN) 4.1 nmol/L↑ (≤ 0.50), and methoxynorepinephrine (NMN) 2.36 nmol/L↑ (≤ 0.90). There were no observed abnormalities in blood and urine routine, ion levels, liver and kidney functions, tumor markers, blood lipid levels, five parameters of thyroid function, circadian cortisol rhythm, 24-h urinary cortisol level, and adrenocorticotropic hormone level.
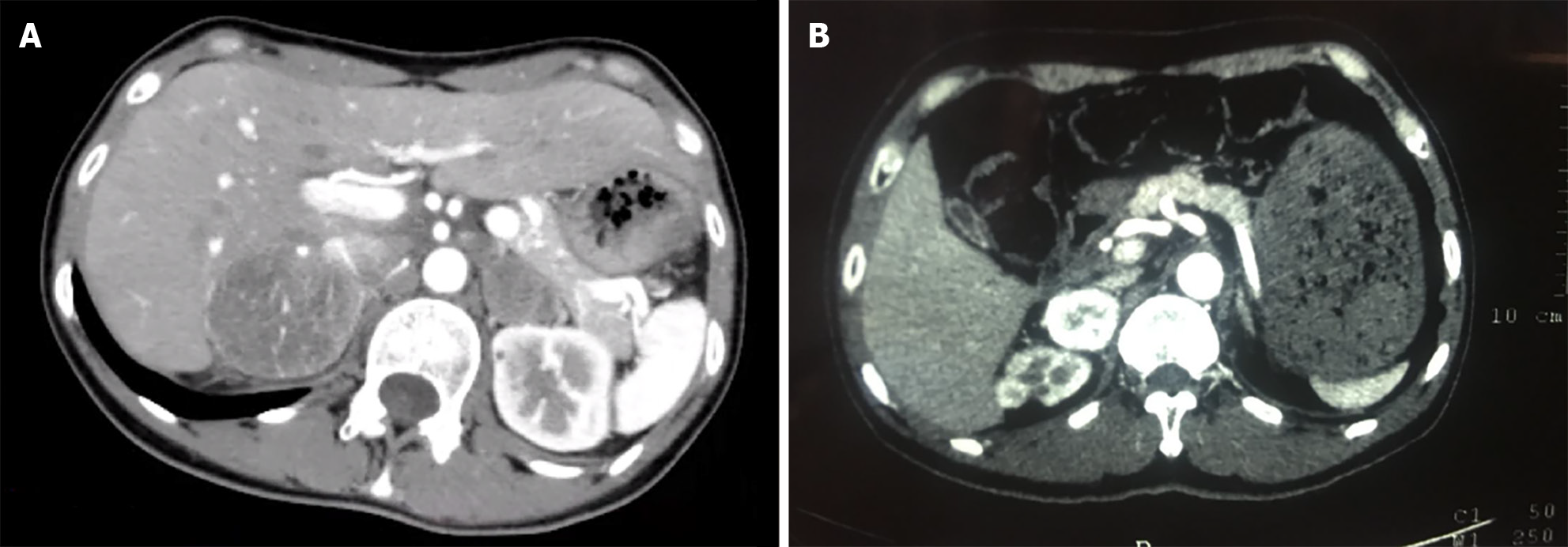
Bilateral adrenal computed tomography (CT): Bilateral adrenal mass occupying lesions (left diameter approximately 29 mm, right diameter approximately 50 mm with a clear boundary, enhanced scan uneven and significantly enhanced, and lower density necrosis within) (Figure 1A).
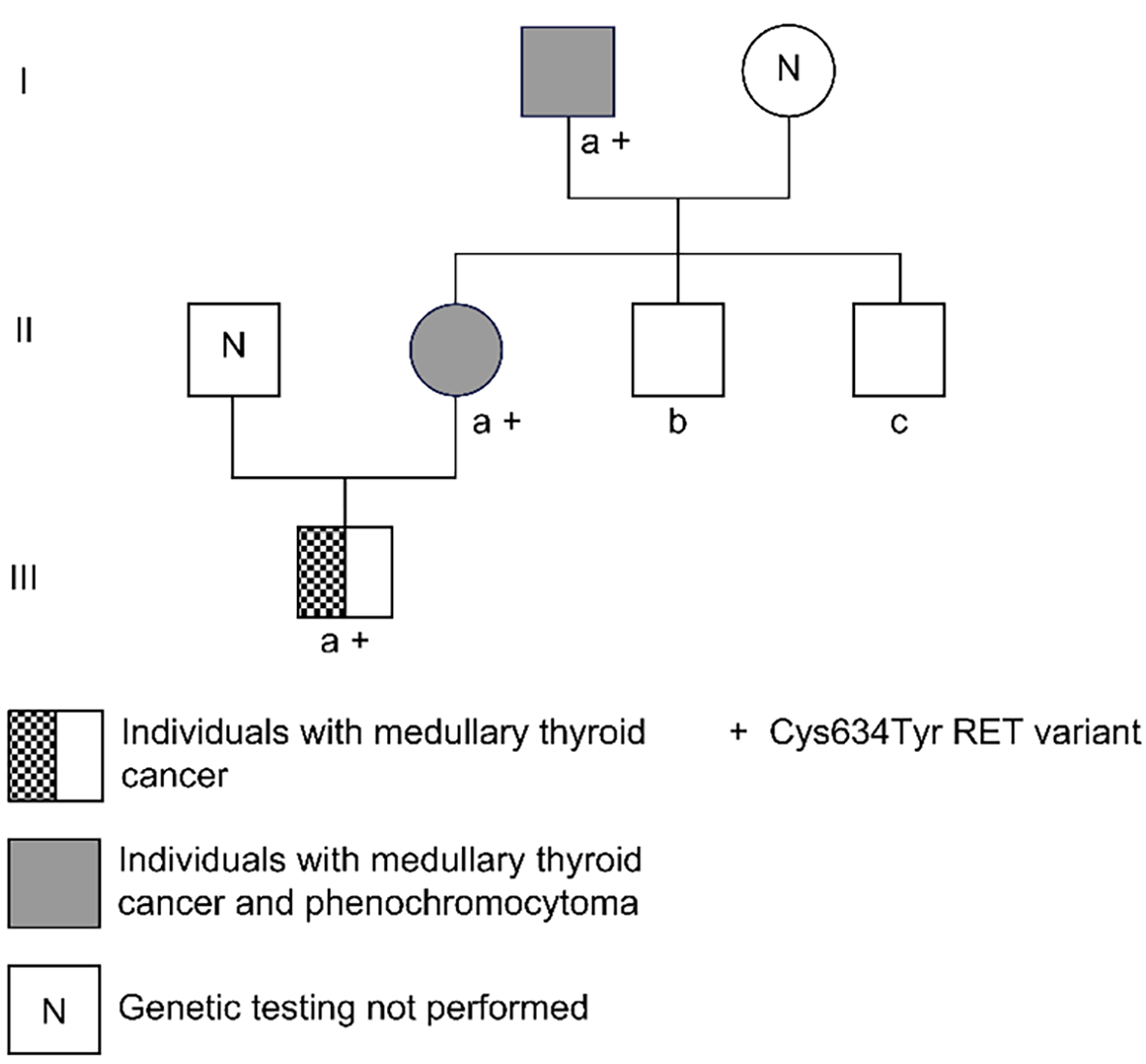
Between 2016 and 2022, a Han Chinese family of three generations and five members with MEN2A were clinically monitored at the Department of Endocrinology, Dongguan TCM Hospital. The family comprised 1 female and 4 male members, ranging in age from 3 to 60 years. This study was approved by the Medical Ethics Committee of Dongguan Hospital of Traditional Chinese Medicine (Approval Number: PJ [2022] 41) and conducted in accordance with the tenets of the Declaration of Helsinki [2013]. Written informed consent for the publication of this report and any accompanying images was obtained from all family members, and legal guardians provided signatures for the children.
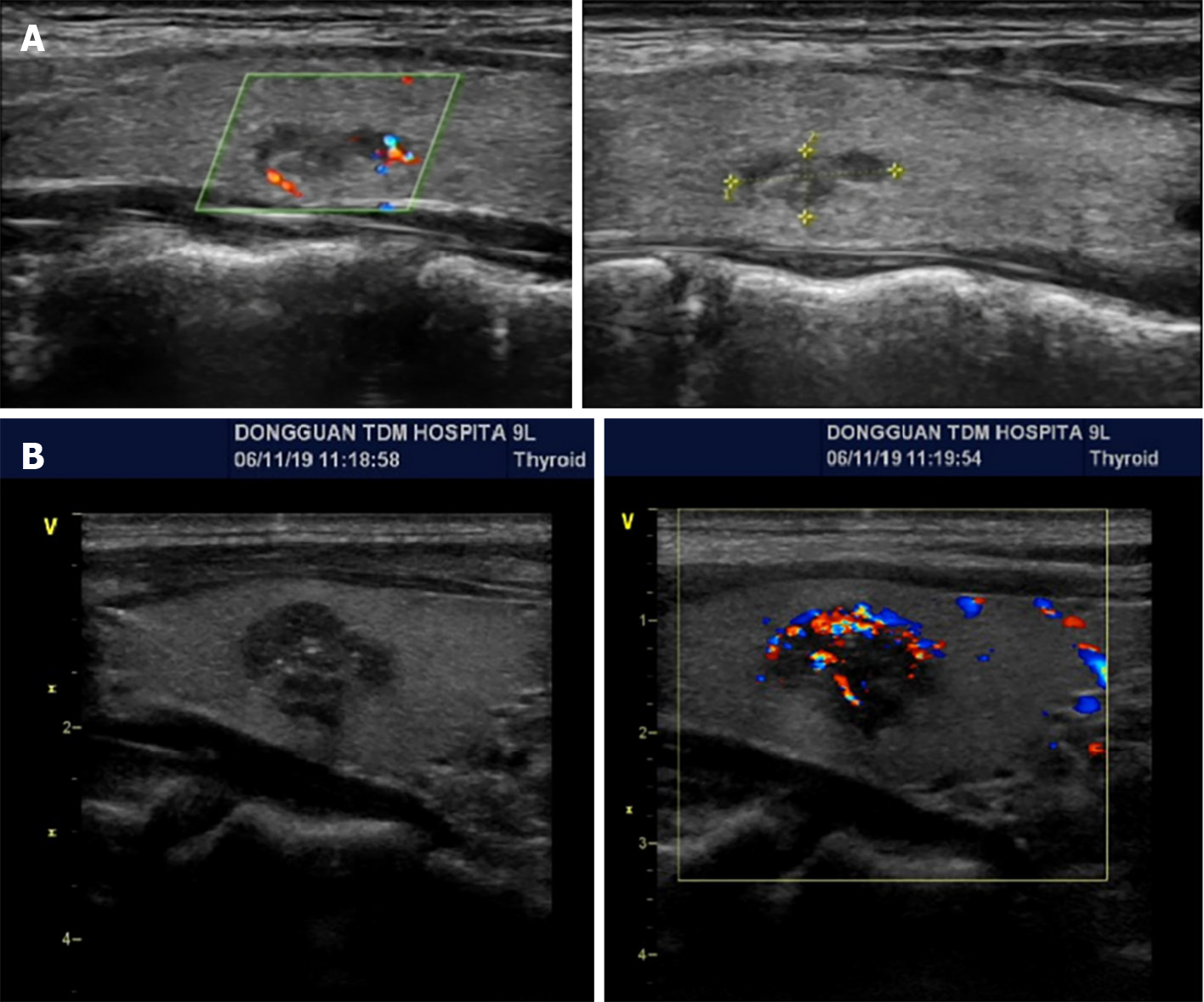
Proband IIa was a 30-year-old female who was hospitalized in August 2016 because of persistent dizziness for over 3 months, coupled with hypertension. The patient's blood pressure was consistently in the range of 180-165/95-105 mmHg. The MNS tests revealed the following: 3-MT level 0.12 nmol/L (< 0.18); MN level 4.1 nmol/L↑ (≤ 0.50); and NMN 2.36 nmol/L↑ (≤ 0.90). The results of further testing, including routine blood and urine tests, ion levels, liver and kidney functions, tumor markers, lipid levels, thyroid function, cortisol rhythm, 24-h urinary cortisol level, and adrenocorticotropic hormone level, were within normal ranges. CT of the adrenal glands revealed space-occupying lesions in the form of bilateral adrenal masses (Figure 1A). Surgery was performed to excise the masses, and enterolysis was also performed. Postoperative pathological examination identified the masses as pheochromocytomas. A thyroid B-ultrasound performed on 10 January 2017 revealed the presence of bilateral thyroid nodules (Figure 2A). The patient's calcitonin level was elevated to 29.49 pg/mL↑ (0-9.52). The parathyroid hormone (PTH) level was determined to be 1.97 pmol/L (1.6-6.9) and the corresponding blood calcium level was 2.41 mmol/L (2-2.5). Thyroglobulin (Tg) was 13.587 ng/mL (0-30). An RET gene test conducted on February 13, 2017 (MM_020975) revealed a clinically significant mutation characterized by a nucleotide (c. T1901A) and amino acid (p.Cys634Tyr) change, supporting MEN2A diagnosis. Considering the results of the patient's gene mutation, the bilateral thyroid masses were diagnosed as medullary thyroid carcinomas. Consequently, a bilateral total thyroidectomy was performed in April 2017. Pathological examination confirmed bilateral medullary thyroid carcinomas. In 2021, uterine fibroids were identified for which hysterectomy was performed. The pathological examination indicated leiomyoma (uterus).
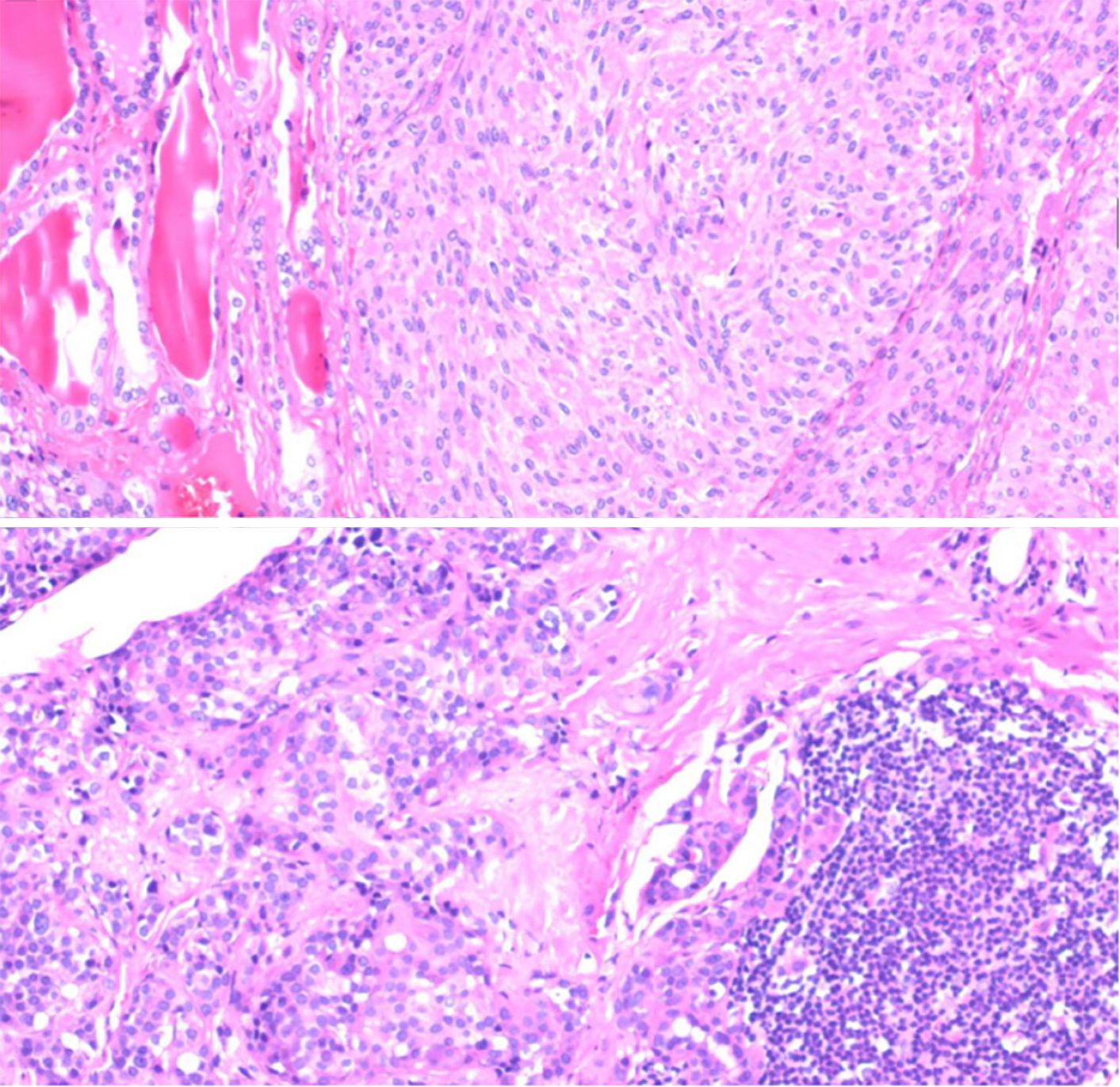
The proband's father, designated Ia, developed the disease at 60 years of age. He had a medical history of hypertension and diabetes for more than a decade. Upon identification of the RET proto-oncogene mutation, adrenal CT in 2018 revealed a right adrenal mass (Figure 1B). The MN and NMN levels were elevated to 4.5 and 2.26 nmol/L↑, respectively, whereas the 3-MT level was 0.1 nmol/L. Patient Ia underwent laparoscopic resection of the right adrenal mass, which was pathologically diagnosed as right pheochromocytoma. Three months after the adrenal surgery, additional screening using thyroid ultrasound revealed bilateral solid nodules within the thyroid (Figure 2B). The calcitonin level was drastically elevated to 972.2 pg/mL↑↑. The patient underwent complete bilateral thyroidectomy accompanied by cervical lymph node resection. Pathological results revealed bilateral medullary thyroid carcinoma with lymph node metastasis (Figure 3). Postoperative follow-up indicated that the dynamic review of MNS, MN, NMN, and calcitonin levels returned to normal levels.
Patients IIc and IId were two younger brothers with no history of hypertension. No suspicious mutations were detected in the RET proto-oncogene of these patients and their blood calcium, PTH, calcitonin, thyroid, and parathyroid levels, color Doppler ultrasound, and adrenal CT/color Doppler ultrasound results were all within the normal ranges.
The son of the patient, referred to as IIIa, was found to harbor a mutation in the RET proto-oncogene at the age of 3 years and 10 months. No noticeable abnormalities were detected on thyroid or parathyroid color Doppler ultrasonography. Prophylactic total thyroidectomy was suggested; however, the patient’s family declined. Upon a 5-year follow-up in 2022, an elevated calcitonin level of 18.93 pg/mL↑ was detected, with a Tg level of 19.6 ng/mL, PTH level of 7.22 pg/mL↓, and calcium level of 1.96 mmol/L↓. Therefore, a bilateral thyroidectomy was performed. Postoperative pathology revealed medullary thyroid carcinoma in the left lobe.
The clinical characteristics of the family are presented in Table 1.
| Patient | Age at onset/follow-up | RET proto-oncogene mutation | 3-MT | MN | NMN | Ct | Ca | PTH | Thyroid and parathyroid color Doppler ultrasound | Adrenal gland examination | Pathological diagnosis |
| Ⅰa | 60 years old | Yes1 | 0.10 | 4.5↑ | 2.26↑ | 972.2↑ | 2.47 | 4.13 | Bilateral thyroid nodules | Adrenal CT: Right adrenal space-occupying mass | Right PHEO, bilateral MTC with lymph node metastasis |
| Ⅱa | 30 years old | Yes1 | 0.12 | 4.1↑ | 2.36↑ | 29.49↑ | 2.41 | 1.97 | Bilateral thyroid nodules | Adrenal CT: Bilateral adrenal space-occupying mass | Bilateral PHEO, bilateral MTC |
| Ⅱc | 27 years old | No | N | N | N | N | N | N | N | Color Doppler ultrasound: N | - |
| Ⅱd | 24 years old | No | N | N | N | N | N | N | N | Color Doppler ultrasound: N | - |
| Ⅲa | 9 years old | Yes1 | - | - | - | 18.93 | 1.96 | 7.22 | N | Color Doppler ultrasound: N | Left MTC |
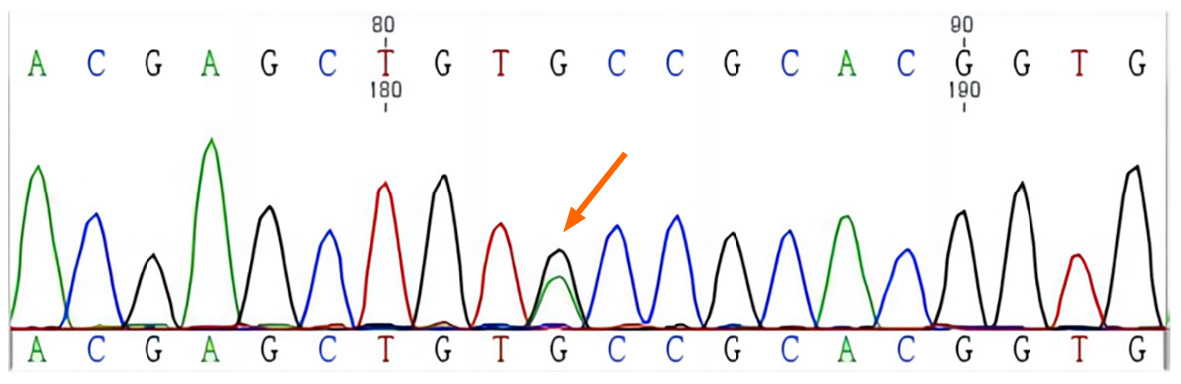
A c.T1901A mutation was identified in the 11th exon of RET, resulting in an amino acid change (p.Cys634Tyr) in the protein. This mutation was also detected in Ia, IIa, and IIIa at the same position (Figures 4 and 5, Table 2)
| Mutation position | Change in nucleic acids | Change in amino acids | RS-ID | Hom/Het |
| EI1/CDS11 | c.G1901A | p.Cys634Tyr | CM941237 | Het |
Three family members were diagnosed with MEN2 and harbored a c.T1901A mutation in the 11th exon of RET.
The patient underwent thyroidectomy and was diagnosed with medullary thyroid carcinoma by postoperative pathology.
The patient's father and his son, who was found to harbor a mutation in the RET proto-oncogene, underwent adrenal and thyroid screening. The father was diagnosed with bilateral pheochromocytoma and medullary thyroid carcinoma with metastasis, while the son was diagnosed with medullary thyroid carcinoma.
There is a strong correlation between the incidence and prognosis of MEN2A and mutations in the RET proto-oncogene. Donis-Keller et al[3] were the first to report that mutations in the RET proto-oncogene cause MEN2A. Three years later, the International RET Mutation Consortium compiled and investigated the clinical and pathological characteristics of RET proto-oncogenes in 477 families with MEN2A, MEN2B, and familial medullary thyroid cancer (FMTC) mutations, confirming the pathogenic role of the RET proto-oncogene in MEN2 syndrome. Located on 10q11.2, the RET proto-oncogene, a tyrosine kinase receptor, comprises 21 exons and approximately 1100 amino acid residues. When RET receptors dimerize after binding to the co-receptor (GDNF family receptor alpha), the phosphorylation of intracellular tyrosine residues is triggered, thus playing a role in cell proliferation regulation, cell differentiation, gene expression, and other cellular processes. This cascade can lead to excessive cell proliferation, resulting in carcinogenesis.
To date, over 1000 cases of MEN2A, both sporadic and familial, have been reported globally, with less than 200 cases reported in China, including more than 80 sporadic and 90 familial cases[4-6]. Approximately 95% of patients with classic MEN2A harbor RET gene mutations at codons 634, 609, 611, and 618 of exon 11 and codon 620 of exon 10; of these, 85% are located in codon 634 of exon 11[7]. In a comprehensive global review on MEN2-related RET pathogenic variants, the pathogenic variants at codon 634 are the most prevalent, found in between 30% and 50% of affected patients; for codon 634 mutations, the relative frequency in China is higher than those found in Brazil, France, Germany, Italy, United Kingdom, Japan, or United States[8].
There are four MEN2A variants: Classic MEN2A, MEN2A with cutaneous lichen amyloidosis, MEN2A with Hirschsprung disease, and MEN2A with FMTC[1,2]. Classic MEN2A is the most common type, manifesting primarily as medullary thyroid cancer (MTC), pheochromocytoma, and hyperparathyroidism[7]. Nearly 100% of patients present with multifocal and bilateral MTC, which is typically the first disorder, usually occurring before 30 years of age[9]. Only 15% of MEN2A cases are symptomatic at the time of diagnosis, and the remaining 85% are identified via screening[10].
The occurrence of MEN2A associated with extra-adrenal and malignant pheochromocytomas[11] is rare. Primary hyperparathyroidism may not be prevalent in MEN2A, potentially appearing in as few as 3%-11% of cases[12]. Most cases (56%-88%) are asymptomatic at the time of diagnosis[13], and complications, such as hypercalcemia crisis, are rare. RET gene testing is especially crucial for early MEN2A detection, offering predictive diagnosis and treatment for family members.
Mortality in MEN 2 is primarily attributed to MTC. The 10-year overall survival for patients with MTC has been estimated to be 64%[14]. Guidelines from the American, British, and European Thyroid Associations endorse RET analysis for all patients with confirmed MTC. Over 50% of patients with MTC with lymph node involvement experience disease relapse leading to death, which is the primary cause of mortality in patients with MEN2A[15]. A large long-term follow-up study demonstrated that prophylactic thyroidectomy in patients with MEN2A harboring the C634Y mutation enables disease cure without inducing any long-term complications[16]. Therefore, the prognosis of MEN2A primarily depends on the status of MTC. Currently, surgery is the only effective treatment for MTC. Prophylactic thyroidectomy in patients with early asymptomatic MEN2A is crucial to prevent carcinogenesis and its complications and improve survival rates.
When considering prophylactic thyroidectomy, the focus is on the RET mutation type, age, and calcitonin level. The American Thyroid Association classifies RET mutations into three categories based on the invasive risk of MTC: Medium, high, and extremely high. The more invasive the tumor, the earlier the prophylactic thyroidectomy should be performed and the larger the range of the thyroid necessitating surgical removal. Based on this categorization, different surgical indications have been proposed for patient treatment depending on the risk level[7,17]. The classic MEN2A 634 chromosomal mutation is a high-risk mutation; high-risk mutations transition from normal to C-cell hyperplasia within an average of 5 years, and to MTC N0 and MTC N1 within 19 and 31 years, respectively[18]. Prophylactic thyroidectomy is recommended before the age of 5 years. However, clinicians must carefully weigh the risk of surgical complications in children, increased recurrence rates during lymph node dissection, and the probability of cure[19,20]. Monitoring calcitonin level is another crucial evaluation standard. A French study of 170 young patients aged < 21 years confirmed the major role of calcitonin testing in prophylactic thyroidectomies, indicating that all preoperative patients with calcitonin levels < 30 pg/mL, regardless of whether their MTC was classified as extremely high-, high-, or medium-risk, were cured after thyroidectomy[21].
Raue and Frank-Raue[22] suggested a three-point management strategy for patients with MEN2: (1) Confirm the pathogenic RET gene mutation type; (2) classify MTC risk groups; and (3) determine preoperative calcitonin levels to ensure that asymptomatic carriers undergo timely MTC removal. Both high- and moderate-risk groups could determine the timing of surgery based on calcitonin level[21-24]. Once the calcitonin levels surpass the upper limit of normal level, surgery can be performed. This approach may postpone thyroidectomy until the patient reaches full growth, thereby reducing surgical complications, such as permanent hypoparathyroidism and recurrent laryngeal nerve injury, and preventing overtreatment.
In the family with MEN2A discussed in this study, three members harbored a heterozygous mutation of nucleotide c.T1901A, which caused the 634th amino acid of the encoded protein to mutate from cysteine to tyrosine. Among the mutations in codon 634, the C634Y mutation is uncommon and may present a more indolent clinical course. In this family with the high-risk C634Y mutation, proband IIa first presented with pheochromocytoma. Given the patient’s age of 30 years, the atypical presentation of calcitonin levels, and color Doppler ultrasound findings, a thyroidectomy was performed. Postoperative pathological examination confirmed the presence of MTC without lymph node metastasis. A 5-year follow-up revealed normal calcitonin levels and thyroid color Doppler ultrasound findings, with no signs of recurrence. Regular follow-ups with abdominal CT and MN levels were also normal, with no pheochromocytoma or pheochromocytic tissue, suggesting a benign pheochromocytoma. The proband's father, who had a history of hypertension and did not participate in regular health examinations, was found to have an RET gene C634Y mutation through genetic testing. This finding led to surgical intervention for the pheochromocytoma and MTC, both of which were pathologically confirmed. However, owing to the advanced age at which testing was conducted, the MTC had already metastasized to the lymph nodes, necessitating stringent monitoring of calcitonin levels to prevent recurrence. The gene mutation carrier IIIa harbors a high-risk mutation. According to the guidelines, total thyroidectomy can be performed before the age of 5 years; however, given the child's growth and developmental needs, along with relatively higher surgical risks, and in accordance with the family's wishes, we opted to closely monitor calcitonin level and thyroid color Doppler ultrasound. After 5 years of follow-up, an increase in calcitonin level was detected when the child turned 9 years old, necessitating a prophylactic thyroidectomy. Subsequent pathological examination confirmed the presence of MTC.
The thyroid conditions of the three aforementioned patients were managed in accordance with the American Thyroid Association guidelines, considering the genotype–phenotype relationship of MEN2A, which effectively guided clinical diagnosis and treatment. At the time of diagnosis of the disease in the proband, she was diagnosed with bilateral pheochromocytomas, whereas her father had a unilateral pheochromocytoma. These findings align with previous reports on MEN2A pheochromocytomas, which can be either unilateral or bilateral, with rare malignancies. None of the three patients exhibited signs of hyperparathyroidism, thereby necessitating long-term monitoring of blood calcium and PTH levels and parathyroid color Doppler ultrasonography.
In this report, we highlight that gene detection in three generations of a family can be used for early screening of related diseases, especially for the diagnosis and treatment of early medullary thyroid cancer. However, owing to the limited number of patients, there are certain limitations, and it will be more meaningful to screen the genes and related diseases in Ia parents and siblings.
In summary, RET proto-oncogene mutations play crucial roles in determining the clinical phenotype and prognosis of patients with MEN2A. In a clinical setting, advancements in genetic research on MEN2A can be leveraged for familial management; assess the invasive risk of MTC based on RET gene mutation types, age, and calcitonin levels; and decide the timing of surgery. This study offers considerable guidance regarding the onset, progression, and prognosis of pheochromocytoma and hyperparathyroidism.
We thank the doctors and nurses at the Department of Endocrinology, Guangzhou University of Chinese Medicine, Dongguan TCM Hospital for their help in this study. We express gratitude to our partner, Beijing Hongwei Testing Co., Ltd, for their excellent cooperation and technical support.
| 1. | Mathiesen JS, Kroustrup JP, Vestergaard P, Madsen M, Stochholm K, Poulsen PL, Krogh Rasmussen Å, Feldt-Rasmussen U, Schytte S, Pedersen HB, Hahn CH, Bentzen J, Gaustadnes M, Ørntoft TF, Hansen TVO, Nielsen FC, Brixen K, Frederiksen AL, Godballe C. Incidence and prevalence of multiple endocrine neoplasia 2B in Denmark: a nationwide study. Endocr Relat Cancer. 2017;24:L39-L42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Mathiesen JS, Kroustrup JP, Vestergaard P, Stochholm K, Poulsen PL, Rasmussen ÅK, Feldt-Rasmussen U, Schytte S, Pedersen HB, Hahn CH, Bentzen J, Möller S, Gaustadnes M, Rossing M, Nielsen FC, Brixen K, Frederiksen AL, Godballe C. Incidence and prevalence of multiple endocrine neoplasia 2A in Denmark 1901-2014: a nationwide study. Clin Epidemiol. 2018;10:1479-1487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Donis-Keller H, Dou S, Chi D, Carlson KM, Toshima K, Lairmore TC, Howe JR, Moley JF, Goodfellow P, Wells SA Jr. Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum Mol Genet. 1993;2:851-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 800] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 4. | Chen L, Zhang JX, Liu DG, Liu HG. A Familial Case of Multiple Endocrine Neoplasia 2A: From Morphology to Genetic Alterations Penetration in Three Generations of a Family. Diagnostics (Basel). 2023;13:955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Lu F, Chen X, Bai Y, Feng Y, Wu J. A large Chinese pedigree of multiple endocrine neoplasia type 2A with a novel C634Y/D707E germline mutation in RET exon 11. Oncol Lett. 2017;14:3552-3558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Du ZF, Li PF, Zhao JQ, Cao ZL, Li F, Ma JM, Qi XP. Genetic diagnosis of a Chinese multiple endocrine neoplasia type 2A family through whole genome sequencing. J Biosci. 2017;42:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Wells SA Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, Raue F, Frank-Raue K, Robinson B, Rosenthal MS, Santoro M, Schlumberger M, Shah M, Waguespack SG; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25:567-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1802] [Cited by in RCA: 1554] [Article Influence: 141.3] [Reference Citation Analysis (0)] |
| 8. | Maciel RMB, Maia AL. GLOBAL ENDOCRINOLOGY: Geographical variation in the profile of RET variants in patients with medullary thyroid cancer: a comprehensive review. Eur J Endocrinol. 2021;186:R15-R30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Elisei R, Romei C. Looking for RET alterations in thyroid cancer: clinical relevance, methodology and timing. Endocrine. 2023;81:206-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 10. | Mathiesen JS, Kroustrup JP, Vestergaard P, Stochholm K, Poulsen PL, Rasmussen ÅK, Feldt-Rasmussen U, Schytte S, Londero SC, Pedersen HB, Hahn CH, Bentzen J, Möller S, Gaustadnes M, Rossing M, Nielsen FC, Brixen K, Frederiksen AL, Godballe C. Survival and Long-Term Biochemical Cure in Medullary Thyroid Carcinoma in Denmark 1997-2014: A Nationwide Study. Thyroid. 2019;29:368-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Mucha L, Leidig-Bruckner G, Frank-Raue K, Bruckner T, Kroiss M, Raue F; German study group for rare thyroid cancer. Phaeochromocytoma in multiple endocrine neoplasia type 2: RET codon-specific penetrance and changes in management during the last four decades. Clin Endocrinol (Oxf). 2017;87:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Machens A, Elwerr M, Lorenz K, Weber F, Dralle H. 100-Year evolution of precision medicine and surgery for multiple endocrine neoplasia type 2A. Endocrine. 2020;68:368-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Twigt BA, Scholten A, Valk GD, Rinkes IH, Vriens MR. Differences between sporadic and MEN related primary hyperparathyroidism; clinical expression, preoperative workup, operative strategy and follow-up. Orphanet J Rare Dis. 2013;8:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Mathiesen JS, Effraimidis G, Rossing M, Rasmussen ÅK, Hoejberg L, Bastholt L, Godballe C, Oturai P, Feldt-Rasmussen U. Multiple endocrine neoplasia type 2: A review. Semin Cancer Biol. 2022;79:163-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 15. | American Thyroid Association Guidelines Task Force; Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF, Gharib H, Moley JF, Pacini F, Ringel MD, Schlumberger M, Wells SA Jr. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009;19:565-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1040] [Cited by in RCA: 812] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 16. | Febrero B, Rodríguez JM, Ríos A, Segura P, Pérez-Sánchez B, Torregrosa N, Hernández AM, Parrilla P. Prophylactic thyroidectomy in multiple endocrine neoplasia 2 (MEN2) patients with the C634Y mutation: A long-term follow-up in a large single-center cohort. Eur J Surg Oncol. 2019;45:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Wells SA Jr, Pacini F, Robinson BG, Santoro M. Multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma: an update. J Clin Endocrinol Metab. 2013;98:3149-3164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 221] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 18. | Machens A, Lorenz K, Weber F, Dralle H. Genotype-specific progression of hereditary medullary thyroid cancer. Hum Mutat. 2018;39:860-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Kluijfhout WP, van Beek DJ, Verrijn Stuart AA, Lodewijk L, Valk GD, van der Zee DC, Vriens MR, Borel Rinkes IHM. Postoperative Complications After Prophylactic Thyroidectomy for Very Young Patients With Multiple Endocrine Neoplasia Type 2: Retrospective Cohort Analysis. Medicine (Baltimore). 2015;94:e1108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Machens A, Dralle H. Advances in risk-oriented surgery for multiple endocrine neoplasia type 2. Endocr Relat Cancer. 2018;25:T41-T52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Rohmer V, Vidal-Trecan G, Bourdelot A, Niccoli P, Murat A, Wemeau JL, Borson-Chazot F, Schvartz C, Tabarin A, Chabre O, Chabrier G, Caron P, Rodien P, Schlumberger M, Baudin E; Groupe Français des Tumeurs Endocrines. Prognostic factors of disease-free survival after thyroidectomy in 170 young patients with a RET germline mutation: a multicenter study of the Groupe Francais d'Etude des Tumeurs Endocrines. J Clin Endocrinol Metab. 2011;96:E509-E518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Raue F, Frank-Raue K. Update on Multiple Endocrine Neoplasia Type 2: Focus on Medullary Thyroid Carcinoma. J Endocr Soc. 2018;2:933-943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Lifante JC, Blanchard C, Mirallié E, David A, Peix JL. Role of preoperative basal calcitonin levels in the timing of prophylactic thyroidectomy in patients with germline RET mutations. World J Surg. 2014;38:576-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Zhang X, Yan D, Wang J, Wan H, Zhang Y, He Y, Liu W, Zhang B. Is new American Thyroid Association risk classification for hereditary medullary thyroid carcinoma applicable to Chinese patients? A single-center study. Chin J Cancer Res. 2017;29:223-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade C
Creativity or Innovation: Grade B
Scientific Significance: Grade C
P-Reviewer: Saberi S, United States S-Editor: Che XX L-Editor: A P-Editor: Yu HG