Published online May 16, 2024. doi: 10.12998/wjcc.v12.i14.2396
Revised: February 15, 2024
Accepted: April 3, 2024
Published online: May 16, 2024
Processing time: 103 Days and 5.7 Hours
Rhabdomyosarcoma (RMS) of the vagina in postmenopausal women is an extre
A 68-year-old multiparous female was admitted to the hospital on October 11, 2023, with the chief complaint of a mass causing vaginal prolapse with incomplete urination that had persisted for 4 months. The vaginal mass was approximately the size of a pigeon egg; after lying down, the vaginal mass retracted. Complete resection was performed, and vaginal pleomorphic RMS was diagnosed based on pathology and immunohistochemical staining features. The patient is currently undergoing chemotherapy. The present study also reviewed the clinical, histolo
When surgery is planned for vaginal RMS, an organ-preserving approach should be considered.
Core Tip: Rhabdomyosarcoma (RMS) of the vagina is an extremely rare malignant tumor in postmenopausal women. Here we describe a 68-year-old female admitted to hospital on October 11, 2023 with the chief complaint of a mass causing vaginal prolapse with incomplete urination that had persisted for 4 months. Complete resection was performed, and vaginal pleomorphic RMS was diagnosed based on pathology and immunohistochemical staining features. The patient is currently undergoing chemotherapy. This study also included review of the current literature to summarize clinical, histological, and immunohistochemical features of the postmenopausal vaginal RMS patients reported to date and latest treatment recommendations.
- Citation: Xu P, Ling SS, Hu E, Yi BX. Pleomorphic rhabdomyosarcoma of the vagina: A case report. World J Clin Cases 2024; 12(14): 2396-2403
- URL: https://www.wjgnet.com/2307-8960/full/v12/i14/2396.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i14.2396
Rhabdomyosarcoma (RMS) is a family of soft tissue tumors that originate from undifferentiated mesenchymal cells, which can differentiate into striated skeletal muscle[1]. The World Health Organization (WHO) has classified RMS into four histologic subtypes, namely embryonic, pleomorphic, spindle cell, and alveolar. Each subtype is linked to a specific genetic mutation profile and prognosis[2].
RMS itself is a rare illness, with an estimated 350 new cases occurring annually in the United States. While it can develop in almost any region of the body, including the head and neck, in up to 29% of cases it has arisen in genitouri
Treatments for vaginal RMS have evolved alongside advancements in medical research that have improved our overall understanding of RMS. In the past, complete removal of affected organs was often the standard approach. However, currently, more conservative and organ-preserving techniques are increasingly being used; these include local resection, radiation therapy, and chemotherapy. The goal of treatment is to achieve effective tumor control while minimizing impact on the patient’s quality of life. According to a study by Andrassy et al[5], local resection may be considered an appropriate approach. Indeed, primary chemotherapy following the initial biopsy provides excellent tumor control in these cases. Complete organ removal, such as vaginectomy or hysterectomy, is typically not necessary except in cases of persistent or recurrent disease.
A 68-year-old multiparous female was admitted to hospital on October 11, 2023, with the chief complaint of a vaginal mass causing prolapse with incomplete urination that had persisted for 4 months. Transvaginal palpitation indicated the vaginal mass to be approximately the size of a pigeon egg. Upon lying in the supine position, the vaginal mass retracted and this was accompanied by incomplete urination. The patient reported some episodes of urinary incontinence upon coughing but denied experiences of frequent urination, urgent urination, or dysuria.
The patient had no history of sexually transmitted diseases nor urinary tract infection.
The patient had a history of bilateral fallopian tubal ligation surgery but no history of cancer, hypertension, or diabetes.
The patient had given birth to 2 children, had no history of abortion, and was menopausal at the age of 41 years. She had a brother who died of lung cancer.
Gynecological examination of the anterior wall of the vagina and the rear of the urethra revealed a mass of 3.5 cm in diameter with medium texture; the bilateral ovarian fallopian tube area was not in contact with the mass. In addition, cervical and uterine atrophy was observed. The patient indicated no tenderness during the examination. The results of stress test and Bonney test were negative.
Tumor marker levels were within normal range, including carbohydrate antigen 125, lactate dehydrogenase, carbohy
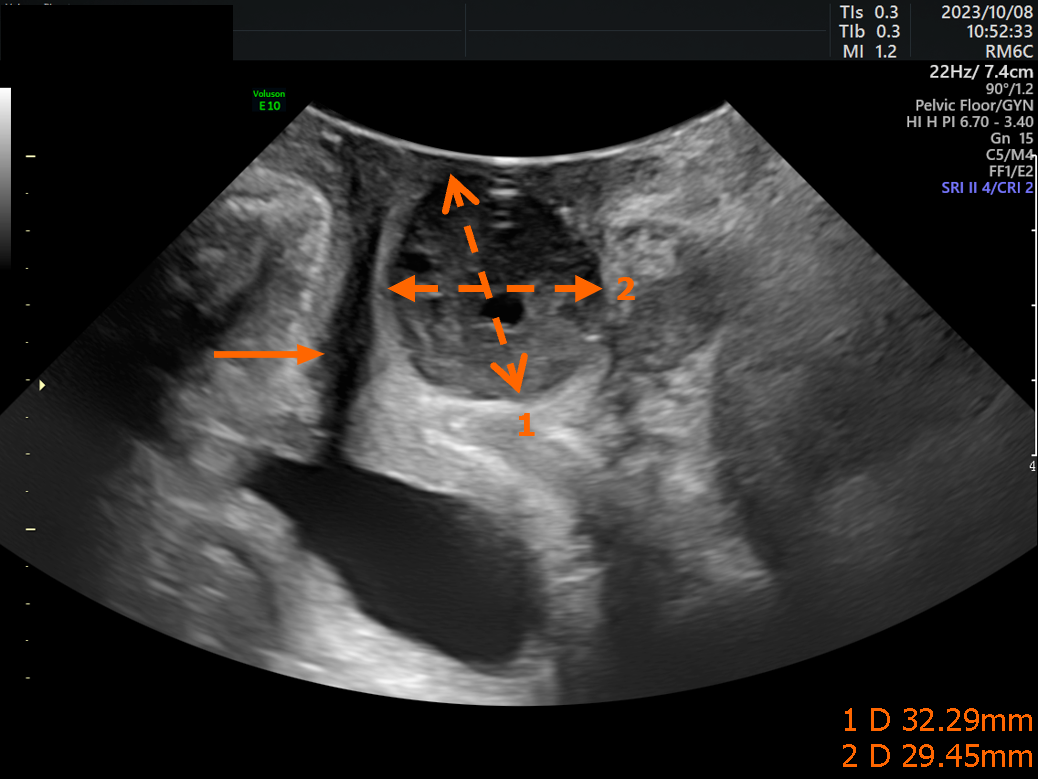
Pelvic floor three-dimensional color Doppler ultrasound examination showed a solid 3.2 cm × 2.9 cm mass involved the posterior urethra (Figure 1). Further imaging examinations, including color Doppler ultrasonography of the uterus and adnexa, showed no abnormal findings.
The patient chose to forego genetic testing, citing economic reasons.
The patient underwent cystoscope examination, complete resection of the anterior vaginal tumor, and vaginal wall repair under general anesthesia on October 12, 2023. Intraoperatively, no other mass was found in the urethra or bladder cavity.
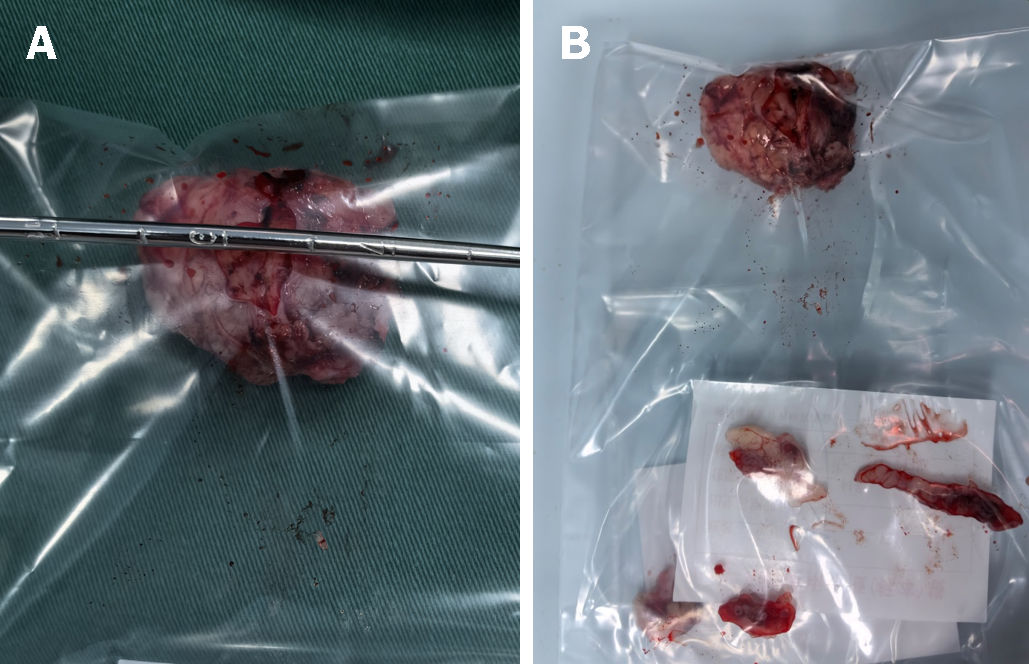
The tumor was not adhered to the urethra and was easily separated from the anterior vaginal wall using normal saline as expansion medium. Resection of the tumor included an adjacent portion of the vaginal wall (1 cm in size) for comprehensive evaluation. Gross examination defined the size of the vaginal mass to be approximately 32 mm × 30 mm × 30 mm (Figure 2A), with no obvious capsule, pale red coloration, medium texture, and resemblance to a uterine leiomyoma (Figure 2B).
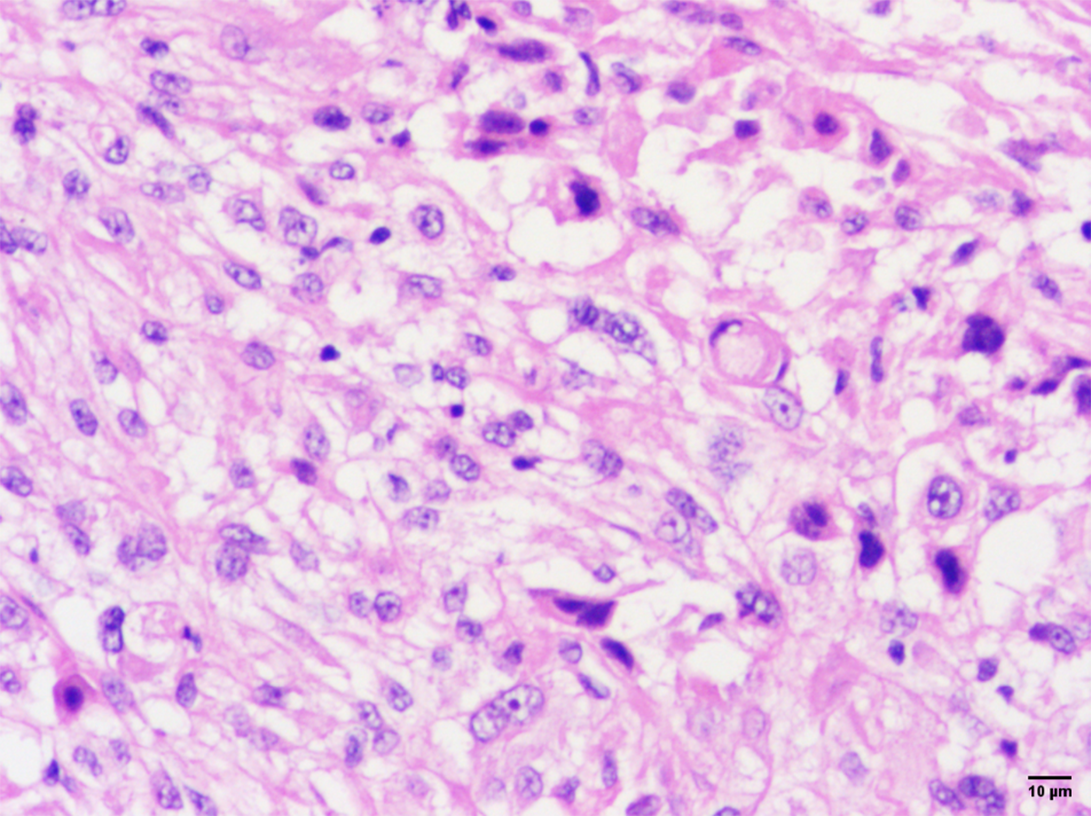
Postoperative pathology revealed pleomorphic RMS, chronic inflammation of the vaginal wall tissue, and no tumor infiltration. Microscopic examination revealed various tumor cell morphologies, including round or ovoid nuclei, deep staining, eosinophilic cytoplasm, tennis racket-like or spider-like tumor cells, and a variable number of multinucleated giant cells with deep-stained nuclei and lax interstitium. The rhabdomyoblasts observed in the pathology of our patient’s tumor displayed a range of differentiation. The predominant cell type was characterized by small and ovoid to spindled shapes, with limited cytoplasm that stained amphophilic. The nuclei of these cells appeared densely hyperchromatic, with irregular nuclear membranes and frequent apoptoses. Additionally, early differentiating rhabdomyoblasts were identified as elongated, bipolar spindled cells with varying amounts of wavy eosinophilic cytoplasm. It is worth noting that terminally differentiated rhabdomyoblasts were only observed in focal areas, as seen in Figure 3 and in agreement with the literature[6].
Immunohistochemical analysis of the tumor cells showed positivity for vimentin, Brm/Swi2-related gene 1, cluster of differentiation 68 (CD68), myosin, integrase interactor 1, desmin (individual cells), epithelial membrane antigen (individual cells), and cytokeratin (CK AE1/AE3) (individual cells) but negativity for CD34, CK5/6, p63, and myoglobin. The Ki-67 index was more than 30%. P53 was wild-type. Immunohistochemical staining for myogenin and myoblast determination protein 1 (myoD1) in the cellular aggregates were indicated rare to patchy positivity. By contrast, desmin staining was positive in the majority of tested tumors and exhibited more extensive staining compared to myogenin and myoD1, as seen in Figure 4 and in agreement with the literature[7].
Hematoxylin and eosin staining (Figure 3) and immunophenotyping (Figure 4) indicated pleomorphic RMS.
Pleomorphic RMS.
The patient is currently undergoing chemotherapy on an administration regimen of once every three weeks for doxorubicin (75 mg/m2 on the 1st day) and ifosfamide (2.5 g/m2/day, from the 1st day to the 3rd day) for a total of six courses of treatment. The main side effects have been II° bone marrow suppression, alopecia, mild nausea, and vomiting, but not to the point of treatment discontinuance. No cardiac toxicity, such as arrhythmia and hemorrhagic cystitis, has occurred, even temporarily.
Follow-up at 2 months has revealed no signs of recurrence and lifetime follow-up is recommended.
Although RMS is one of the most common soft tissue sarcomas in girls under the age of 5 years, it is an extremely rare malignant tumor of the vagina in postmenopausal women. According to a 4-decade retrospective study conducted in the United States which analyzed 144 cases of lower genital tract (vulva, vagina, cervix) RMS from 1973 to 2013, the average age of the patients was 16 years. Moreover, it was determined that vulvovaginal RMS was most common in prepubertal girls (89.1%), occurring to a much lesser extent in adolescents (3.0%), premenopausal women (2.3%), and postmenopausal women (4.6%)[2]. Among the four WHO subtypes of RMS, embryonal is the most frequently observed; the relatively uncommon pleomorphic variant tends to occur in adults and that of the spindle cell/sclerosing variant is more commonly seen in children[8]. The pathological diagnosis of our case was pleomorphic RMS.
The first report of vaginal RMS in postmenopausal women dates back to 1970 when it was described by Hilgers et al[9]. Shy et al[10] systematically summarized the cases of vaginal rhabdomyosarcoma before 1995. In 2004, Suzuki et al[11] reported a 70-year-old postmenopausal woman with history of endometrial cancer surgery, who was suffering from vaginal RMS. She was given three sessions of intravaginal radiation therapy but at 6 months after the initial treatment, the patient died from progression of the disease. We have founded 6 case reports of postmenopausal women with vaginal rhabdomyosarcoma in English, as is known in Table 1. The 5-year overall survival (OS) rate of women diagnosed with RMS in the lower genital tract is reported to be greater than 90%[12,13]. Several factors are correlated with improved OS, including younger age, lack of distant cancer spread, embryonal histology, absence of lymph node metastasis, and previous cancer-directed surgery[2].
| Ref. | Year | Age | Symptom | Stage | Surgery | Radio-therapy | Chemo-therapy | Survival |
| Hilgers et al[9] | 1970 | 60 | Bleeding | Ⅳ | TV + TAH | No | Yes | DOD at 59 months |
| Davis and Franklin[23] | 1975 | 61 | N | Ⅱ | TV + TAH + BSO | No | No | NED at 96 months |
| Hays et al[24] | 1988 | 72 | N | Ⅳ | Biopsy | Yes | Yes | DOD at 32 months |
| Shy et al[10] | 1995 | 62 | Bleeding | Ⅰ | Excision + BSO | Yes | No | NED at 12 months |
| Suzuki et al[11] | 2004 | 70 | Mass | Ⅳ | Biopsy | Yes | No | DOD at 6 months |
| Present case | 2023 | 68 | Mass | Ⅰ | Excision | No | Yes | NED at 4 months |
Over the past 30 years, there has been a revolutionary shift in the treatment of vaginal RMS to minimize long-term side effects from the treatment itself and to maintain organ function. This transformation has been driven by a growing awareness of the potential adverse effects of cancer treatments such as those induced by radical surgery and external beam radiotherapy. A more conservative and multidisciplinary approach has been adopted, which involves limited surgical intervention, local radiotherapy (brachytherapy), and chemotherapy. This combined approach has yielded promising results, with an 18% local failure rate and a 5-year OS rate of 91%. Thus, the adopted conservative treatment strategy has effectively reduced the risk of local recurrence and improved the long-term outcome of patients[5,13,14]. According to a retrospective study conducted at a single institution, the survival rate for adult RMS patients was not significantly lower than that for children with RMS if similar treatments were applied[7].
The initial evaluation of vaginal RMS typically involves pelvic magnetic resonance imaging (MRI), cystoscopy, vaginoscopy, bimanual rectovaginal examination, and color Doppler ultrasound. Local biopsy is recommended. When the tumor is small, localized, and well-defined, resection is preferred if it can be completely removed without causing significant damage to nearby normal structures. Routine assessment of surgical lymph nodes is not advised[12]. Complete removal of the tumor is correlated with positive prognosis when the patient receives subsequent chemotherapy[12]. In an international pooled analysis, 33 patients who received chemotherapy after surgical local resection of vaginal RMS but who did not undergo radiotherapy had a 10-year event-free survival of 79% and an OS rate of 97%[13]; thus, radiotherapy is not considered a necessary part of the treatment routine for vaginal RMS. Vincristine, dactinomycin, doxorubicin, and cyclophosphamide are among the most frequently utilized chemotherapeutic drugs, and more recently, iphosphamide and etoposide have also been included in treatment regimens[15].
Intracavitary brachytherapy (BT) was first described by Flamant et al[14] as a treatment for RMS of the female lower genital tract. Their patients who had received chemotherapy and BT achieved outcomes that were at least as effective as for those who had undergone radical surgery, such as total vaginectomy and hysterectomy. The BT had been applied alone or in combination with external beam radiotherapy, and the subsequent preservation of gynecological function allowed for fertility preservation with a local control rate of 94%[14]. These findings were later refined by Lautz et al[12], who showed that patients with histologically proven complete responses to chemotherapy did not require any further local control, whereas patients with residual disease could be treated effectively with chemotherapy and BT[12,16,17].
Unfortunately, our patient did not undergo a pelvic MRI examination, only Doppler ultrasound imaging evaluation. Postoperative pathology of our case revealed pleomorphic RMS with chronic inflammation of the vaginal wall tissue but no tumor infiltration. Careful and complete local resection of the tumor was possible and allowed for preservation of vaginal function. The patient has tolerated the subsequent chemotherapy well and will continue to attend follow-up.
There are great differences in chemotherapy regimens for different pathological types of rhabdomyosarcomas. Rhabdomyosarcoma can be classified into pleomorphic rhabdomyosarcoma and non-pleomorphic rhabdomyosarcoma, and the treatment approaches differ between the two. Non-pleomorphic rhabdomyosarcoma includes embryonal rhabdomyosarcoma, alveolar rhabdomyosarcoma, and spindle cell/sclerosing rhabdomyosarcoma. The chemotherapy regimen based on vincristine, actinomycin D, and cyclophosphamide is commonly used for non-pleomorphic rhabdomyosarcoma. According to the NCCN Clinical Practice Guidelines in Oncology (Soft Tissue Sarcoma, Version 2.2022)[18], doxorubicin-based combination chemotherapy is recommended for the chemotherapy of pleomorphic rhabdomyosarcoma., such as “doxorubicin + ifosfamide”, “epirubicin + ifosfamide”, “doxorubicin + dacarbazine”, “doxorubicin + ifosfamide + mesna”, and “mesna + doxorubicin + ifosfamide + dacarbazine”. Some studies have shown that post
The prognosis of RMS depends on the patient's age, tumor location in the body, pathological type, tumor size, distant metastasis, and tumor residual size after initial surgery. The incidence of polymorphic RMS increases with age, and the prognosis of adult polymorphic RMS is poor. Studies have shown that polymorphic RMS and growth in poor sites are more common in adults, with an expected 5-year OS of 27% in adults and 63% in children[22]. The good sites of tumor growth were the head and neck (non-meningeal), urogenital tract (non-bladder and prostate), bile duct area, and other adverse sites. Due to the small number of clinical cases, the data is limited. The case reported by our team occurred in postmenopausal women, but the malignant tumor grew in a good location, the tumor was less than 5 cm, the malignant tumor was completely resected, the vaginal wall margin was negative, and there was no distant metastasis. The patient has received doxorubicin-based combined chemotherapy. She is still in the process of continuous follow-up, and we expect her to have a good clinical outcome.
Vaginal pleomorphic RMS is a rare tumor, but good therapeutic effects can be achieved. Early detection of this uncommon malignancy in adult patients can significantly enhance the patient’s chances of survival. Any abnormal vaginal mass should be promptly investigated through pelvic examination and appropriate imaging; however, subsequent biopsy and pathological analysis is necessary to obtain a definitive diagnosis of RMS. The current treatment for vaginal RMS following resection is primary chemotherapy. When local treatment is planned, an organ-preserving approach should be considered, which is similar to that used for other primary sites.
The authors are grateful to the staff of the Pathology Department and Imaging Department of Jinhua People’s Hospital, specifically Professor Zhen-Wei Xie, for their guidance.
| 1. | Solomon LA, Zurawin RK, Edwards CL. Vaginoscopic resection for rhabdomyosarcoma of the vagina: a case report and review of the literature. J Pediatr Adolesc Gynecol. 2003;16:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Nasioudis D, Alevizakos M, Chapman-Davis E, Witkin SS, Holcomb K. Rhabdomyosarcoma of the lower female genital tract: an analysis of 144 cases. Arch Gynecol Obstet. 2017;296:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Zhan XK, Zhang S, Cao BW, Wang JW, Li JL, Sun YK, Zhang W, Yang L, Zhou AP, Chi YH, Li YX, Ma JH, Li CL. Clinicopathological characteristics and treatment outcomes of Chinese patients with genitourinary embryonal rhabdomyosarcoma. World J Surg Oncol. 2015;13:190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Filipas D, Fisch M, Stein R, Gutjahr P, Hohenfellner R, Thüroff JW. Rhabdomyosarcoma of the bladder, prostate or vagina: the role of surgery. BJU Int. 2004;93:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Andrassy RJ, Wiener ES, Raney RB, Hays DM, Arndt CA, Lobe TE, Lawrence W, Anderson JR, Qualman SJ, Crist WM. Progress in the surgical management of vaginal rhabdomyosarcoma: a 25-year review from the Intergroup Rhabdomyosarcoma Study Group. J Pediatr Surg. 1999;34:731-4; discussion 734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Devins KM, Young RH, Ghioni M, Burandt E, Bennett JA, Oliva E. Embryonal Rhabdomyosarcoma of the Uterine Cervix: A Clinicopathologic Study of 94 Cases Emphasizing Issues in Differential Diagnosis Staging, and Prognostic Factors. Am J Surg Pathol. 2022;46:1477-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 7. | Ferrari A, Dileo P, Casanova M, Bertulli R, Meazza C, Gandola L, Navarria P, Collini P, Gronchi A, Olmi P, Fossati-Bellani F, Casali PG. Rhabdomyosarcoma in adults. A retrospective analysis of 171 patients treated at a single institution. Cancer. 2003;98:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 274] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 8. | Jadhav T, Madakshira MG, Garud S. Embryonal rhabdomyosarcoma of the uterine cervix in an adult female. Autops Case Rep. 2023;13:e2023419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 9. | Hilgers RD, Malkasian GD Jr, Soule EH. Embryonal rhabdomyosarcoma (botryoid type) of the vagina. A clinicopathologic review. Am J Obstet Gynecol. 1970;107:484-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 43] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Shy SW, Lee WH, Chen D, Ho SY. Rhabdomyosarcoma of the vagina in a postmenopausal woman: report of a case and review of the literature. Gynecol Oncol. 1995;58:395-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Suzuki Y, Sakurai H, Hasegawa M, Kanuma T, Segawa A, Nakano T. A case of rhabdomyosarcoma of the vagina in an elderly woman. Eur J Gynaecol Oncol. 2004;25:509-511. [PubMed] |
| 12. | Lautz TB, Martelli H, Fuchs J, Chargari C, Smeulders N, Granberg CF, Wolden SL, Sparber-Sauer M, Hawkins DS, Bisogno G, Koscielniak E, Rodeberg DA, Seitz G; INSTRuCT group. Local treatment of rhabdomyosarcoma of the female genital tract: Expert consensus from the Children's Oncology Group, the European Soft-Tissue Sarcoma Group, and the Cooperative Weichteilsarkom Studiengruppe. Pediatr Blood Cancer. 2023;70:e28601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Minard-Colin V, Walterhouse D, Bisogno G, Martelli H, Anderson J, Rodeberg DA, Ferrari A, Jenney M, Wolden S, De Salvo G, Arndt C, Merks JHM, Gallego S, Schwob D, Haie-Meder C, Bergeron C, Stevens MCG, Oberlin O, Hawkins D; International Society of Pediatric Oncology Sarcoma Committee, the Children's Oncology Group, the Italian Cooperative Soft Tissue Sarcoma Group, and the European pediatric Soft tissue sarcoma Study Group. Localized vaginal/uterine rhabdomyosarcoma-results of a pooled analysis from four international cooperative groups. Pediatr Blood Cancer. 2018;65:e27096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Flamant F, Gerbaulet A, Nihoul-Fekete C, Valteau-Couanet D, Chassagne D, Lemerle J. Long-term sequelae of conservative treatment by surgery, brachytherapy, and chemotherapy for vulval and vaginal rhabdomyosarcoma in children. J Clin Oncol. 1990;8:1847-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 54] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Narayanan G, Rajan V, Soman LV. Rhabdomyosarcoma of the Vagina in an Adolescent Girl. J Pediatr Adolesc Gynecol. 2017;30:649-651. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Fuchs J, Paulsen F, Bleif M, Lamprecht U, Weidner N, Zips D, Neunhoeffer F, Seitz G. Conservative surgery with combined high dose rate brachytherapy for patients suffering from genitourinary and perianal rhabdomyosarcoma. Radiother Oncol. 2016;121:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Morcos M, Vogel J, Garcia JR, Gomez-Lobo V, Bartolac S. Treatment of pediatric vaginal rhabdomyosarcoma with the use of a real-time tracked custom applicator. Brachytherapy. 2022;21:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | von Mehren M, Kane JM, Agulnik M, Bui MM, Carr-Ascher J, Choy E, Connelly M, Dry S, Ganjoo KN, Gonzalez RJ, Holder A, Homsi J, Keedy V, Kelly CM, Kim E, Liebner D, McCarter M, McGarry SV, Mesko NW, Meyer C, Pappo AS, Parkes AM, Petersen IA, Pollack SM, Poppe M, Riedel RF, Schuetze S, Shabason J, Sicklick JK, Spraker MB, Zimel M, Hang LE, Sundar H, Bergman MA. Soft Tissue Sarcoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:815-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 247] [Reference Citation Analysis (0)] |
| 19. | DeLaney TF, Spiro IJ, Suit HD, Gebhardt MC, Hornicek FJ, Mankin HJ, Rosenberg AL, Rosenthal DI, Miryousefi F, Ancukiewicz M, Harmon DC. Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2003;56:1117-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 221] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 20. | Kraybill WG, Harris J, Spiro IJ, Ettinger DS, DeLaney TF, Blum RH, Lucas DR, Harmon DC, Letson GD, Eisenberg B; Radiation Therapy Oncology Group Trial 9514. Phase II study of neoadjuvant chemotherapy and radiation therapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. J Clin Oncol. 2006;24:619-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Kraybill WG, Harris J, Spiro IJ, Ettinger DS, DeLaney TF, Blum RH, Lucas DR, Harmon DC, Letson GD, Eisenberg B. Long-term results of a phase 2 study of neoadjuvant chemotherapy and radiotherapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. Cancer. 2010;116:4613-4621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, Ganjoo KN, George S, Gonzalez RJ, Heslin MJ, Kane JM, Keedy V, Kim E, Koon H, Mayerson J, McCarter M, McGarry SV, Meyer C, Morris ZS, O'Donnell RJ, Pappo AS, Paz IB, Petersen IA, Pfeifer JD, Riedel RF, Ruo B, Schuetze S, Tap WD, Wayne JD, Bergman MA, Scavone JL. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:536-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 476] [Article Influence: 68.0] [Reference Citation Analysis (1)] |
| 23. | Davis PC, Franklin EW 3rd. Cancer of the vagina. South Med J. 1975;68:1239-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade D
Novelty: Grade C
Creativity or Innovation: Grade B
Scientific Significance: Grade C
P-Reviewer: Sugai S, Japan S-Editor: Che XX L-Editor: A P-Editor: Chen YX