Published online May 6, 2024. doi: 10.12998/wjcc.v12.i13.2182
Peer-review started: February 1, 2024
First decision: February 28, 2024
Revised: March 8, 2024
Accepted: March 28, 2024
Article in press: March 28, 2024
Published online: May 6, 2024
Processing time: 83 Days and 18.4 Hours
Liver metastases (LM) is the primary factor contributing to unfavorable outcomes in patients diagnosed with gastric cancer (GC). The objective of this study is to analyze significant prognostic risk factors for patients with GCLM and develop a reliable nomogram model that can accurately predict individualized prognosis, thereby enhancing the ability to evaluate patient outcomes.
To analyze prognostic risk factors for GCLM and develop a reliable nomogram model to accurately predict individualized prognosis, thereby enhancing patient outcome assessment.
Retrospective analysis was conducted on clinical data pertaining to GCLM (type III), admitted to the Department of General Surgery across multiple centers of the Chinese PLA General Hospital from January 2010 to January 2018. The dataset was divided into a development cohort and validation cohort in a ratio of 2:1. In the development cohort, we utilized univariate and multivariate Cox regression analyses to identify independent risk factors associated with overall survival in GCLM patients. Subsequently, we established a prediction model based on these findings and evaluated its performance using receiver operator characteristic curve analysis, calibration curves, and clinical decision curves. A nomogram was created to visually represent the prediction model, which was then externally validated using the validation cohort.
A total of 372 patients were included in this study, comprising 248 individuals in the development cohort and 124 individuals in the validation cohort. Based on Cox analysis results, our final prediction model incorporated five independent risk factors including albumin levels, primary tumor size, presence of extrahepatic metastases, surgical treatment status, and chemotherapy administration. The 1-, 3-, and 5-years Area Under the Curve values in the development cohort are 0.753, 0.859, and 0.909, respectively; whereas in the validation cohort, they are observed to be 0.772, 0.848, and 0.923. Furthermore, the calibration curves demonstrated excellent consistency between observed values and actual values. Finally, the decision curve analysis curve indicated substantial net clinical benefit.
Our study identified significant prognostic risk factors for GCLM and developed a reliable nomogram model, de
Core Tip: This study identifies pivotal prognostic factors and introduces a nomogram model for predicting individualized prognosis in gastric cancer liver metastases (GCLM). The developed model, supported by comprehensive validation, showcases substantial potential for improving patient outcome evaluation. Notably, the incorporation of five independent risk factors demonstrates promising predictive accuracy, paving the way for enhanced clinical decision-making in managing GCLM patients, ultimately offering valuable insights for personalized treatment strategies.
- Citation: Chang ZY, Gao WX, Zhang Y, Zhao W, Wu D, Chen L. Establishment and evaluation of a prognostic model for patients with unresectable gastric cancer liver metastases. World J Clin Cases 2024; 12(13): 2182-2193
- URL: https://www.wjgnet.com/2307-8960/full/v12/i13/2182.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i13.2182
Among all malignant tumor types, gastric cancer (GC) has the highest morbidity and fatality rates. It is one of the most frequent malignant tumors in the world[1]. With the development of treatment technology, the prognosis of patients with GC continues to improve. However, the 5-year overall survival rate (OS) of GC is only about 5%-20%[2]. Therefore, many studies have focused on exploring and analyzing the factors affecting the prognosis of GC patients, such as tumor size and distant metastases. Liver is the most common distant metastases organ. The incidence of GC with liver metastases (GCLM) is 5%-34%[3], which is the main cause of poor prognosis of GC patients[4]. Although the comprehensive treatment technology has made some progress, the prognosis of GCLM is still not ideal[5]. Therefore, effective individualized treatment and comprehensive prognosis evaluation for GCLM patients are of great significance for the implementation of clinical strategies.
Based on a large number of clinical data, nomogram prediction models are widely used to evaluate the prognosis of patients with various types of cancer by combining multiple independent prognostic evaluation factors and quantifying individual survival risk[6-8]. Previous studies have explored the clinical prognostic factors of GCLM patients, but due to the small case size and incomplete research content, the analysis of the prognosis of patients is limited.
Due to the great differences in pathological types, clinical manifestations, tumor size and clinical stage among different types of GCLM patients, the prediction of disease prognosis and the selection of diagnosis and treatment methods are still controversial in clinical practice. Chinese type for GCLM (C-GCLM)[9] is a new clinical classification standard proposed by Chinese experts, which has a high reference value for clinical diagnosis and treatment decisions. This study focused on patients with C-GCLM type III, namely unresectable patients, and developed a prediction model to improve the ability to evaluate the individualized prognosis of patients.
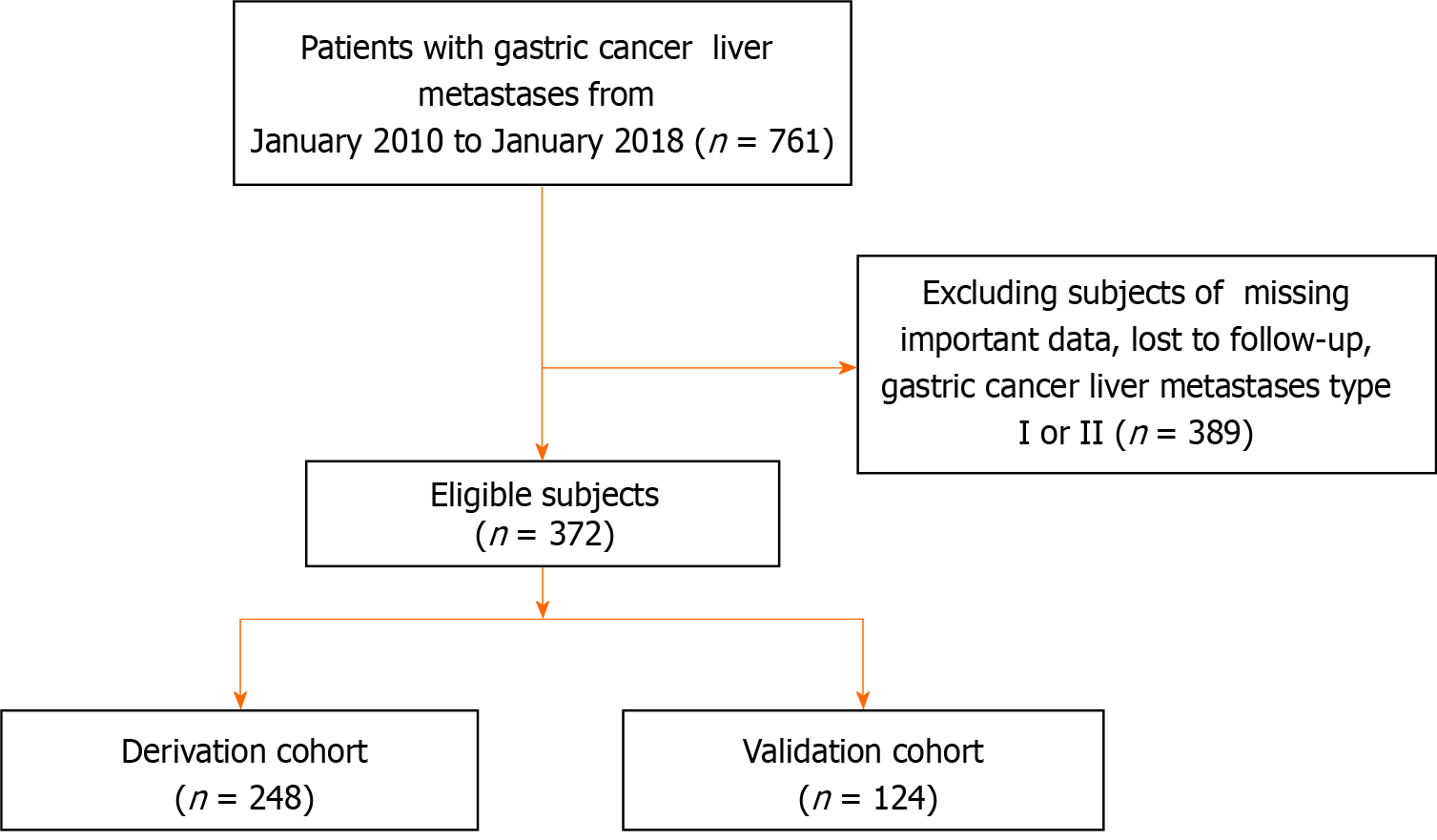
A total of 761 individuals diagnosed with GCLM were selected for this study from January 2010 to January 2018 at multiple centers within the Chinese PLA General Hospital's General Surgery Department. Following the exclusion of participants who were lost during follow-up, GCLM Type I and Type II, or lacking essential clinical data, a final cohort of 372 patients was included (Figure 1). The Ethics Committee of the Chinese PLA General Hospital approved this study (S2023-724-02), and all participant information was anonymized prior to analysis.
Obtaining demographic information and clinical data from electronic medical record systems, Age, gender, height, weight, drinking habits, tumor size, tumor location, metastases size, metastases location, aspartate aminotransferase, alanine aminotransferase, hemoglobin, albumin, γ-glutamyl transferase, chemotherapy, surgery, etc. All the above data were collected and reviewed by uniformly trained professionals.
The classification of GCLM was formulated by the consensus of Chinese experts[9], and the specific classification criteria were as follows: Type I: (1) The invasion depth of the primary tumor of GC was ≤ T4a, and the lymph node metastases was within the D2 dissection range (Bulky N2 was not included); Bulky N2-presence of at least one lymph node ≥ 3 cm in diameter or at least three adjacent lymph nodes ≥ 1.5 cm in diameter along the hepatic, celiac, or splenic arteries; and (2) 1-3 LM; the maximum diameter of the metastatic lesions was ≤ 4 cm or they were confined to one lobe of the liver and did not involve important blood vessels or bile ducts. Type II: (1) The invasion depth of the primary tumor was T4b, or Bulky N2, or Bulky No. 16a2, b1-abdominal aortic lymph nodes; and (2) the number and size of LM were beyond the scope of Type I, but surgical techniques for removal are possible. Type III: (1) Primary GC significantly invaded adjacent tissues or organs; regional lymph nodes such as mesenteric or paraaortic lymph nodes were fixed, fused, or unresectable and confirmed by imaging studies or biopsy; and (2) LM were divided into type III a, bilobar multiple diffuse metastases without extrahepatic metastases, and type III b, LM with one or more extrahepatic organs with or without peritoneal metastases. The difference between the date of GCLM diagnosis and the date of death or the final follow-up was known as overall survival.
There were two cohorts created: One for derivation and the other for validation, with a 2:1 ratio. Utilizing chi-square analyses, categorical variables were compared and are shown as percentages (%). Continuous variable data were presented as the median and interquartile range (25th, 75th). Mann-Whitney U test was applied for comparing differences between groups for continuous variables. The 20 clinical factors underwent univariate Cox regression analysis to evaluate their individual associations with the outcome. Subsequently, significant prognostic variables (P < 0.05) related to GCLM were incorporated into multivariate Cox regression to identify independent risk factors for predicting patient prognosis. A nomogram was created to visualize the model and calculate 1-, 3-, and 5-years overall survival rates. The predictive accuracy of the prediction model was evaluated using receiver operating characteristic (ROC) curves in both deve
This study analyzed 372 individuals diagnosed with GCLM, with an average age of 60 years. The group consisted of 306 males and 66 females. Table 1 displays the patients' fundamental characteristics. 96 (25.8%) patients underwent surgery, 72 (19.4%) patients had concurrent extrahepatic metastases, and 60 (16.1%) patients received chemotherapy. All participants were assigned randomly to the development group (n = 248) and the validation group (n = 124), as a ratio of 2:1, prior to further analysis. According to the results, there were no appreciable variations based on gender, age, tumor site, primary tumor dimensions, surgical procedures, radiotherapy or chemotherapy between the modeling and validation groups (all P > 0.05).
| Variables | Derivation cohort | Validation cohort | P value |
| n | 248 | 124 | |
| Age (yr) | 60.0 (52-67) | 60.0 (53-67) | 0.198 |
| Sex | 0.427 | ||
| Men | 204 (82.3) | 102 (82.3) | 0.219 |
| Women | 44 (17.7) | 22 (17.7) | 0.274 |
| BMI, kg/m2 | 23.6 (21.1-25.7) | 23.6 (20.9-25.8) | 0.295 |
| Albumin, g/L | 36.2 (33.5-40.0) | 36.4 (33.6-40.3) | 0.187 |
| Hemoglobin, g/L | 117.0 (95-135.3) | 117.0 (95-135) | 0.109 |
| CEA, ng/mL | 9.7 (2.5-59.3) | 9.6 (2.4-59) | 0.159 |
| AFP, μg/L | 3.5 (2.2-8.2) | 3.4 (2.2-8.) | 0.213 |
| ALT, U/L | 18.6 (11.8-35.2) | 18.7 (11.9-35.4) | 0.308 |
| AST, U/L | 22.8 (15.4-47.9) | 22.7 (15.4-47.7) | 0.103 |
| GGT, U/L | 22.5 (10.3-46.2) | 22.4 (10.3-46.0) | 0.235 |
| TG, mmol/L | 1.1 (0.9-1.4) | 1.1 (0.9-1.4) | 0.122 |
| TC, mmol/L | 4.2 (3.5-4.8) | 4.2 (3.4-4.8) | 0.169 |
| Drinking habit | 0.155 | ||
| Yes | 91 (36.8) | 46 (37) | |
| No | 157 (63.2) | 78 (63) | |
| Primary tumor size, cm | 3.4 (2.4-5) | 3.4 (2.5-5) | 0.213 |
| Surgery | 0.409 | ||
| Yes | 64 (25.9) | 32 (25.8) | |
| No | 184 (74.1) | 92 (74.2) | |
| Extrahepatic metastases | 0.504 | ||
| Yes | 48 (19.4) | 24 (19.4) | |
| No | 200 (80.6) | 100 (80.6) | |
| Chemotherapy | 0.306 | ||
| Yes | 40 (16.1) | 20 (16.1) | |
| No | 208 (83.9) | 104 (83.9) | |
| Primary site | 0.186 | ||
| Proximal | 60 (24.2) | 30 (24.2) | |
| Gastric body | 75 (30.3) | 37 (29.8) | |
| Distal | 101 (40.7) | 51 (41.1) | |
| Multiple or whole stomach/anastomosis | 12 (4.8) | 6 (4.8) | |
| Metastases size (max) | 3.3 (2.2-5.7) | 3.3 (2.2-5.6) | 0.279 |
| Metastases site | 0.127 | ||
| Left liver | 24 (9.6) | 12 (9.6) | |
| Right liver | 39 (15.7) | 19 (15.3) | |
| Whole liver | 182 (73.4) | 91 (73.4) | |
| Hilar | 2 (0.9) | 1 (0.9) | |
| Other | 1 (0.4) | 1 (0.8) |
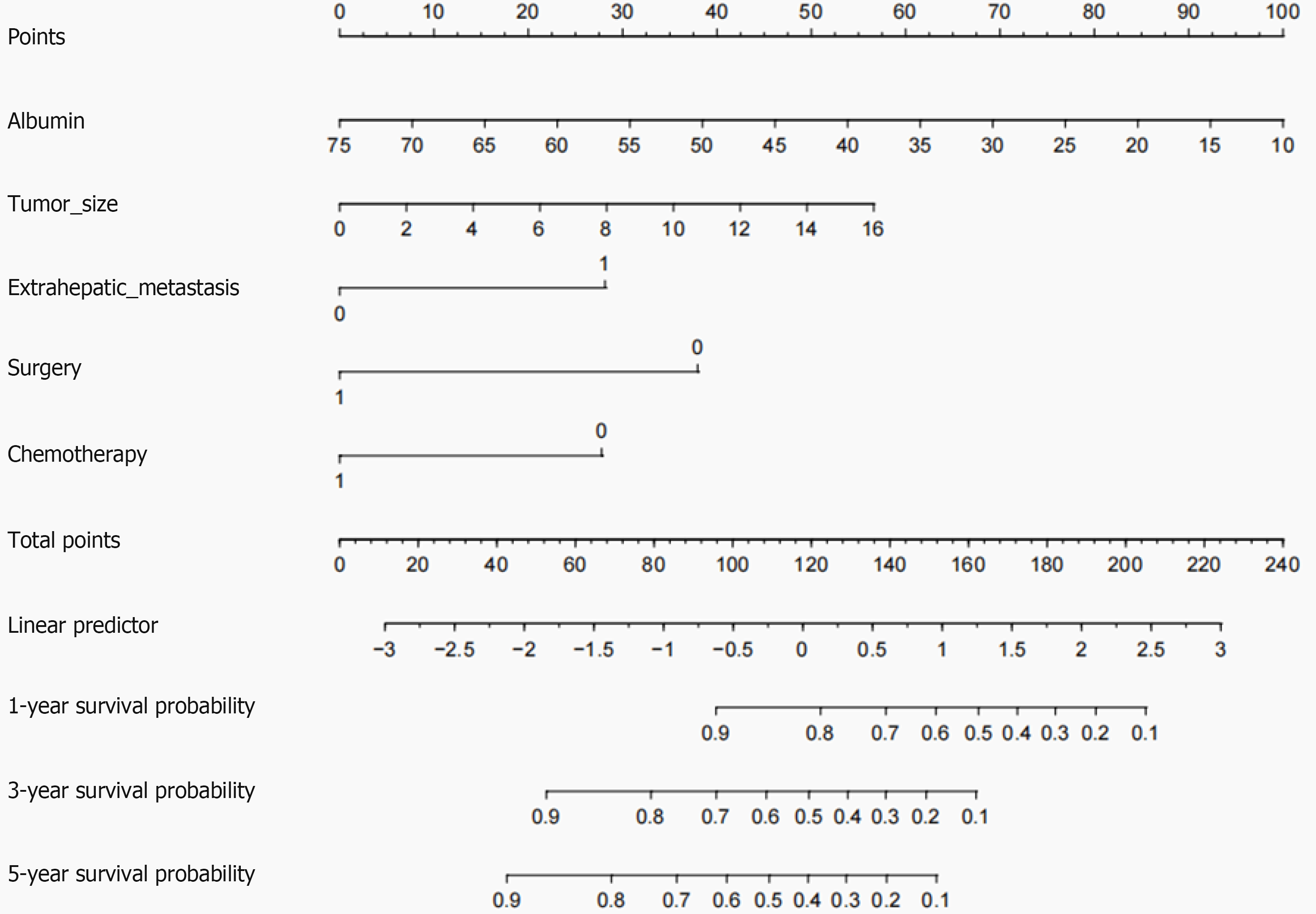
In the development cohort, a total of 20 clinical factors were included in univariate Cox regression analysis, 6 significant risk factors (all P < 0.05) for OS in GCLM patients were screened: Body mass index, albumin levels, primary tumor size, presence of extrahepatic metastases, surgical intervention, and chemotherapy, respectively. Age and sex are important clinical factors for this study. Although they did not show statistical significance in the univariate Cox regression, we still included them in the multivariate Cox regression for analysis. Finally, 8 clinical factors were included in multivariate Cox regression analysis. Multivariate Cox regression analysis revealed that albumin [P = 0.016, hazard ratio (HR) 95%CI: 0.95 0.91-0.99], primary tumor size (P = 0.031, HR 95%CI: 1.12 1.05-1.25), surgical intervention (P = 0.005, HR 95%CI: 0.38 0.19-0.70), presence of extrahepatic metastases (P = 0.004, HR 95%CI: 2.20 1.30-3.64) and administration of chemotherapy (P = 0.010, HR 95%CI: 0.45, 0.24-0.85) were identified as independent prognostic factors for patients diagnosed with GCLM (Table 2). Based on the above results, a nomogram was drawn using 5 variables. By utilizing the respective scale associated with every risk factor on the nomogram, we derived individual scores for each factor and obtained a cumulative score by summing them up. By further comparing the percentage at the bottom, the predictive value of the 1-, 3-, and 5-years OS of C-GCLM type III patients could be obtained (Figure 2).
| Variables | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (yr) | 0.96 (0.75-1.23) | 0.108 | 0.84 (0.69-1.02) | 0.074 |
| Sex, n (%) | 0.96 (0.82-1.11) | 0.557 | ||
| Men | Reference | |||
| Women | 0.89 (0.76-1.05) | 0.286 | ||
| BMI, kg/m2 | 0.97 (0.96-0.99) | 0.045 | 1.19 (0.87,1.64) | 0.158 |
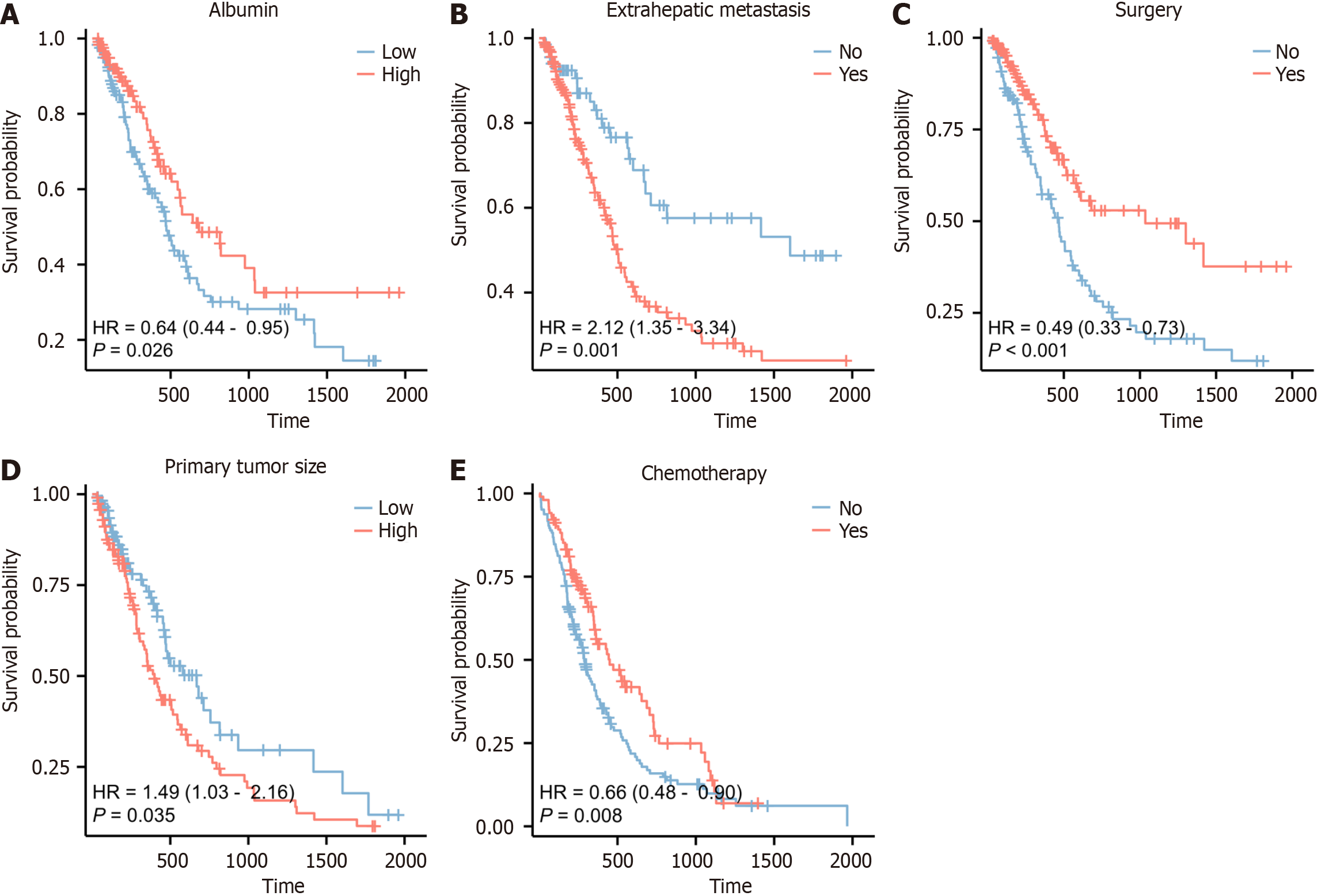
| Albumin, g/L | 0.64 (0.44-0.95) | 0.016 | 0.95 (0.91, 0.99) | 0.016 |
| Hemoglobin, g/L | 1.02 (0.98-1.06) | 0.361 | ||
| CEA, ng/ml | 0.98 (0.92-1.04) | 0.170 | ||
| AFP, μg/L | 0.46 (0.10-2.05) | 0.251 | ||
| ALT, U/L | 0.91 (0.83-1.01) | 0.224 | ||
| AST, U/L | 1.01 (0.43-2.33) | 0.668 | ||
| GGT, U/L | 0.99 (0.94-1.06) | 0.145 | ||
| TG, mmol/L | 0.76 (0.47-1.25) | 0.130 | ||
| TC, mmol/L | 0.88 (0.41-1.88) | 0.254 | ||
| Drinking habit | ||||
| Yes | Reference | |||
| No | 1.19 (0.49-2.88) | 0.638 | ||
| Primary tumor size, cm | 1.49 (1.03-2.16) | 0.009 | 1.12 (1.05-1.25) | 0.031 |
| Surgery | ||||
| Yes | 0.49 (0.33-0.73) | 0.001 | 0.38 (0.19-0.70) | 0.005 |
| No | Reference | Reference | ||
| Extrahepatic metastases | ||||
| Yes | 2.12 (1.35-3.34) | 0.001 | 2.20 (1.30-3.64) | 0.004 |
| No | Reference | Reference | ||
| Chemotherapy | ||||
| Yes | 0.66 (0.48-0.90) | 0.006 | 0.45 (0.24-0.85) | 0.010 |
| No | Reference | Reference | ||
| Primary site | ||||
| Proximal | Reference | |||
| Gastric body | 1.48 (0.51-3.25) | 0.213 | ||
| Distal | 1.07 (0.89-1.30) | 0.320 | ||
| Multiple or whole stomach/anastomosis | 1.00 (0.57-1.74) | 0.971 | ||
| Metastases size(max) | 0.93 (0.48-1.84) | 0.119 | ||
| Metastases site | ||||
| Left liver | Reference | |||
| Right liver | 1.31 (0.72-2.38) | 0.192 | ||
| Whole liver | 0.65 (0.30-1.38) | 0.183 | ||
| Hilar | 0.55 (0.18-1.65) | 0.610 | ||
| Other | 1.56 (0.67-3.62) | 0.214 | ||
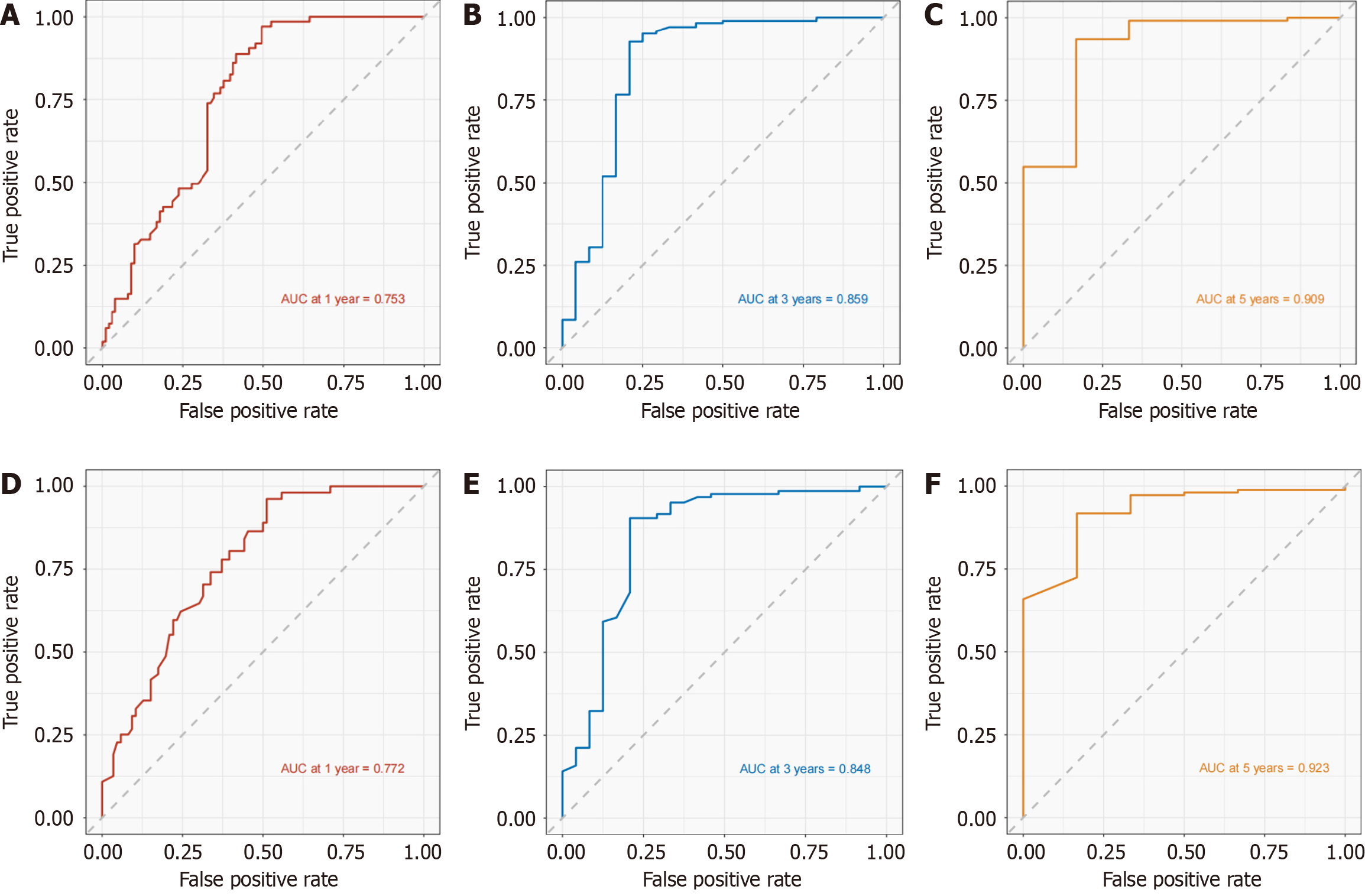
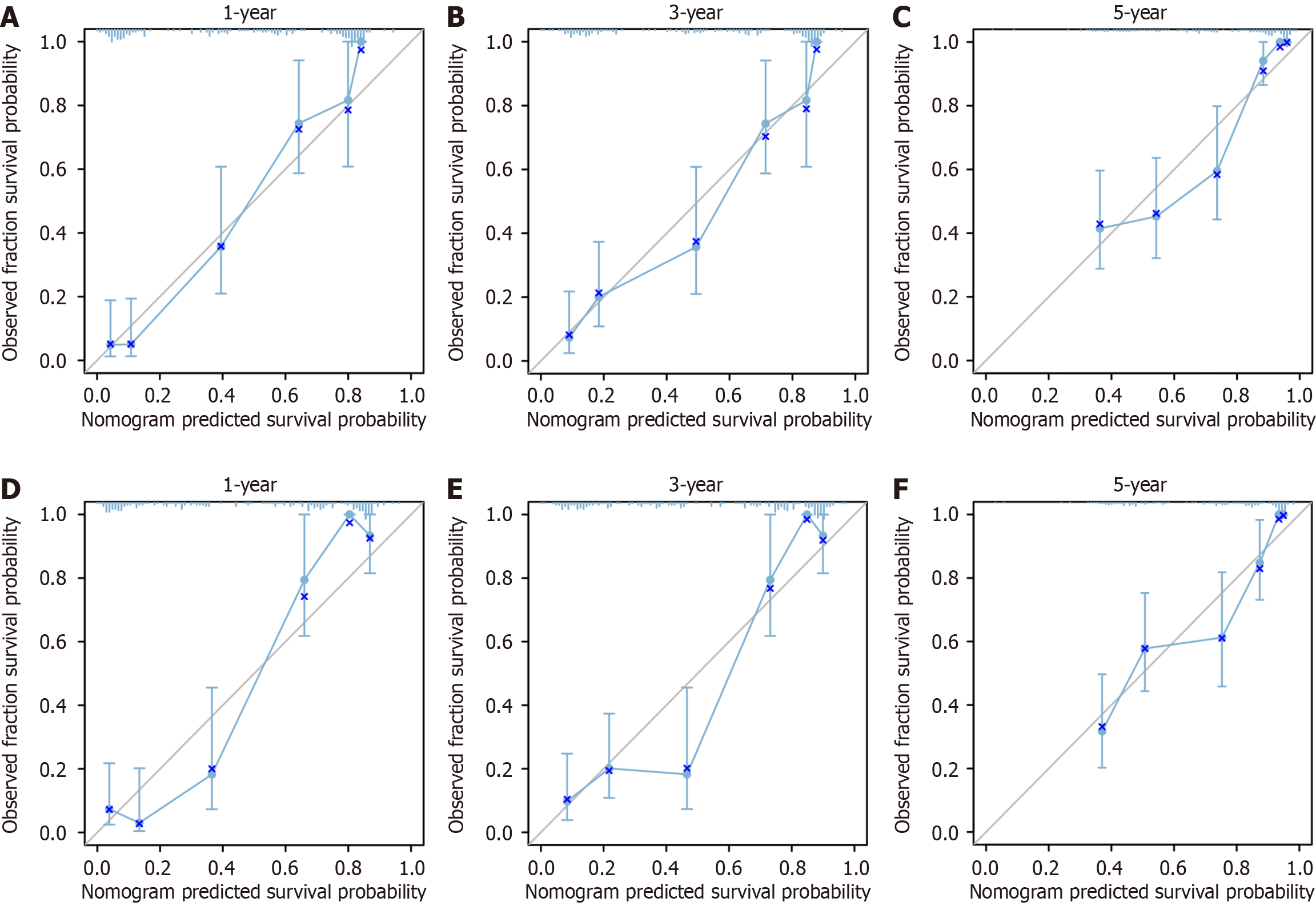
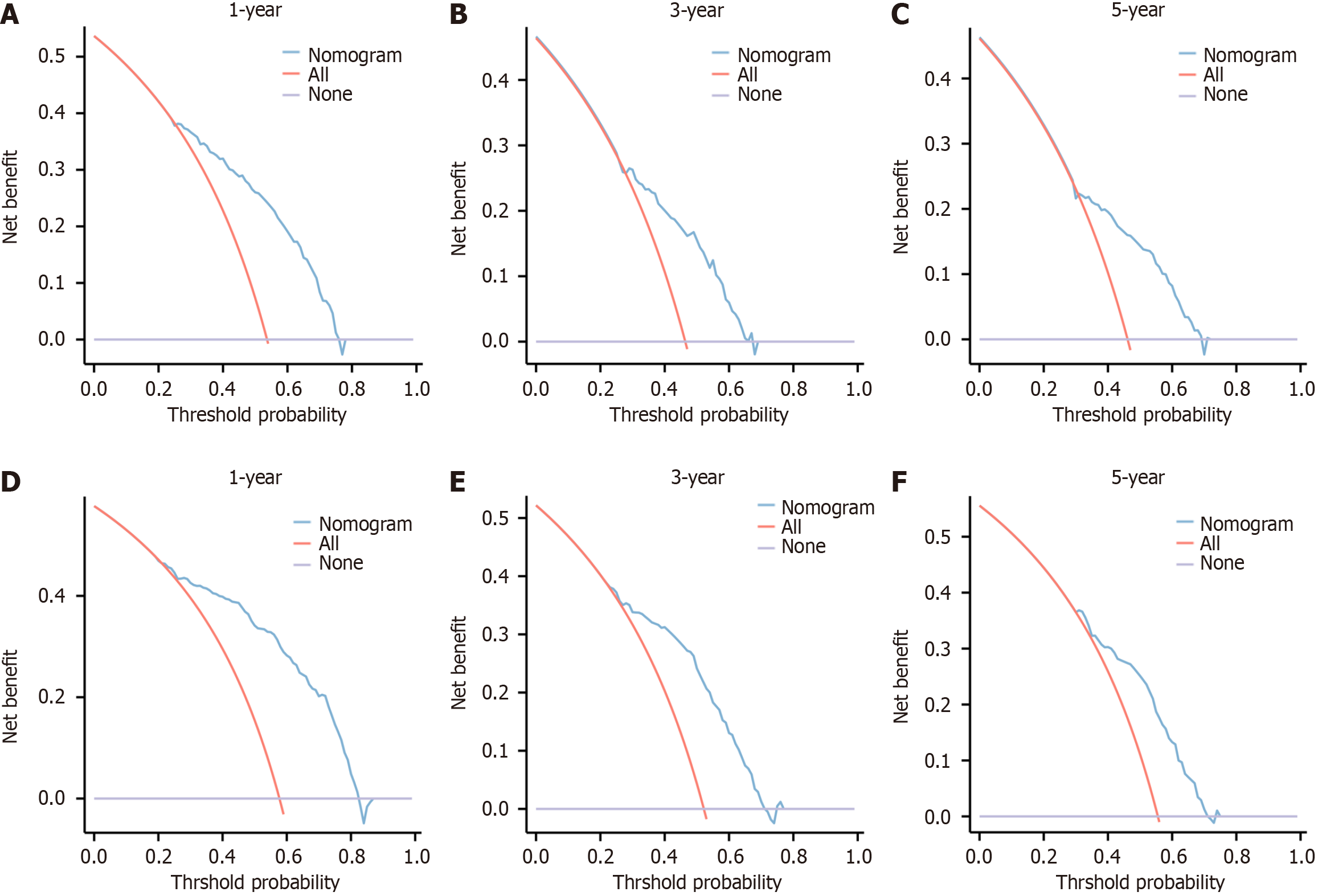
The nomogram in the development cohort correctly predicted overall survival after 1-, 3-, and 5-years, with area under the curve values of 0.753, 0.859, and 0.909, respectively, according to the ROC curve. In the validation cohort, the values of 0.772, 0.848, and 0.923 were observed, all exceeding the threshold of 0.7 (Figure 3), suggesting a favorable predictive capacity of the nomogram. Patient overall survival rates from the development and validation cohorts were used to create the calibration curve at 1, 3, and 5 years. The findings indicated a strong concordance between the OS predicted by the nomogram model and the actual observation, as evidenced by the close alignment of their prediction curve with the 45° diagonal. This indicated that the constructed model had good discrimination ability and accuracy (Figure 4). Decision curve analysis was conducted in both the development and validation cohorts, revealing favorable net benefits for 1-, 3-, and 5-year overall survival rates. These findings indicate that the predictive model holds certain clinical value when it comes to forecasting OS in patients with unresectable GCLM (Figure 5).
The impact of each individual factor on overall survival was further examined. Patients in the low-risk group had a significantly higher OS than those in the high-risk group, according to the Kaplan-Meier curve survival analysis (P < 0.05) (Figure 6).
In this study, a cohort of 372 individuals diagnosed with C-GCLM type III were assigned to two groups in a random manner, maintaining a ratio of 2:1. The development group was utilized to assess the correlation between potential factors that may pose risks and outcomes related to survival, as well as build a prognostic model. On the other hand, the validation group served to confirm the effectiveness of the developed model in predicting future events. Independent prognostic factors influencing the overall survival rate of patients with GCLM were identified through multivariate Cox regression analysis, including albumin levels, size of the primary tumor, presence of extrahepatic metastases, utilization of surgical treatment and administration of chemotherapy. Additionally, a nomogram model was developed to assess the survival rates at 1-, 3-, and 5-year intervals for patients with C-GCLM type III. The findings demonstrated that the proposed model exhibited satisfactory prognostic discrimination ability and survival prediction capability, indicating its potential in facilitating clinical decision-making.
GC is a highly invasive cancer, and LM is the most common distant metastases mode[10], and the prognosis is poor. Multidisciplinary comprehensive treatment has become the main treatment mode of GCLM. However, the prognosis and treatment effect of patients with unresectable GCLM are still controversial. Therefore, it is essential to construct a reliable, efficient and easy to generalize prognostic model to improve the survival rate of GCLM patients. Most of the previous studies that proposed survival prediction models for GC have limited samples, limited predictors, or difficult to obtain evaluation indicators, which greatly limits the clinical application of these models. Chau et al[11] established a four-factor prognostic model including performance status, LM, peritoneal metastases and alkaline phosphatase level. A meta study involving 1304 GCLM patients found that surgical resection of GCLM had better 5-year overall survival and 10-year overall survival than medical control alone[12]. In addition, Ma et al[13] developed and verified a nomogram prognostic scoring model including 9 variables, and simplified metastatic or recurrent GC into low, medium and high risk subgroups according to the survival rate to evaluate the prognosis. However, the applicability or reliability of these models in GCLM patients are limited. This study attempts to construct a clinical prediction model with good prediction ability and convenience, so that clinicians can make appropriate treatment according to individualized prediction and achieve better prognosis of patients.
The influence of the independent risk factors included in this study on the prognosis of patients with GCLM has also been confirmed in other studies. Nationwide retrospective studies from the United Kingdom have shown that gastrectomy and hepatectomy for GCLM may confer a survival advantage for selected patients[14]. A systematic review showed that the median OS of patients who underwent gastrectomy combined with liver resection was significantly longer than that of patients who received palliative care (23.7 vs 7.6 months)[15]. Similar to the findings of our investigation, surgical intervention demonstrates a beneficial impact on patient prognosis. This could be attributed to the presence of primary lesions, which potentially stimulate para-cancerous tissues surrounding metastatic lesions to create a tumor microenvironment that facilitates the infiltration, spread, and proliferation of cancer cells[16]. Albumin is often used as an indicator of clinical nutritional status, and low albumin is an indication of cachexia, which is usually associated with poor prognosis of cancer patients[17,18]. A cohort study involving 147 patients with metastatic GC found that the score of hemoglobin, albumin, lymphocyte and platelet composition had good prognostic value in advanced GC[19]. In the prediction model of recurrent or metastatic GC established by Ma et al[13], albumin is also a risk factor affecting the prognosis[13]. Tumor size directly affects the survival of GC patients[20-22]. In this study, we found that tumor size was an independent prognostic factor in patients with unresectable LM from GC and was inversely associated with OS in our model. Chemotherapy is one of the main treatment methods for patients with GCLM. With the wide application of new chemotherapeutic drugs in recent years, preoperative neoadjuvant chemotherapy has been increasingly used in advanced or metastatic GC, which provides support for reducing postoperative recurrence and prolonging survival time for patients with multiple GCLM. A Japanese study showed that chemotherapy can be applied to patients who underwent R2 resection of LM (macroscopic residual tumor after resection), and the general condition and major organ function of these patients should be ensured before receiving chemotherapy[23]. For GCLM patients who cannot undergo radical resection at the time of initial diagnosis, preoperative chemotherapy can reduce the stage of the primary tumor, so as to obtain a high R0 resection rate (R0 resection refers to the absence of cancer cells at the surgical margin under the microscope)[24]. The metastases of GCLM outside the liver indicates that the patient has entered a more advanced stage of the tumor, suggesting a worse prognosis. GCLM is usually multifocal, and can be accompanied by extrahepatic metastases (peritoneum, lymph node, etc.). Ueda et al[25] showed that peritoneal metastases and lymph node metastases were independent risk and prognostic factors of GCLM. The outcomes of advanced GC patients with distant metastases were poor, with lung, bone, and brain metastases being 4 months, 3 months, 4 months, and 3 months, respectively[26]. This study found that the survival time of patients with extrahepatic metastases was significantly reduced, and simultaneous hepatectomy can be attempted in GCLM patients without extrahepatic metastases.
At present, with the promotion of multidisciplinary treatment mode, GCLM has gradually changed from a single-discipline treatment mode to a multidisciplinary treatment mode[9]. In addition to surgery, the treatment of unresectable GC also includes chemotherapy, immunosuppressant, molecular targeted drugs and so on[27]. In the process of treatment, we should correctly evaluate the patient's condition and take the patient as the center. According to the individual differences of patients, we should study and formulate an individualized treatment plan of "one person, one policy", so as to improve the quality of life of patients and prolong the survival time of patients as far as possible.
The strength of this study is that it is the first prediction model for OS in patients with GCLM type III, which has multi-center and large sample data, and has been internally and externally validated, reflecting good performance. However, it has the following limitations: (1) As a retrospective cohort study, selection bias is inevitable; (2) the data came from Chinese patients, and there may be limitations in generalization to other countries and ethnic groups; (3) with the in-depth study of tumor biological behavior and invasion mechanism, a variety of new tumor treatment methods have emerged, such as immunotherapy, targeted drugs and targeted gene therapy, and have achieved good results. However, the collection of relevant data in this study was not complete, which may cause certain bias; and (4) Helicobacter pylori (H. pylori) infection is prevalent in most cases of GC, but our analysis concentrated on factors directly impacting the prognosis of patients with GCLM, aiming to provide a targeted and detailed investigation in this specific context. Therefore, the relationship with H. pylori was not included in our study.
In conclusion, it is significant for clinicians to conduct precision medicine and individualized medicine by evaluating the prognosis of patients with unresectable GCLM and constructing the corresponding prognostic model. The nomogram model developed in this study offers a convenient, accurate, and user-friendly tool for clinicians to predict and evaluate the prognosis of GCLM patients.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68447] [Article Influence: 13689.4] [Reference Citation Analysis (201)] |
| 2. | Thrift AP, El-Serag HB. Burden of Gastric Cancer. Clin Gastroenterol Hepatol. 2020;18:534-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 1076] [Article Influence: 179.3] [Reference Citation Analysis (7)] |
| 3. | Sano T, Coit DG, Kim HH, Roviello F, Kassab P, Wittekind C, Yamamoto Y, Ohashi Y. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer. 2017;20:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 354] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 4. | Kerkar SP, Kemp CD, Avital I. Liver resections in metastatic gastric cancer. HPB (Oxford). 2010;12:589-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Li Q, Li H, Jiang H, Feng Y, Cui Y, Wang Y, Ji Y, Yu Y, Li W, Xu C, Yu S, Zhuang R, Liu T. Predictive factors of trastuzumab-based chemotherapy in HER2 positive advanced gastric cancer: a single-center prospective observational study. Clin Transl Oncol. 2018;20:695-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Chen T, Xu L, Ye L, Qiu H, Hu Y, Liu H, Zhou Z, Li G, Yu J. A new nomogram for recurrence-free survival prediction of gastrointestinal stromal tumors: Comparison with current risk classification methods. Eur J Surg Oncol. 2019;45:1109-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Bakhti SZ, Latifi-Navid S, Safaralizadeh R. Helicobacter pylori-related risk predictors of gastric cancer: The latest models, challenges, and future prospects. Cancer Med. 2020;9:4808-4822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Valentini V, van Stiphout RG, Lammering G, Gambacorta MA, Barba MC, Bebenek M, Bonnetain F, Bosset JF, Bujko K, Cionini L, Gerard JP, Rödel C, Sainato A, Sauer R, Minsky BD, Collette L, Lambin P. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol. 2011;29:3163-3172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 417] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 9. | Zhang K, Chen L. Chinese consensus on the diagnosis and treatment of gastric cancer with liver metastases. Ther Adv Med Oncol. 2020;12:1758835920904803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Sekiguchi M, Oda I, Matsuda T, Saito Y. Epidemiological Trends and Future Perspectives of Gastric Cancer in Eastern Asia. Digestion. 2022;103:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 11. | Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer--pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004;22:2395-2403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 403] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 12. | Montagnani F, Crivelli F, Aprile G, Vivaldi C, Pecora I, De Vivo R, Clerico MA, Fornaro L. Long-term survival after liver metastasectomy in gastric cancer: Systematic review and meta-analysis of prognostic factors. Cancer Treat Rev. 2018;69:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Ma T, Wu Z, Zhang X, Xu H, Feng Y, Zhang C, Xie M, Yang Y, Zhang Y, Feng C, Sun G. Development and validation of a prognostic scoring model for mortality risk stratification in patients with recurrent or metastatic gastric carcinoma. BMC Cancer. 2021;21:1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Markar SR, Mackenzie H, Mikhail S, Mughal M, Preston SR, Maynard ND, Faiz O, Hanna GB. Surgical resection of hepatic metastases from gastric cancer: outcomes from national series in England. Gastric Cancer. 2017;20:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Liao YY, Peng NF, Long D, Yu PC, Zhang S, Zhong JH, Li LQ. Hepatectomy for liver metastases from gastric cancer: a systematic review. BMC Surg. 2017;17:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | van der Wal GE, Gouw AS, Kamps JA, Moorlag HE, Bulthuis ML, Molema G, de Jong KP. Angiogenesis in synchronous and metachronous colorectal liver metastases: the liver as a permissive soil. Ann Surg. 2012;255:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1011] [Cited by in RCA: 1083] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 18. | Crumley AB, Stuart RC, McKernan M, McMillan DC. Is hypoalbuminemia an independent prognostic factor in patients with gastric cancer? World J Surg. 2010;34:2393-2398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 19. | Duzkopru Y, Kocanoglu A, Dogan O, Sahinli H, Cilbir E, Altinbas M. Hemoglobin, albumin, lymphocyte, and platelet score as a predictor of prognosis in metastatic gastric cancer. World J Gastrointest Oncol. 2023;15:1626-1635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 20. | Wang HM, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Lu J. Tumor size as a prognostic factor in patients with advanced gastric cancer in the lower third of the stomach. World J Gastroenterol. 2012;18:5470-5475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Park JM, Ryu WS, Kim JH, Park SS, Kim SJ, Kim CS, Mok YJ. Prognostic factors for advanced gastric cancer: stage-stratified analysis of patients who underwent curative resection. Cancer Res Treat. 2006;38:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Zu H, Wang F, Ma Y, Xue Y. Stage-stratified analysis of prognostic significance of tumor size in patients with gastric cancer. PLoS One. 2013;8:e54502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1409] [Article Influence: 281.8] [Reference Citation Analysis (2)] |
| 24. | Yu P, Zhang Y, Ye Z, Chen X, Huang L, Du Y, Cheng X. Treatment of Synchronous Liver Metastases from Gastric Cancer: A Single-Center Study. Cancer Manag Res. 2020;12:7905-7911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Ueda K, Iwahashi M, Nakamori M, Nakamura M, Naka T, Ishida K, Ojima T, Yamaue H. Analysis of the prognostic factors and evaluation of surgical treatment for synchronous liver metastases from gastric cancer. Langenbecks Arch Surg. 2009;394:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Qiu MZ, Shi SM, Chen ZH, Yu HE, Sheng H, Jin Y, Wang DS, Wang FH, Li YH, Xie D, Zhou ZW, Yang DJ, Xu RH. Frequency and clinicopathological features of metastasis to liver, lung, bone, and brain from gastric cancer: A SEER-based study. Cancer Med. 2018;7:3662-3672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 27. | Guan WL, He Y, Xu RH. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol. 2023;16:57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 588] [Reference Citation Analysis (5)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Toyoshima O, Japan S-Editor: Liu H L-Editor: A P-Editor: Zhao S