Published online May 6, 2024. doi: 10.12998/wjcc.v12.i13.2275
Peer-review started: December 23, 2023
First decision: January 30, 2024
Revised: February 9, 2024
Accepted: March 28, 2024
Article in press: March 28, 2024
Published online: May 6, 2024
Processing time: 123 Days and 23.8 Hours
Thymic carcinoid (TC) is a rare entity among anterior mediastinal malignancies. TCs are neuroendocrine carcinomas that constitute approximately 2%–5% of all thymic epithelial tumors.
The study reported a rare TC with multiple bone metastases. A 77-year-old man presented with a 2-month history of lower back pain and weight loss of 5 kg. Magnetic resonance imaging scans revealed damage to the lumbar spine, sacro
These findings demonstrated that anlotinib is a promising treatment regimen for patients with TC and multiple bone metastases.
Core Tip: Here, we report a 77-year-old man diagnosed with thymic carcinoid (TC) with multiple bone metastases, and the study reports the rapid contraction of thymoid carcinoma after therapy with the multitargeted tyrosine kinase inhibitor anlotinib. The treatment has shown good efficacy, with a long stable disease benefit of more than 2.5 years (until March 2022). These findings demonstrated that anlotinib is a promising treatment regimen for TC tumors with multiple bone metastases.
- Citation: Chen CQ, Huang MY, Pan M, Chen QQ, Wei FF, Huang H. Thymic carcinoid with multiple bone metastases: A case report. World J Clin Cases 2024; 12(13): 2275-2280
- URL: https://www.wjgnet.com/2307-8960/full/v12/i13/2275.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i13.2275
Thymic carcinoid (TC) tumors are malignant and clinically rare tumors originating from thymic neuroendocrine cells. These tumors are different from thymomas and thymic carcinomas and have their own clinical and pathological characteristics. These tumors were first described as separate entities by Rosai and Higa[1] in 1972 and were named TC tumors. The latest World Health Organization classification from 2015 grouped lung and thymic neuroendocrine tumors within one unique group[2]. TC tumors, also known as highly differentiated neuroendocrine tumors, are divided into typical carcinoid tumors and atypical carcinoid tumors according to their morphology, among which atypical carcinoid tumors have a greater degree of malignancy and invasion. There is a lack of understanding of thymic carcinogenesis and no clear standardized diagnosis or treatment strategies due to the rarity of this disease; therefore, further knowledge is required for its clinical diagnosis and treatment. This report describes a 77-year-old man diagnosed with TC with multiple bone metastases, and the study reports the rapid contraction of thymoid carcinoma after therapy with the multitargeted ty
A 77-year-old man presented with a complaint of a 2-month history of lower back pain and weight loss of 5 kg.
Initial symptoms included two months of lower back pain and a weight loss of 5 kg.
He had a past medical history of coronary heart disease, hypertension and lacunar cerebral infarction. The patient’s past medical history was not significant for night sweats, fevers, facial flushing, or diarrhea.
The patient denied any family history of malignant tumors.
Physical examination revealed marked tenderness to percussion over the lumbar 2 vertebra, an Eastern Cooperative Oncology Group score above 1 and a pain score above 4. His physical examination revealed no signs of adenopathy or organomegaly.
Laboratory tests showed an increase in serum neuron specific enolase (NSE) levels, reaching 32.05 mg/L (normal range < 13 mg/L); alpha-fetoprotein 3.29 ng/mL; alkaline phosphatase 95 U/L; β2-MB 2.15 mg/L.
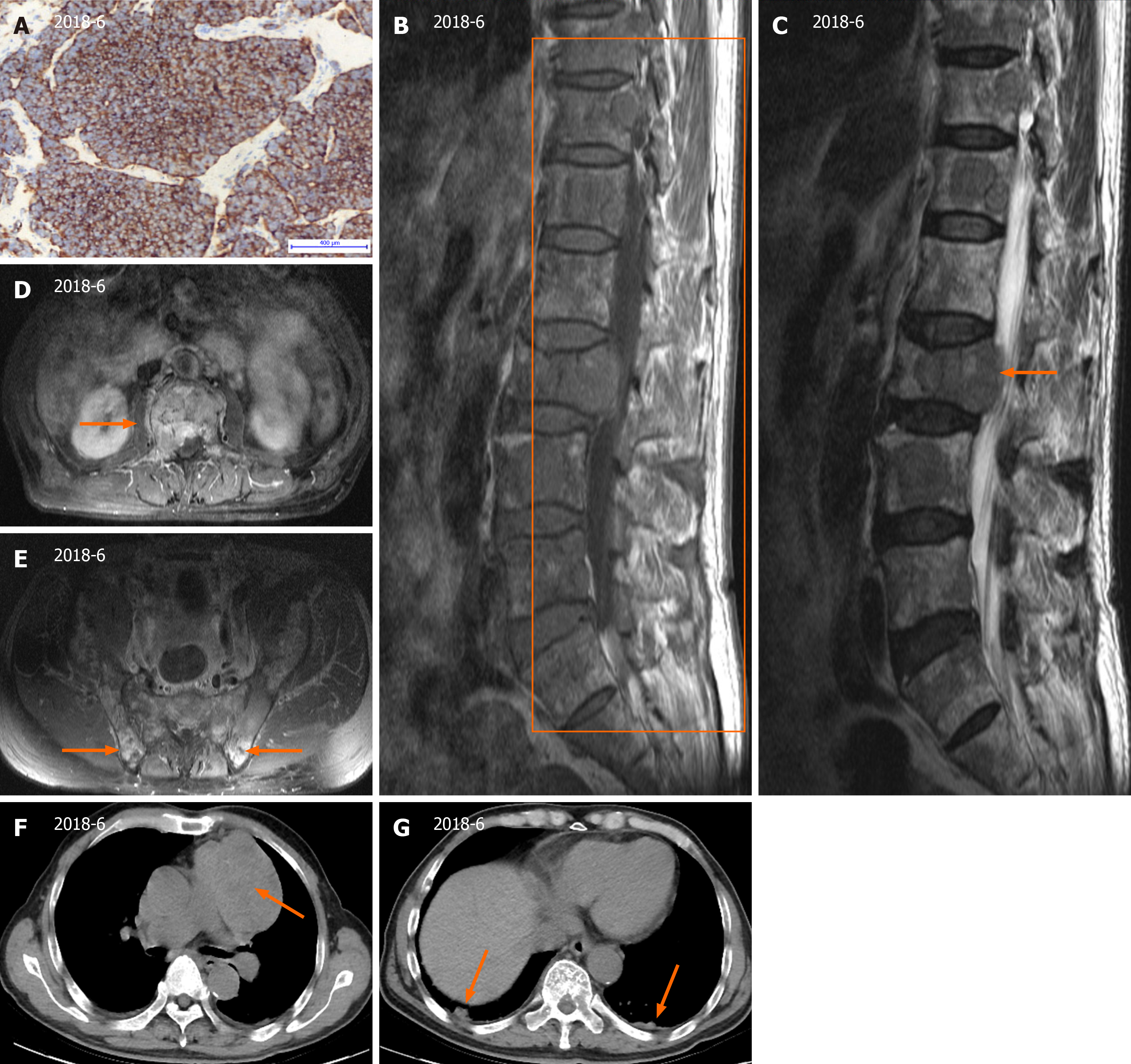
The original percutaneous needle biopsy of the mediastinal mass revealed predominantly single small, round to oval cells with scant cytoplasm and some loose clusters with a mitotic rate > 2/10 high power fields (Figure 1A). Immunohistochemical staining showed positive for common neuroendocrine markers, such as chromogranin A, cytokeratin-Pan, CD56 and synaptophysin, but negative results for cytokeratin7 and thyroid transcription factor-1. The Ki67 index of the tumor moderately increased.
Magnetic resonance imaging of the lumbar region of the spine revealed multifocal metastatic lesions, an L2 vertebral compression fracture, and an L1/2-L5/S1 fusion with degenerative disc disease (Figure 1B and C). On computed to
In summary, the patient was diagnosed with moderately differentiated neuroendocrine carcinoma (atypical carcinoid).
Tumor cells extensively invade the lung and various bones and cannot be removed surgically. The patient was ad
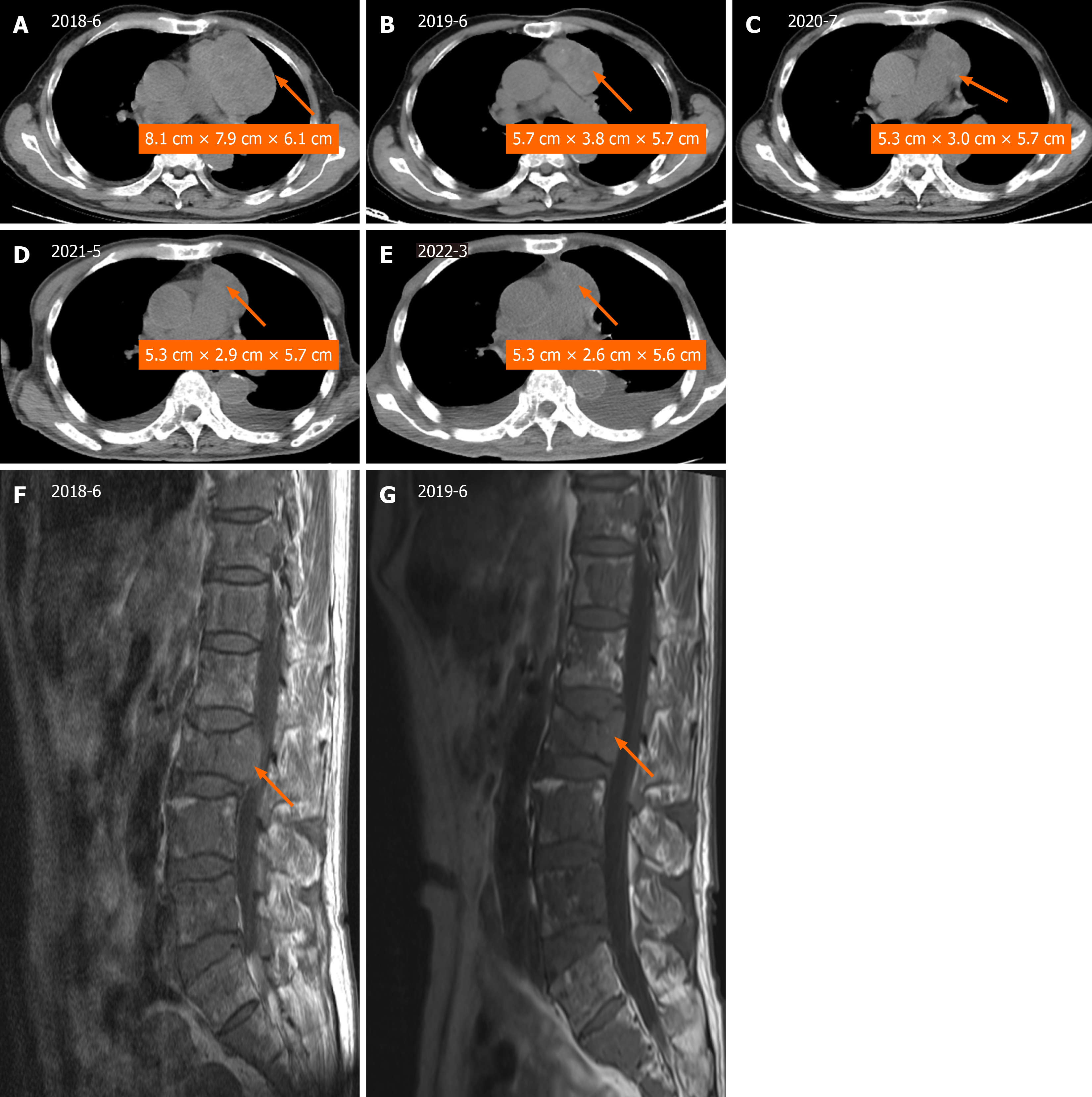
Treatment has shown good efficacy, with a long stable disease (SD) benefit of more than 2.5 years (until March 2022) (Figure 2). During the year of anlotinib-targeted therapy, a follow-up CT scan of the chest taken in June 2019 demon
| Date | 2018-06 | 2018-08 | 2019-06 | 2020-07 | 2021-05 | 2022-03 |
| NSE (ng/mL) | 32.05 | 111.6 | 51.6 | 35.83 | 41.51 | 25.34 |
TCs are uncommon lesions. The clinical characteristics of TC tumors are male predominance, difficult diagnosis, high malignancy, frequent recurrence and extrathoracic metastasis over a long period of time[3]. In fact, TC tumors are amine precursor uptake and decarboxylation tumors and thus can exhibit endocrine function. TCs associated with Cushing’s syndrome are more common than other types of carcinoids and account for 29% to 38% of all TCs[4]. In addition, TCs are usually slow-growing tumors that cause symptoms only when they begin to exert pressure on adjacent structures. Some patients have no clinical symptoms at all, and only thymic masses are found at physical examination. Symptoms can vary greatly, as in our case, in which the disease was acutely manifested as lumbar pain caused by lumbar metastasis compression and nerve root stimulation. Such cases have not been previously reported.
TC tumors have a greater rate of metastatic disease than carcinoid tumors in other locations. The 5-year survival rate of TCs is 60%, and recurrence is common[3]. The prognosis of these tumors is poor, even in patients whose tumors appear favorable in terms of resectability and histology. With the development of molecular pharmacology, targeted therapy has gradually become possible[5]. In fact, in this case, the patient was assessed as inoperable due to tumor invasion into the mediastinum and multiple bone metastases from thymic cancer, and a new adjuvant strategy was adopted to induce tu
Anlotinib, a multiple tyrosine kinase receptor, is a potent anti-angiogenic and anti-tumor drug that inhibits signal transduction vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptors, fibroblast growth factor receptor, and c-KIT[6]. Anlotinib has been shown to inhibit the osteosarcoma cell lines by affecting cell proliferation and the protein levels of the VEGFR2 and MET signaling pathways[7]. Additionally, anlotinib inhibits cell viability and induces apoptosis in lung cancer cells, which in turn enhances the cytotoxicity of anlotinib and amplifies its antiangiogenic effect through Janus kinase 2/signal transducer and activator of transcription 3/vascular endothelial growth factor A signaling[8]. In human trials, Han et al[9] and Zhang et al[10] reported that, compared with placebo, an
However, in our patient, anlotinib therapy was administered with the neoadjuvant intent of increasing the disease con
This report demonstrates that anlotinib is a promising treatment regimen for TC tumors with multiple bone metastases.
| 1. | Rosai J, Higa E. Mediastinal endocrine neoplasm, of probable thymic origin, related to carcinoid tumor. Clinicopathologic study of 8 cases. Cancer. 1972;29:1061-1074. [PubMed] [DOI] [Full Text] |
| 2. | Marx A, Chan JK, Coindre JM, Detterbeck F, Girard N, Harris NL, Jaffe ES, Kurrer MO, Marom EM, Moreira AL, Mukai K, Orazi A, Ströbel P. The 2015 World Health Organization Classification of Tumors of the Thymus: Continuity and Changes. J Thorac Oncol. 2015;10:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 439] [Article Influence: 43.9] [Reference Citation Analysis (2)] |
| 3. | Bakhos CT, Salami AC, Kaiser LR, Petrov RV, Abbas AE. Thymic Neuroendocrine Tumors and Thymic Carcinoma: Demographics, Treatment, and Survival. Innovations (Phila). 2020;15:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Filosso PL, Ruffini E, Solidoro P, Roffinella M, Lausi PO, Lyberis P, Oliaro A, Guerrera F. Neuroendocrine tumors of the thymus. J Thorac Dis. 2017;9:S1484-S1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Liang Y, Ji S, Yu X, Chen J. [Updates on medical treatment for neuroendocrine neoplasm]. Zhongguo Aizheng Zazhi. 2022;32:757-764. [DOI] [Full Text] |
| 6. | Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, Zhao F, Ahmad R, Zhao J. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 490] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 7. | Wang G, Sun M, Jiang Y, Zhang T, Sun W, Wang H, Yin F, Wang Z, Sang W, Xu J, Mao M, Zuo D, Zhou Z, Wang C, Fu Z, Duan Z, Hua Y, Cai Z. Anlotinib, a novel small molecular tyrosine kinase inhibitor, suppresses growth and metastasis via dual blockade of VEGFR2 and MET in osteosarcoma. Int J Cancer. 2019;145:979-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Liang L, Hui K, Hu C, Wen Y, Yang S, Zhu P, Wang L, Xia Y, Qiao Y, Sun W, Fei J, Chen T, Zhao F, Yang B, Jiang X. Autophagy inhibition potentiates the anti-angiogenic property of multikinase inhibitor anlotinib through JAK2/STAT3/VEGFA signaling in non-small cell lung cancer cells. J Exp Clin Cancer Res. 2019;38:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 152] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 9. | Han B, Li K, Wang Q, Zhang L, Shi J, Wang Z, Cheng Y, He J, Shi Y, Zhao Y, Yu H, Chen W, Luo Y, Wu L, Wang X, Pirker R, Nan K, Jin F, Dong J, Li B, Sun Y. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2018;4:1569-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 497] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 10. | Zhang RS, Liu J, Deng YT, Wu X, Jiang Y. The real-world clinical outcomes and treatment patterns of patients with unresectable locally advanced or metastatic soft tissue sarcoma treated with anlotinib in the post-ALTER0203 trial era. Cancer Med. 2022;11:2271-2283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Cheng Y, Wang Q, Li K, Shi J, Liu Y, Wu L, Han B, Chen G, He J, Wang J, Lou D, Yu H, Qin H, Li XL. Overall survival (OS) update in ALTER 1202: Anlotinib as third-line or further-line treatment in relapsed small-cell lung cancer (SCLC). Ann Oncol. 2019;30:v711. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Huang J, Xiao J, Fang W, Lu P, Fan Q, Shu Y, Feng J, Zhang S, Ba Y, Zhao Y, Liu Y, Bai C, Bai Y, Tang Y, Song Y, He J. Anlotinib for previously treated advanced or metastatic esophageal squamous cell carcinoma: A double-blind randomized phase 2 trial. Cancer Med. 2021;10:1681-1689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mallin MM, United States S-Editor: Che XX L-Editor: A P-Editor: Xu ZH