Published online May 16, 2024. doi: 10.12998/wjcc.v12.i14.2404
Revised: February 7, 2020
Accepted: April 3, 2024
Published online: May 16, 2024
Processing time: 114 Days and 21.4 Hours
Human cystic echinococcosis (CE) is a life-threatening zoonosis caused by the Echinococcus granulosus (sensu lato). Hepatocellular carcinoma (HCC) is a leading cause of cancer-related mortality in the world. The coexistence of CE and HCC is exceedingly rare, and only several well-documented cases have been reported. In addition to this coexistence, there is no report of the coexistence of CE, HCC, and liver abscess to date. Herein, we aimed to report a case of coexistence of liver abscess, hepatic CE, and HCC.
A 65-year-old herdsman presented to the department of interventional therapy with jaundice, right upper abdominal distension and pain for 10 d. Laboratory test showed that he had positive results for HBsAg, HBeAb, HBcAb, and echinococcosis IgG antibody. The test also showed an increased level of alpha fetopro
This is the first reported case on the coexistence of liver abscess, hepatic CE, and HCC. Individualized treatment and multidisciplinary discussions should be performed in this setting. Therefore, treatment and diagnosis should be based on the characteristics of liver abscess, hepatic CE, and HCC, and in future clinical work, it is necessary to be aware of the possibility of this complex composition of liver diseases.
Core Tip: This is the first reported case of coexistence of liver abscess, hepatic cystic echinococcosis (CE) and hepatocellular carcinoma (HCC). Transarterial chemoembolization was performed for HCC and percutaneous liver puncture drainage was then performed to relieve the liver abscess. The subtype of CE is CE4, as its blood supply is exceedingly poor, so wait-and-watch can be used in this setting. Individualized treatment and multidisciplinary discussions should be performed in this setting.
- Citation: Hu YW, Zhao YL, Yan JX, Ma CK. Coexistence of liver abscess, hepatic cystic echinococcosis and hepatocellular carcinoma: A case report. World J Clin Cases 2024; 12(14): 2404-2411
- URL: https://www.wjgnet.com/2307-8960/full/v12/i14/2404.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i14.2404
Human cystic echinococcosis (CE) is a life-threatening zoonosis caused by the Echinococcus granulosus (sensu lato)[1]. During the adult stage, the parasite can infect canids, sheep, and other animals before releasing parasite eggs in the environment through their feces. Food and water contaminated by their feces may induce human infection though the ingestion of parasite eggs[2]. With CE, 70% of cases manifest primarily in the liver, while the lung is the second-most common location[3]. The lesions can be asymptomatic for years. As the lesions progress, symptoms such as pain in the upper abdomen, fatigue, and fever can be obvious. Unfortunately, with the appearance of symptoms, the lesions often progress to the late stages. The diagnosis of CE is mainly based on its imaging characteristics rather than its biopsy results, so imaging techniques, such as ultrasound, are important for diagnosing CE[4].
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related mortality in the world, with more than 840000 newly reported cases annually[5]. Hepatitis B virus (HBV) is a major cause of HCC in Asia, and when treating patients with HBV-induced HCC, both anti-cancer and anti-virus measures should be taken[6,7]. The prognosis of HCC is poor, and the treatment of HCC is usually limited because most cases are diagnosed during later stages[8]. The coexistence of hepatic CE and HCC is an exceedingly rare condition and, to date, only a few well-documented cases have been reported[9-13]. Besides, the impact of the coexistence of hepatic CE and HCC is still unknown. The coexistence of liver abscess, hepatic CE, and HCC may be another rare setting. Here, we report clinical data on the diagnosis and treatment of a rare case of liver abscess, hepatic CE, and HBV-induced HCC.
A 65-year-old herdsman presented to the department of interventional therapy with jaundice, right upper abdominal distension, and pain on January 4, 2021.
The patient started experiencing jaundice and right upper abdominal distension 10 d ago. He took some unrecognizable herbs without any therapeutic effect.
Unremarkable.
His family history was unremarkable. He had several risk factors for contracting echinococcosis: He was a pastoralist in a rural area, regularly drank water of unknown origin and had contact with dogs.
He had a negative Murphy sign and no history of previous alcohol abuse, smoking, or gallstones. His vital signs were within normal range (blood pressure, 124/78 mmHg; respiration, 17 breaths/min; and heart rate, 88 beats per min).
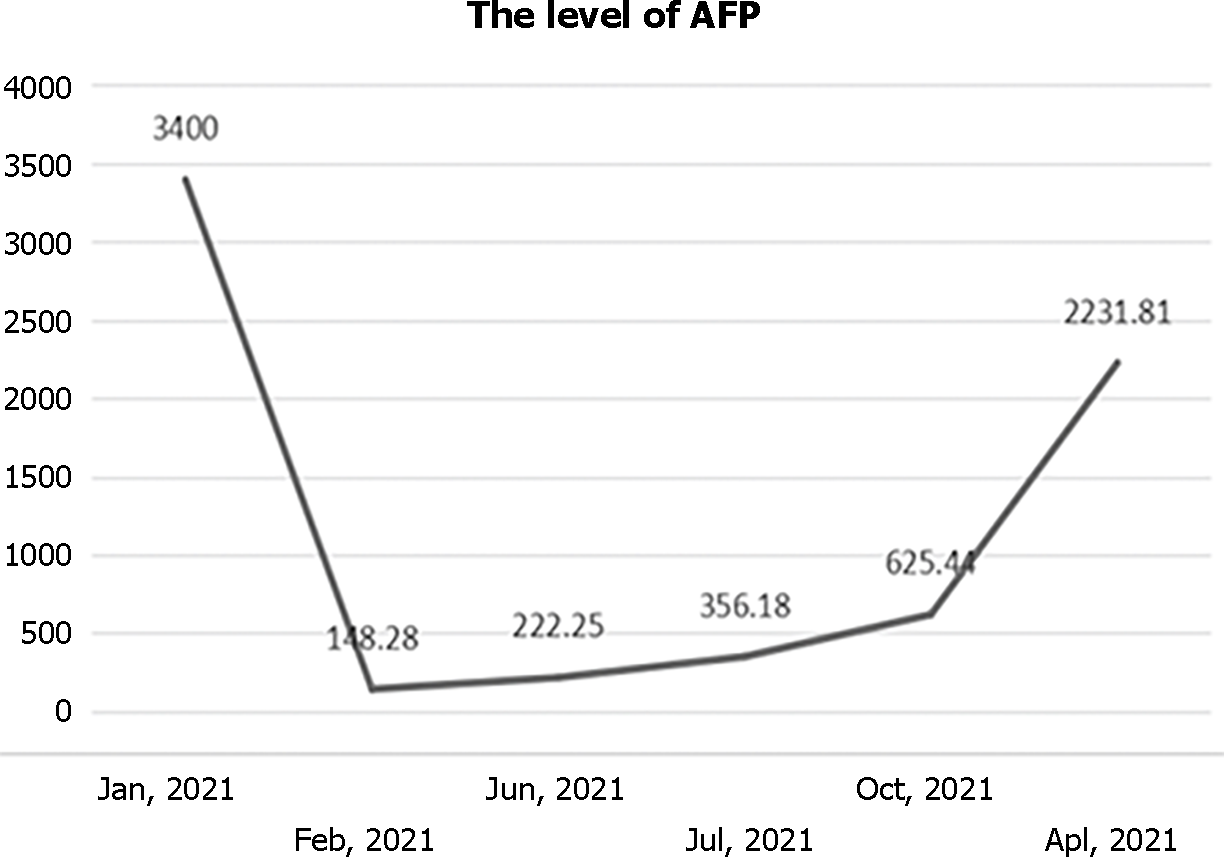
Laboratory test results were positive for HBsAg, HBeAb, HBcAb, and echinococcosis IgG antibody. The results showed an increase in the levels of direct bilirubin at 4.9 μmol/L and alpha fetoprotein (AFP) of 3400 ng/mL.
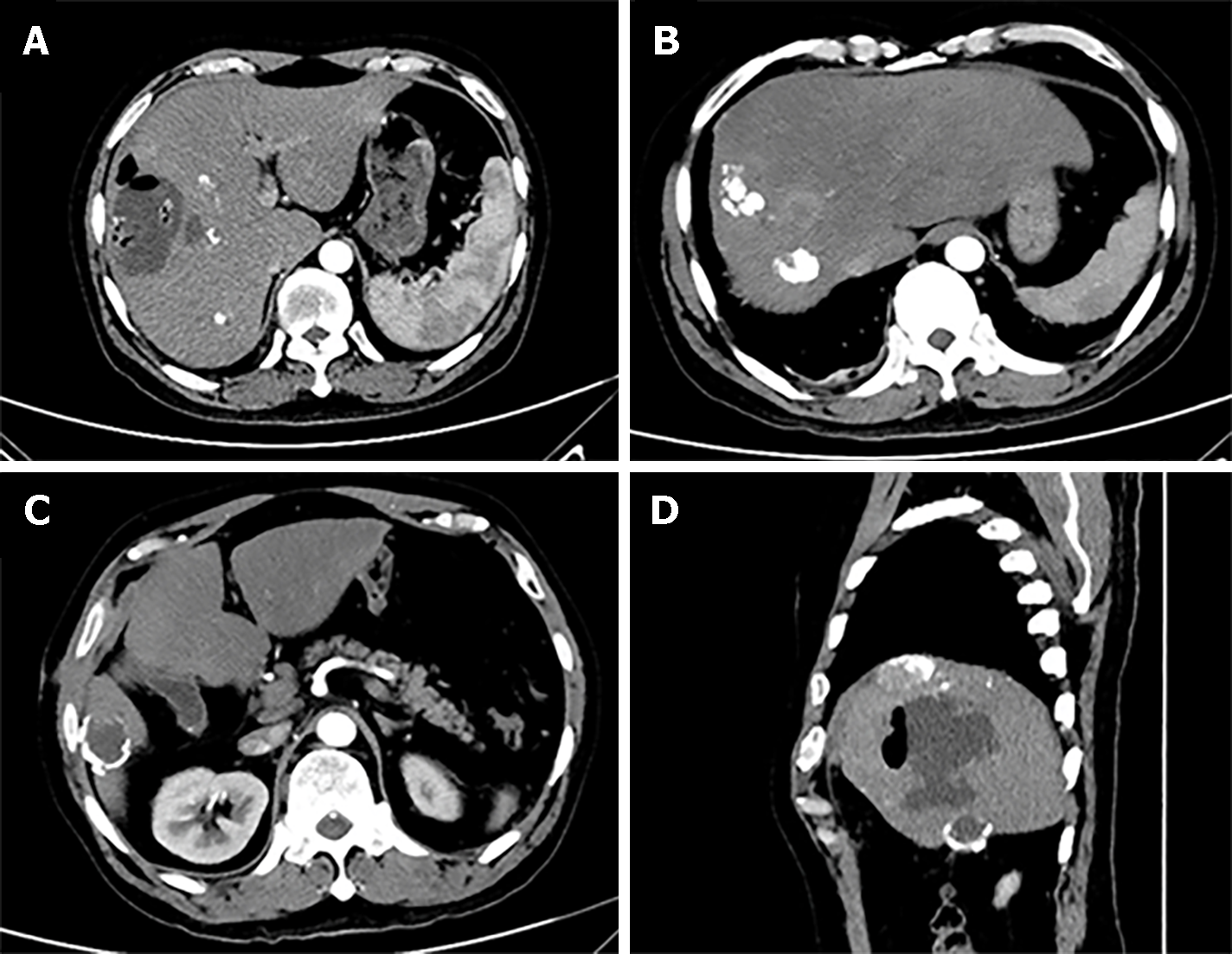
An abdominal computed tomography (CT) scan revealed an uneven enhanced lesion of the liver that measured 7.25 cm × 6.92 cm at the arterial phase with enhancement, located in the S4/8 segment of the liver (Figure 1A). The CT scan also revealed a 3.2 cm mass in the S6 segment of the liver with a thick calcified wall (Figure 1B). Magnetic resonance imaging (MRI) revealed that lesions can be observed in the S4/8 segment of the liver, with long T1 and mixed with T2 signals (Figure 1C and D).
The diagnosis of CE (at S6 segment, subtype: CE4) and HCC (at S4/8 segment) was established according to the World Health Organization Informal Working Group on Echinococcosis (WHO-IWGE) PNM classification system[14] and the European Association for the Study of the Liver (EASL) Clinical Practice Guidelines for HCC and clinical data.
Since the coexistence of CE and HCC was relatively complex, multidisciplinary discussions were performed. Based on the CT scans, CE was considered inactive (CE4 subtype; the blood supply in CE4 subtype cases is exceedingly poor) and the HCC lesion needed to be controlled, so transarterial chemoembolization (TACE) was performed (Figure 2). We used a guide wire for superselective entry into the hepatic artery and infusion of 50 mg of loplatin, 20 mg of epirubicin, and 4 mg of raltitrexed along the catheter for perfusion chemotherapy. We then used a microcatheter for selective entry into the tumor blood supply artery, through which we injected 10 mg of epirubicin, 14 mL of lipiodol suspension, and 10 mL of polyethylene microsphere suspension with a diameter of 150 μm. After embolization treatment, angiography showed lipiodol deposition in the lesion area of the cancer and the disappearance of most of the tumor blood vessels. Considering the positive results for HBsAg, HBeAb, and HBcAb, entecavir was added as an antiviral treatment. When the treatment was completed, the patient was discharged from the hospital.
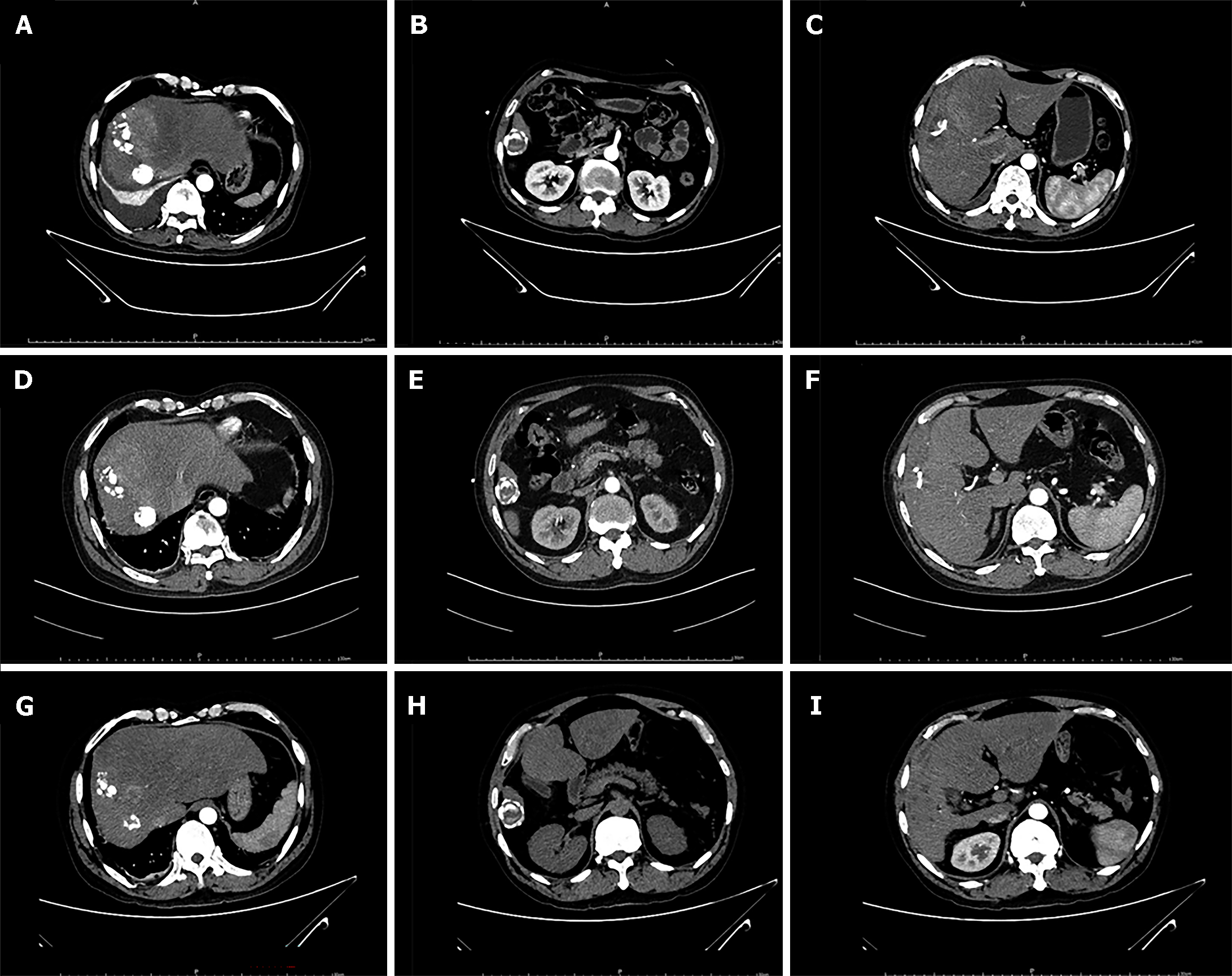
On May 7, 2021, the patient presented to our hospital with generalized abdominal pain, nausea, vomiting, and fever for 3 d. His vital signs were within the normal range. An abdominal CT scan revealed that liver abscess appeared in addition to previous HCC and CE lesions (Figure 3). HCC and CE were stable. Blood culture was performed before liver abscess drainage, with no positive results. Percutaneous liver puncture drainage was then performed to relieve the liver abscess (Figure 4); thus, purulent fluid was drained out. The liver abscess was completely resolved, and the patient was discharged with no evidence of lesion recurrence.
During the next follow-up, the HCC lesion and CE were stable, and the liver function was within normal range. The medical images of CE and HCC are shown in Figure 5. Additionally, the AFP level did not show a significant increase in the follow-up (Figure 6). However, this patient was lost to follow-up after his last follow-up in April 2022.
The prevalence of the coexistence of hepatic CE and HCC is rather low. Bo et al[15] reviewed 3300 patients with hepatic CE and 815 patients with HCC from an echinococcosis epidemic area, and they found that the coexistence incidence rate of CE and HCC was 0.39%[15]. In addition, in the few reported cases where HCC and CE coexist, there have been no reports of simultaneous liver abscess, leaving evidence-based and standard options of treatments unavailable. Regarding the treatment of this condition, we made multidisciplinary discussions according to the patient characteristics and current guidelines. According to the EASL Clinical Practice Guidelines, when treating HCC, only patients with single lesions with a maximum diameter of less than 5 cm are eligible to receive surgical resection[16], while wait-and-watch can be used when treating the CE4 subtype of CE according to the WHO-IWGE PNM classification system. Thus, during the first period of hospitalization for this patient, we mainly treated him for HCC. TACE is a standard treatment option for intermediate-stage HCC (especially for some subgroups of the barcelona clinic liver cancer-B stage HCC). Compared to open surgery, we used superselective embolization for the tumor supply arteries, which can only intervene in the tumor lesion without causing significant impact on the CE[17]. During the second period of hospitalization, percutaneous liver puncture drainage was performed to relieve liver abscess. The reason for choosing percutaneous liver puncture drainage is that the patient’s CE and HCC were in a stable state, and puncture drainage under local anesthesia will not signi
The diagnosis of CE is mainly based on the CT scan or MRI, as CT and MRI could aid in identifying the cyst and assess its location, size, and morphology[18]. CT is the gold standard for the diagnosis of CE, as it could visualize the cystic lesion and walls. Previous studies also found that CT scans could help differentiate between CE and other lesions within the liver, such as abscess and HCC[19]. Serological tests could also help with the diagnosis of CE[20]. In all, the diagnosis of this case was based on the medical image, history, and serological tests.
This case is of a patient with HCC, with HBV infection. HBV showed various mechanisms to accelerate tumor formation, initially by activating various pathways, including the WNT pathway, PI3K/MAPK pathways, and JAK/STAT pathway[21,22]. The culmination of these pathways lead to HBV-related liver disease, which undoubtedly stands as the primary risk factor for the emergence of HCC, so entecavir was added as an antiviral treatment for the patient.
In addition to the coexistence of hepatic CE and HCC, previous studies found that there may be a connection between echinococcosis and cancer[23,24]. Bo et al[15] found that echinococcus may have an anti-tumor effect on HCC, reducing tumor progression and improving survival time. In our presented case, we also speculated that one of the reasons why HCC and CE remained stable during the follow-up period may be the anti-cancer effect of CE. It has been reported that the anti-cancer effect of echinococcosis can be found in serum antigens[25,26]. There are also significant differences in immune recognition ability for different subgroups of CE and serology is often negative in these cases[27]; hence, we speculated that the anti-cancer effects of different subtypes of CE is different.
Similar to all published cases thus far, HCC and CE in our patient presented as a single lesion. Whether this is a coincidence or is because of the specific mechanisms of the coexistence of CE and HCC still need to be explored in future research. Notably, because no surgical procedures were performed for treatment, our diagnoses were based on imaging data rather than histological evidence.
Similar to any studies, this study had some limitations. First, although the diagnosis was made according to current guidelines, there is still a lack of histological testing for CE and HCC. Second, this case can only represent the experience of our center. If other clinicians encounter similar cases, personalized treatment still needs to be carried out.
The coexistence of liver abscess, hepatic CE, and HCC is rare. We reported a case of liver abscess that occurred during the follow-up period of a patient with HCC and hepatic CE. Multidisciplinary discussions were performed, and individualized treatment was given. TACE and percutaneous liver puncture drainage were performed for HCC and liver abscess, respectively. Treatment and diagnosis should be based on the characteristics of liver abscess, hepatic CE, and HCC, and in future clinical work, it is necessary to be aware of the possibility of this complex combination of liver diseases.
| 1. | McManus DP, Gray DJ, Zhang W, Yang Y. Diagnosis, treatment, and management of echinococcosis. BMJ. 2012;344:e3866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 269] [Article Influence: 19.2] [Reference Citation Analysis (1)] |
| 2. | Paternoster G, Boo G, Wang C, Minbaeva G, Usubalieva J, Raimkulov KM, Zhoroev A, Abdykerimov KK, Kronenberg PA, Müllhaupt B, Furrer R, Deplazes P, Torgerson PR. Epidemic cystic and alveolar echinococcosis in Kyrgyzstan: an analysis of national surveillance data. Lancet Glob Health. 2020;8:e603-e611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Dezsényi B, Somorácz Á, Danka J, Kucsera I, Barth TFE, Casulli A. Human cystic echinococcosis in Hungary (2000-2014): a retrospective case series analysis from a single-center study. Infection. 2018;46:477-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Manzano-Román R, Sánchez-Ovejero C, Hernández-González A, Casulli A, Siles-Lucas M. Serological Diagnosis and Follow-Up of Human Cystic Echinococcosis: A New Hope for the Future? Biomed Res Int. 2015;2015:428205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 5. | Yan JX, Deng MJ, Kong SY, Li T, Lei ZW, Zhang LS, Zhuang YT, He X, Wang HW, Fan HN, Guo YX. Transarterial chemoembolization in combination with programmed death-1/programmed cell death-ligand 1 immunotherapy for hepatocellular carcinoma: a mini review. iLIVER. 2022;1:22-234. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 6. | Begum TF, Patil VS, Zhu L, Yeh MC, González E, Fraser MA, Lu W, Zhu S, Rubio-Torio N, Ma GX, Tan Y. Assessing Physicians' Recommendations for Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) Testing Among Minority Populations in Greater Philadelphia and New York City. J Community Health. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 7. | Rabaan AA, Bello KE, Irekeola AA, Kaabi NAA, Halwani MA, Yousuf AA, Alshengeti A, Alfaraj AH, Khamis F, Al-Subaie MF, AlShehail BM, Almuthree SA, Ibraheem NY, Khalifa MH, Alfaresi M, Fares MAA, Garout M, Alsayyah A, Alshehri AA, Alqahtani AS, Alissa M. Prevalence of Hepatocellular Carcinoma in Hepatitis B Population within Southeast Asia: A Systematic Review and Meta-Analysis of 39,050 Participants. Pathogens. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 8. | Stuart KE, Anand AJ, Jenkins RL. Hepatocellular carcinoma in the United States. Prognostic features, treatment outcome, and survival. Cancer. 1996;77:2217-2222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 9. | Kalifu B, Meng Y, Maimaitinijiati Y, Ma ZG, Tian GL, Wang JG, Chen X. Radical resection of hepatic polycystic echinococcosis complicated with hepatocellular carcinoma: A case report. World J Clin Cases. 2021;9:659-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Wang Z, Yang JY, Xia P, Zhu HH, Gai ZG. Misdiagnosis of hepatic cystic echinococcosis complicated with hepatocellular carcinoma: A case report. Medicine (Baltimore). 2022;101:e32291. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Li H, Song T, Shao Y, Wen H. Cystic echinococcosis accompanied by hepatocellular carcinoma in a female herdsman. Int J Clin Exp Med. 2015;8:2985-2988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Karadas S, Dulger AC, Gonullu H, Bulut G, Beyazal M. Coexistence of hepatocelluler carcinoma and cyst hydatid disease of the liver. J Pak Med Assoc. 2014;64:1075-1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Guo J, Ma C, Song X, Tang F, Guo L, Mao J, Li Y. Hepatocellular Carcinoma Complicated by Echinococcal Cyst: A Case Report. Front Surg. 2021;8:816501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Smego RA Jr, Sebanego P. Treatment options for hepatic cystic echinococcosis. Int J Infect Dis. 2005;9:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 151] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Bo R, Yasen A, Shao Y, Zhang W, Lin R, Jiang T, Wen H, Xiao H, Aji T. Co-existence of hepatocellular carcinoma and cystic echinococcosis. Infect Agent Cancer. 2020;15:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6427] [Article Influence: 803.4] [Reference Citation Analysis (9)] |
| 17. | Liu Y, Wang YQ, Wei ZQ, Wang TX, Yang SZ, Xiang CH, Wang XD, Gong L, Dong JH, Lu Q, Zhang YW. Exploratory study of microparticle transcatheter arterial chemoembolization combined with resection for huge hepatocellular carcinoma. iLIVER. 2022;1:35-42. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Kosmidis CS, Papadopoulos K, Mystakidou CM, Sevva C, Koulouris C, Varsamis N, Mantalovas S, Lagopoulos V, Magra V, Theodorou V, Ouzouni S, Iason Katsios N, Axi P, Sapalidis K, Kesisoglou I. Giant Echinococcosis of the Liver with Suppuration: A Case Report and Review of the Literature. Medicina (Kaunas). 2023;59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 19. | Hammami A, Hellara O, Mnari W, Loussaief C, Bedioui F, Safer L, Golli M, Chakroun M, Saffar H. Unusual presentation of severely disseminated and rapidly progressive hydatic cyst: Malignant hydatidosis. World J Hepatol. 2015;7:633-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (2)] |
| 20. | Moro P, Schantz PM. Echinococcosis: a review. Int J Infect Dis. 2009;13:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 710] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 21. | Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1222] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 22. | Lan SH, Wu SY, Zuchini R, Lin XZ, Su IJ, Tsai TF, Lin YJ, Wu CT, Liu HS. Autophagy suppresses tumorigenesis of hepatitis B virus-associated hepatocellular carcinoma through degradation of microRNA-224. Hepatology. 2014;59:505-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 162] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 23. | Atayde VD, Jasiulionis MG, Cortez M, Yoshida N. A recombinant protein based on Trypanosoma cruzi surface molecule gp82 induces apoptotic cell death in melanoma cells. Melanoma Res. 2008;18:172-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Berriel E, Russo S, Monin L, Festari MF, Berois N, Fernández G, Freire T, Osinaga E. Antitumor activity of human hydatid cyst fluid in a murine model of colon cancer. ScientificWorldJournal. 2013;2013:230176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Turhan N, Esendagli G, Ozkayar O, Tunali G, Sokmensuer C, Abbasoglu O. Co-existence of Echinococcus granulosus infection and cancer metastasis in the liver correlates with reduced Th1 immune responses. Parasite Immunol. 2015;37:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Osinaga E. Expression of cancer-associated simple mucin-type O-glycosylated antigens in parasites. IUBMB Life. 2007;59:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Erginöz E, Ergün S, Tunç E, Pekmezci S. Popliteal Echinococcosis: A Long Journey from the Liver. Acta Parasitol. 2023;68:463-467. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Oprea VD, Romania S-Editor: Liu H L-Editor: A P-Editor: Chen YX