Published online May 16, 2024. doi: 10.12998/wjcc.v12.i14.2412
Revised: March 3, 2024
Accepted: April 1, 2024
Published online: May 16, 2024
Processing time: 104 Days and 20.5 Hours
Rectal mucinous adenocarcinoma (MAC) is a rare pathological type of rectal can
In this report, the clinical data, diagnosis and treatment process, and postope
Subcutaneous soft tissue metastasis of rectal MAC is rare, and it can suggest that the tumor is disseminated, and it can appear even earlier than the primary ma
Core Tip: Mucinous adenocarcinoma (MAC) is a relatively rare pathological subtype of colorectal cancer. Patients with rectal MAC are more likely to have abdominal lymph node metastasis, peritoneal metastasis, and abdominal implantation and have a worse prognosis and lower survival rate. Early detection, diagnosis, and treatment of rectal MAC can improve the prognosis of patients. We present a rare case of left waist subcutaneous soft tissue metastasis, hoping to provide some experience for the early clinical diagnosis and treatment of this disease.
- Citation: Gong ZX, Li GL, Dong WM, Xu Z, Li R, Lv WX, Yang J, Li ZX, Xing W. Waist subcutaneous soft tissue metastasis of rectal mucinous adenocarcinoma: A case report. World J Clin Cases 2024; 12(14): 2412-2419
- URL: https://www.wjgnet.com/2307-8960/full/v12/i14/2412.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i14.2412
Colorectal cancer is the third most common malignant tumor in the world, and the incidence and mortality of colorectal cancer in China and the world are increasing. In 2020, the report of China Cancer statistics shows that the incidence and mortality of colorectal cancer rank second and fifth, respectively, among all malignant tumors, with 555000 new cases and 286000 deaths of colorectal cancer occurring in China in 2020[1]. Globally, the fatality rate is the fourth highest among all malignancies[2]. Mucinous adenocarcinoma (MAC) is a relatively rare pathological subtype of colorectal cancer, account
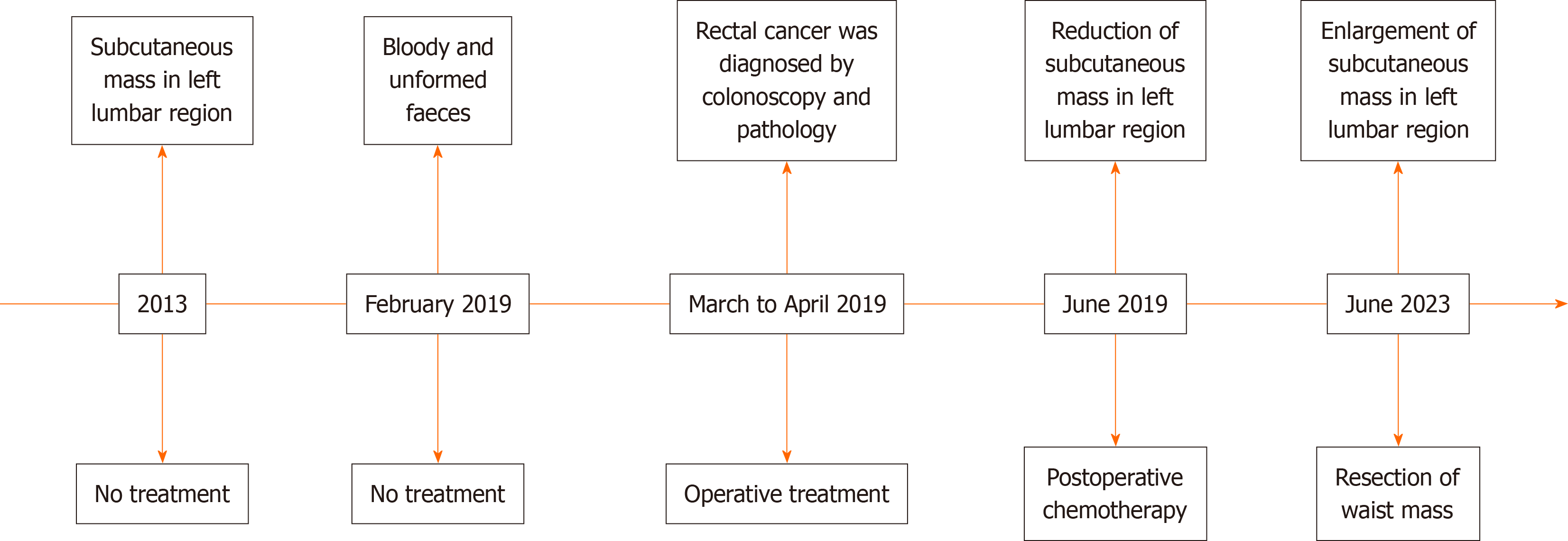
On June 17, 2023, a 49-year-old man was admitted to our hospital with a chief complaint of a mass at the left waist for more than 10 years.
The mass was an approximately 1 cm × 1.5 cm mass bulging from his left waist at the beginning, without pain, rupture, and skin ulceration. Two weeks prior, the mass was approximately 2 cm × 3 cm and caused the patient to have subcu
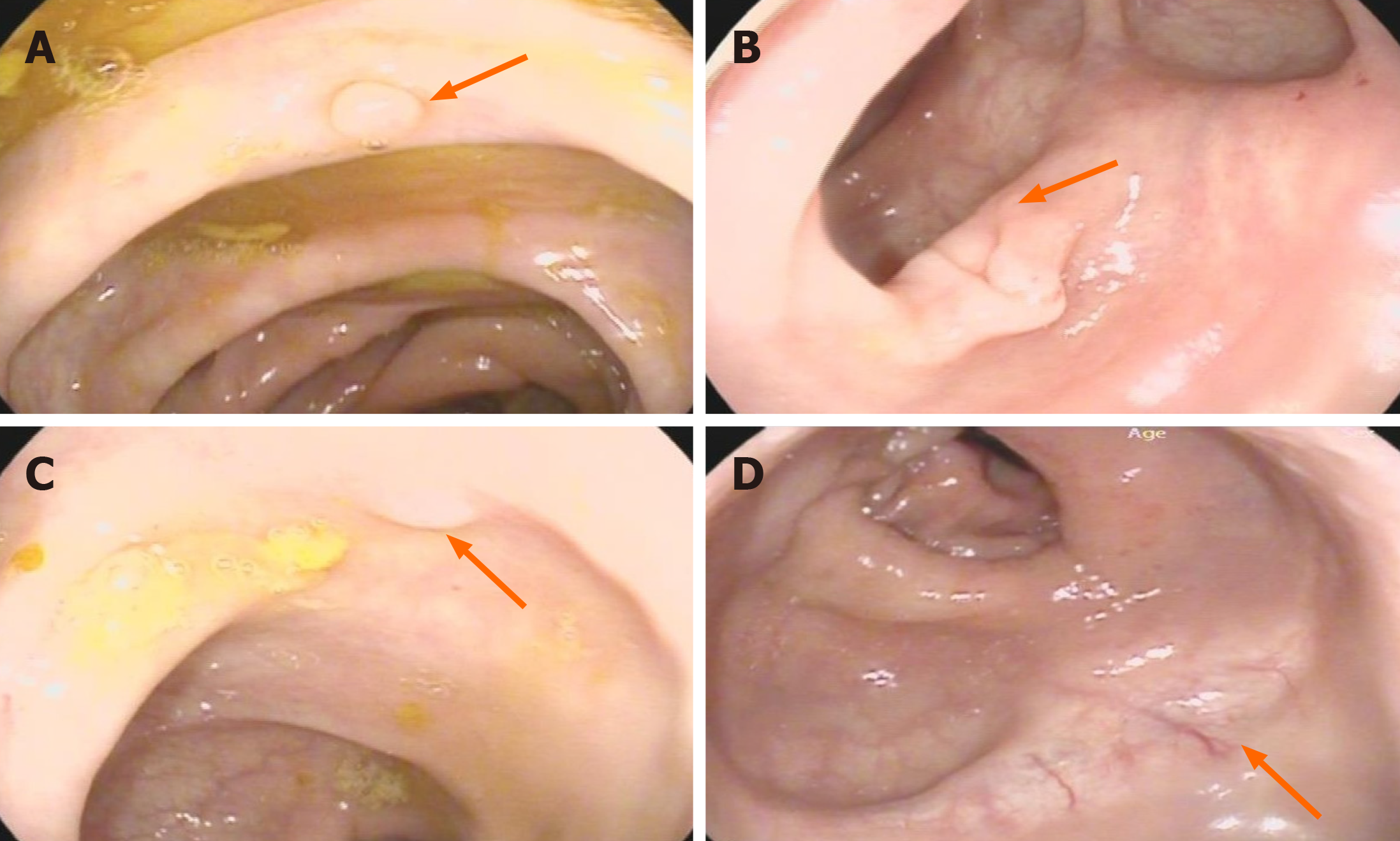
His medical history included radical Dixon resection of rectal cancer (7-9 cm from the anal margin) in April 2019. The diagnosis of postoperative pathology was ulcerative low-differentiated adenocarcinoma and local MAC infiltrating the subserosal fibrous adipose tissue, with 3 of 14 mesorectal lymph nodes involved. A subsequent biopsy and the immunohistochemistry (IHC) findings were as follows: CKI8 (++), CDX2 (++), p53 (++), MUC2 (+), MLHI (+), PMS2 (+), Ki-67 (+ 10%), CD56 (+), p40 (-), Vimentin (-), Syn (-), and CgA (-). The diagnosis of pathology and clinical was reported as stage T3N1M0 and IIIB. The left waist mass became smaller when the patient underwent one cycle of chemotherapy with Ca
The patient had no relevant personal or family history.
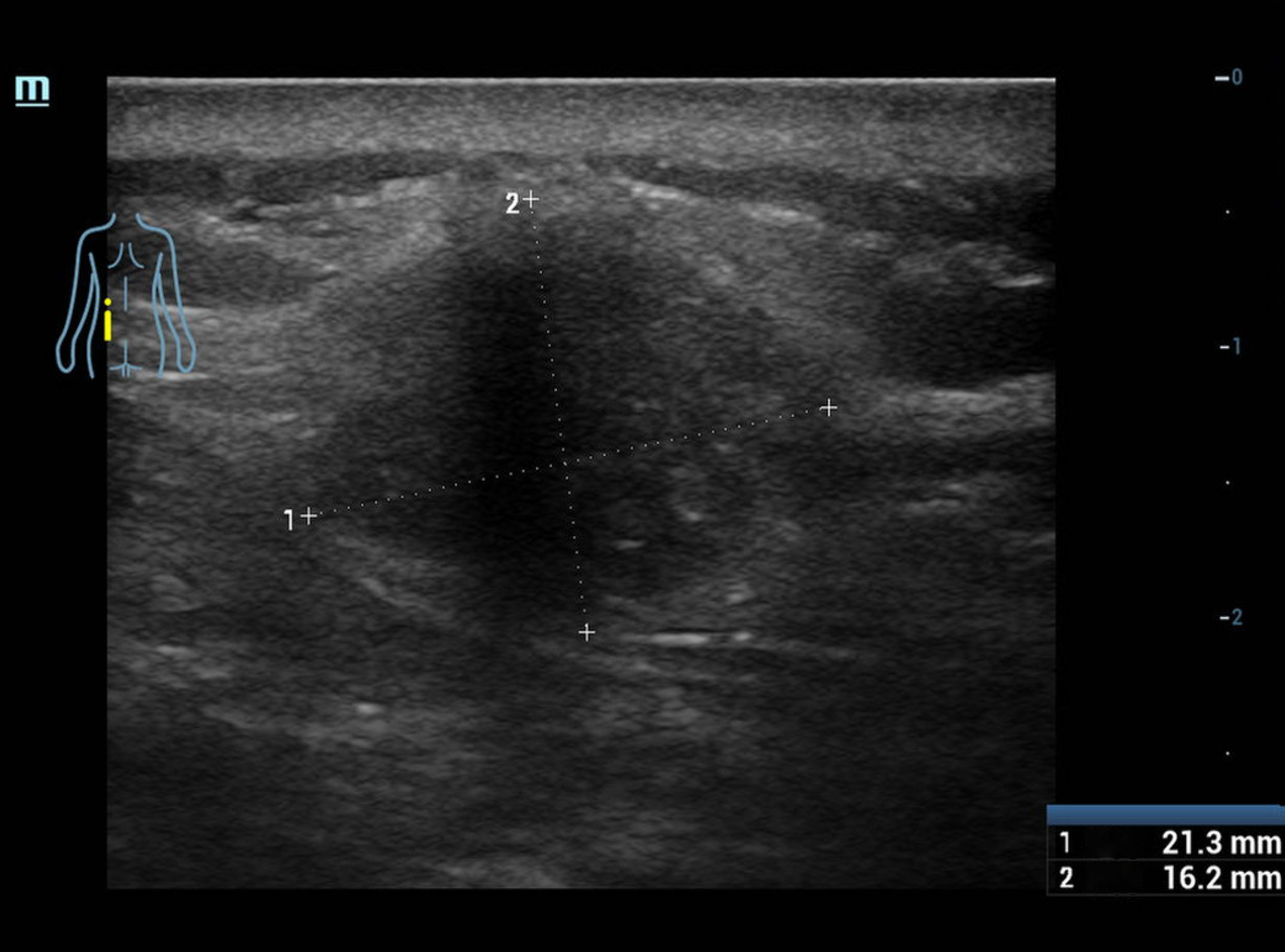
Physical examination revealed a firm and relatively clear border subcutaneous tissue mass with a size of approximately 2 cm × 3 cm at the left waist without skin redness, swelling or warming in this patient.
Laboratory examination revealed the following: White blood cell (WBC) 10.49 × 109/L, interleukin-2 (7.05 pg/mL), in
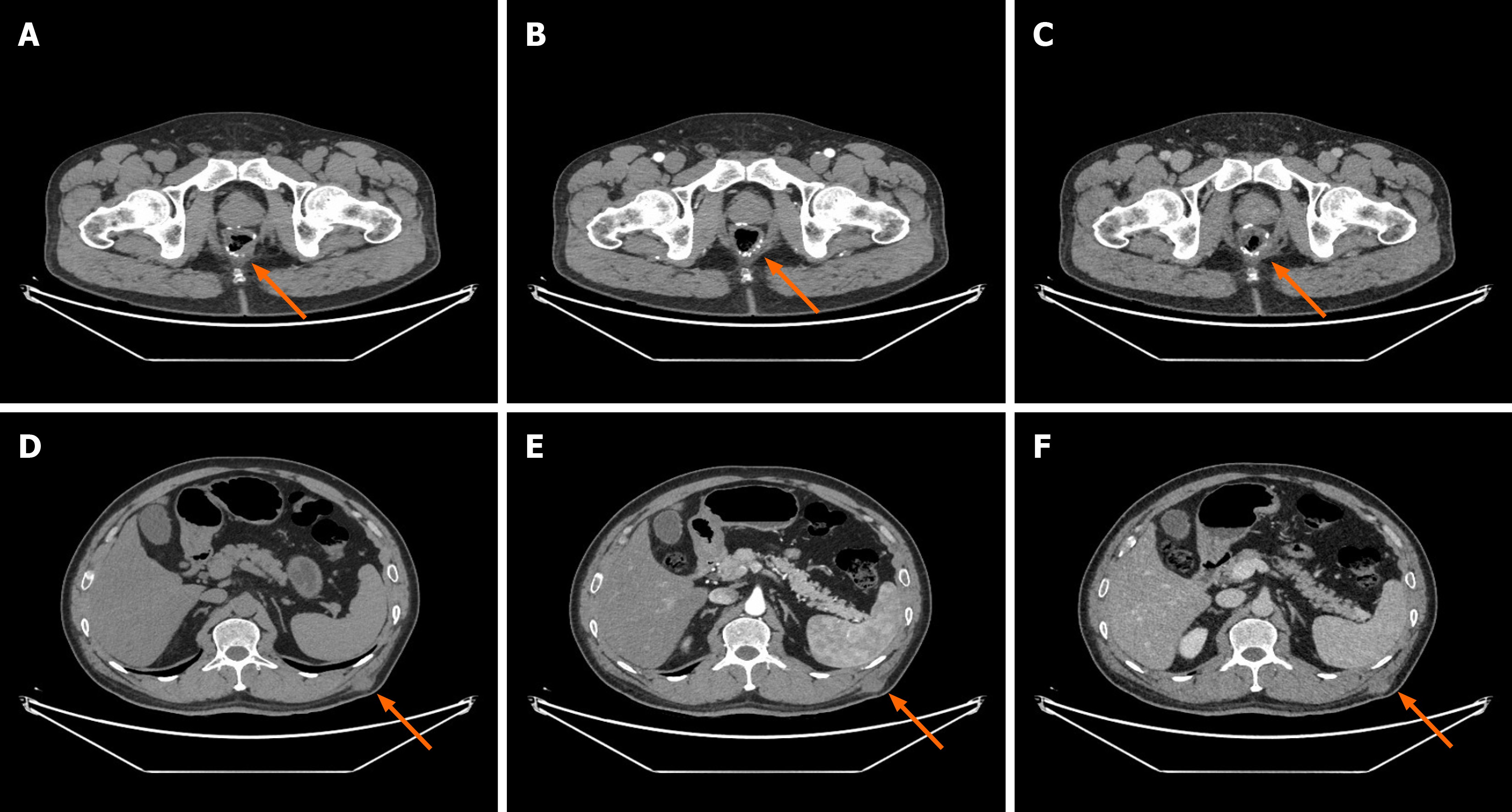
Imaging examination and contrast-enhanced abdominal computed tomography (CT) revealed a soft tissue mass in the subcutaneous tissue plane of the left waist without obvious enhancement in the arterial phase and portal phase, no ab
According to auxiliary examination results and clinical history, skin soft tissue malignancy was considered.
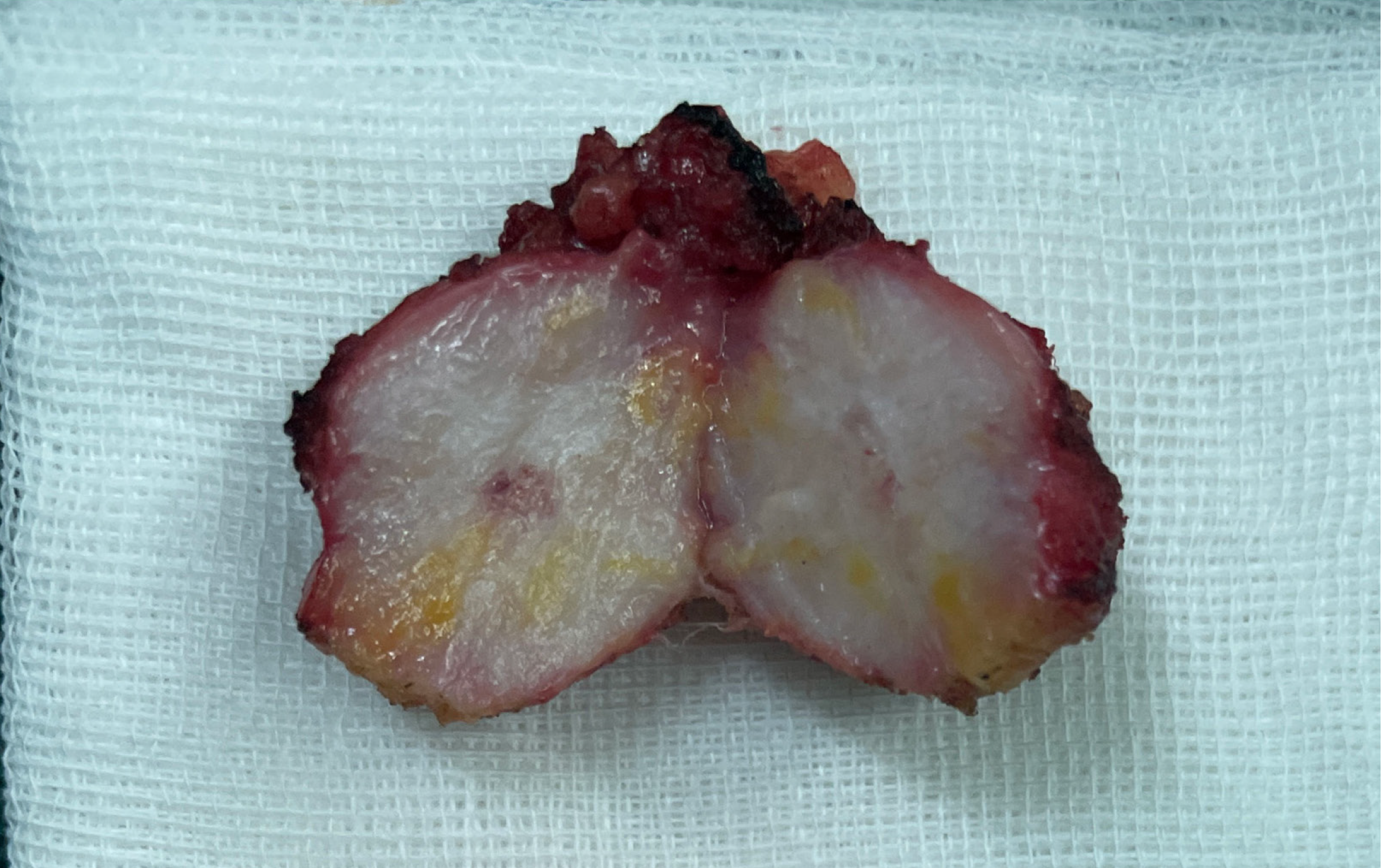
The patient underwent left waist mass resection in our hospital on June 21, 2023. The intraoperative discovery was that the tumor was located in the subcutaneous fat and fascia layer, with a size of 2.5 cm × 2 cm and a relatively clear border, and the bottom of the tumor was adhered to the muscle layer. The mass and part of the adherent muscle tissue were completely resected (Figure 5). Postoperative analgesia and anti-infection treatment were given to the patient.
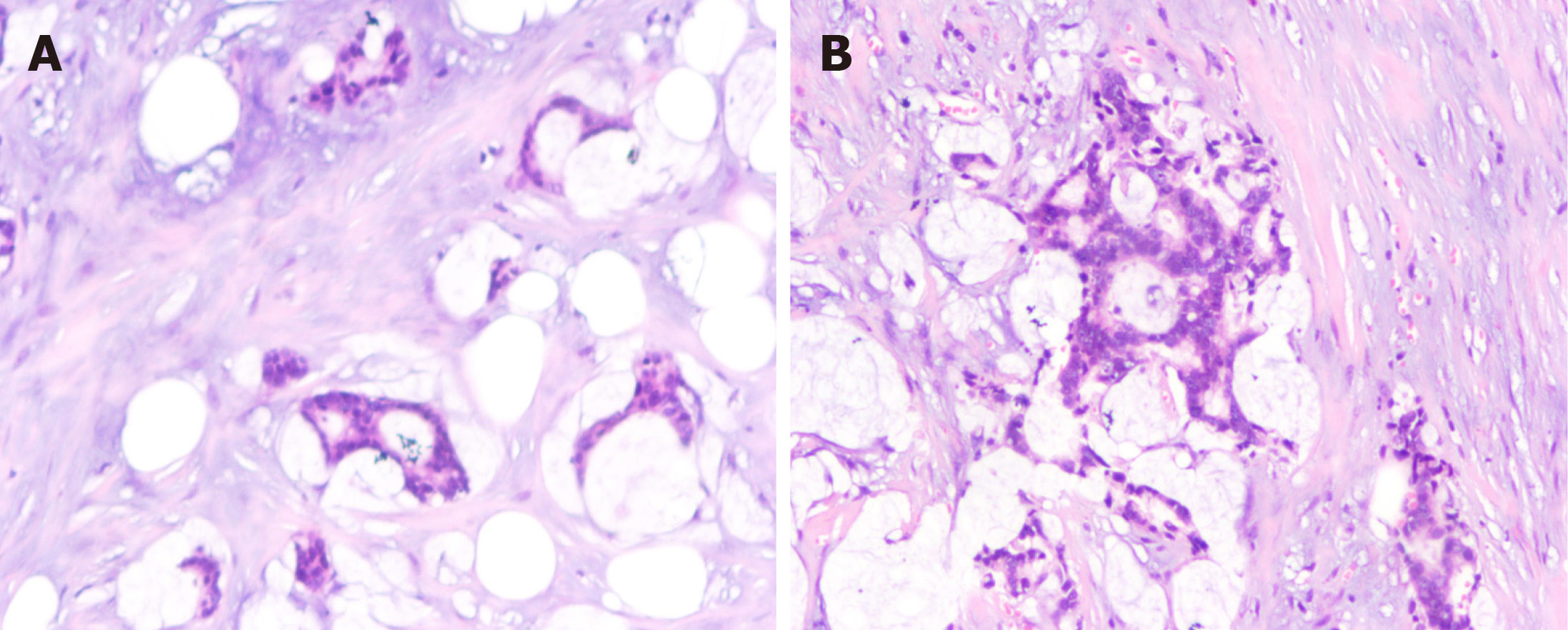
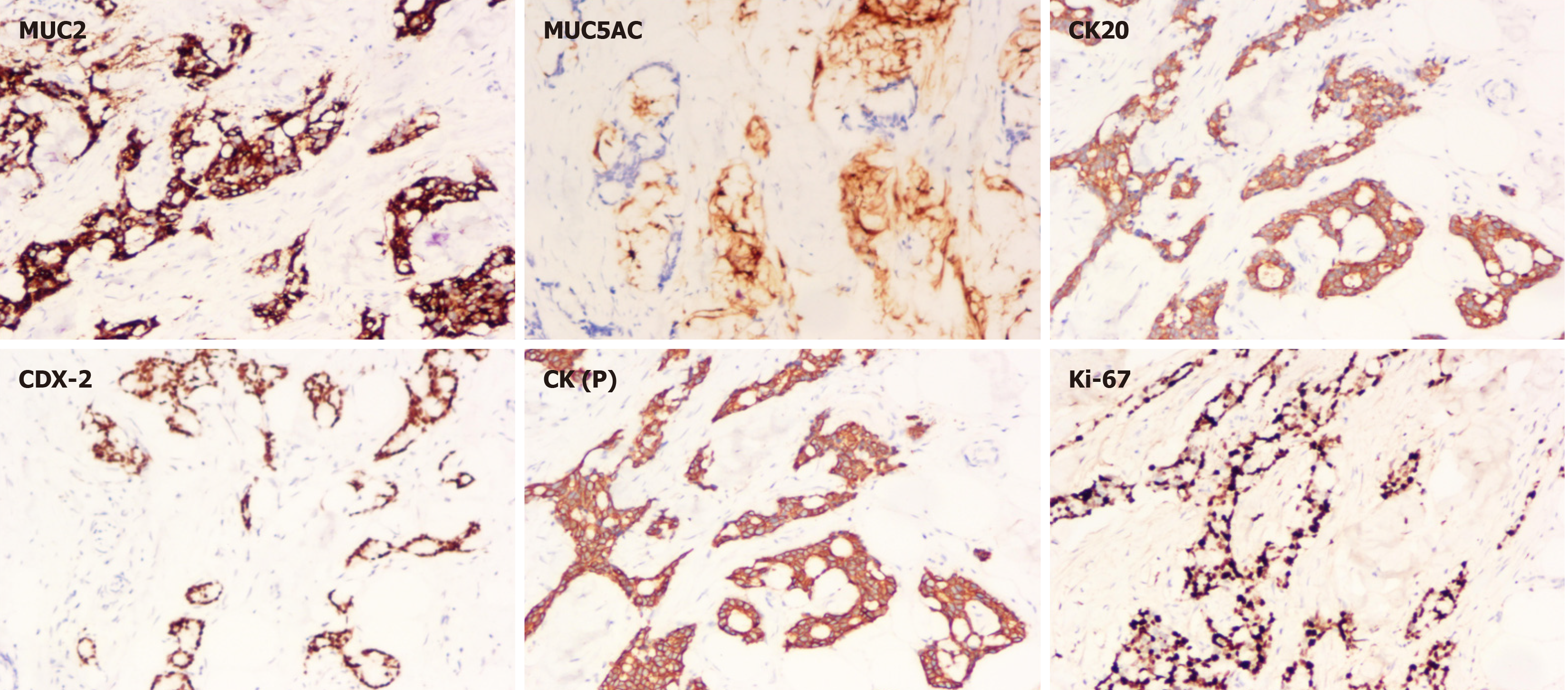
The postoperative pathologic diagnosis of the left waist mass (hematoxylin and eosin) was MAC, nerve invasion and transfer (Figure 6). Subsequent biopsy and IHC showed the following: MUC2 (+), MUC5AC (+), CK20 (+), CDX-2 (+), CK (P) (+), and Ki-67 (index 80%) (Figure 7). The patient recovered well after surgery with good wound healing and was successfully discharged from the hospital. The patient had no local tumor recurrence or distant metastasis at the 3-month follow-up.
MAC is a relatively rare pathological subtype of rectal cancer. It is frequently diagnosed by pathological paraffin sec
| Ref. | Age (yr) | Sex | Histology | Stage | Primary cancer treatment | Interval months | Skin mets location | Skin mets morphology | Skin mets treatment | Survival (follow-up time in months) |
| Hayashi et al[14], 2003 | 55 | M | Adenocarcinoma mucinous | - | LAR | 4 | Perineum | Nodules | None | - |
| Sarid et al[15], 2004 | 60 | F | Adenocarcinoma mucinous | IIIB | NR + LAR + ACR | 16 | Chest | Ulcers | WLE | No (56) |
| Tan et al[16], 2006 | 70 | M | Adenocarcinoma mucinous | IIIB | LAR + AC | 24 | Back | Nodules | WLE, C | - |
| Saladzinskas et al[17], 2010 | 64 | M | Adenocarcinoma mucinous | IIA | NR + LAR | 42 | Face | Ulcers | WLE | Yes (7) |
| Balta et al[18], 2012 | 46 | M | Adenocarcinoma mucinous | IIIB | Colostomy | 12 | Perineum | Ulcers | None | - |
| de Miguel Valencia et al[19], 2013 | 55 | M | Adenocarcinoma mucinous | IIIB | NCR + APR + AC | 18 | Multiple | Nodules | None | No (-) |
| Dehal et al[10], 2015 | 47 | M | IV | CR | 1 | Perineum | Nodules | R | Yes (12) |
The incidence of rectal MAC is low. However, the malignancy rate is high, the local recurrence and distant metastasis rates are higher, the prognosis is poor, and the survival of patients is affected. Distant subcutaneous soft tissue metastasis of rectal MAC is rare. However, for patients with a history of malignant tumors, these should be considered to be ma
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68750] [Article Influence: 13750.0] [Reference Citation Analysis (201)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56700] [Article Influence: 7087.5] [Reference Citation Analysis (135)] |
| 3. | Chand M, Yu S, Swift RI, Brown G. Mucinous carcinoma of the rectum: a distinct clinicopathological entity. Tech Coloproctol. 2014;18:335-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Numata M, Shiozawa M, Watanabe T, Tamagawa H, Yamamoto N, Morinaga S, Watanabe K, Godai T, Oshima T, Fujii S, Kunisaki C, Rino Y, Masuda M, Akaike M. The clinicopathological features of colorectal mucinous adenocarcinoma and a therapeutic strategy for the disease. World J Surg Oncol. 2012;10:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Huang A, Yang Y, Shi JY, Li YK, Xu JX, Cheng Y, Gu J. Mucinous adenocarcinoma: A unique clinicopathological subtype in colorectal cancer. World J Gastrointest Surg. 2021;13:1567-1583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (2)] |
| 6. | Hugen N, Brown G, Glynne-Jones R, de Wilt JH, Nagtegaal ID. Advances in the care of patients with mucinous colorectal cancer. Nat Rev Clin Oncol. 2016;13:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 7. | Debunne H, Ceelen W. Mucinous differentiation in colorectal cancer: molecular, histological and clinical aspects. Acta Chir Belg. 2013;113:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Kocer B, Soran A, Erdogan S, Karabeyoglu M, Yildirim O, Eroglu A, Bozkurt B, Cengiz O. Expression of MUC5AC in colorectal carcinoma and relationship with prognosis. Pathol Int. 2002;52:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Molavi D, Argani P. Distinguishing benign dissecting mucin (stromal mucin pools) from invasive mucinous carcinoma. Adv Anat Pathol. 2008;15:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Dehal A, Patel S, Kim S, Shapera E, Hussain F. Cutaneous Metastasis of Rectal Cancer: A Case Report and Literature Review. Perm J. 2016;20:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Attili VS, Rama Chandra C, Dadhich HK, Sahoo TP, Anupama G, Bapsy PP. Unusual metastasis in colorectal cancer. Indian J Cancer. 2006;43:93-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Kumari N, Dwarakanath BS, Das A, Bhatt AN. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016;37:11553-11572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 790] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 13. | Li LX, Zhang B, Gong RZ. Insights into the role of tumor abnormal protein in early diagnosis of cancer: A prospective cohort study. Medicine (Baltimore). 2020;99:e19382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Hayashi H, Shimizu T, Shimizu H. Scrotal metastases originating from colorectal carcinoma. Clin Exp Dermatol. 2003;28:226-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Sarid D, Wigler N, Gutkin Z, Merimsky O, Leider-Trejo L, Ron IG. Cutaneous and subcutaneous metastases of rectal cancer. Int J Clin Oncol. 2004;9:202-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Tan KY, Ho KS, Lai JH, Lim JF, Ooi BS, Tang CL, Eu KW. Cutaneous and subcutaneous metastases of adenocarcinoma of the colon and rectum. Ann Acad Med Singap. 2006;35:585-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 17. | Saladzinskas Z, Tamelis A, Paskauskas S, Pranys D, Pavalkis D. Facial skin metastasis of colorectal cancer: a case report. Cases J. 2010;3:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Balta I, Vahaboglu G, Karabulut AA, Yetisir F, Astarci M, Gungor E, Eksioglu M. Cutaneous metastases of rectal mucinous adenocarcinoma mimicking granuloma inguinale. Intern Med. 2012;51:2479-2481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | de Miguel Valencia MJ, Fraile González M, Yagüe Hernando A, Oteiza Martínez F, Ciga Lozano MA, Armendáriz Rubio P, de Miguel Velasco M, Ortiz Hurtado H. [Cutaneous metastases of rectal cancer]. An Sist Sanit Navar. 2013;36:557-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Teramoto-Matsubara OT, Mexico S-Editor: Zheng XM L-Editor: A P-Editor: Xu ZH