Published online Jan 6, 2024. doi: 10.12998/wjcc.v12.i1.188
Peer-review started: October 11, 2023
First decision: November 22, 2023
Revised: December 5, 2023
Accepted: December 13, 2023
Article in press: December 13, 2023
Published online: January 6, 2024
Processing time: 83 Days and 3.7 Hours
In this study, we retrospectively analysed macrophage infiltration and podocyte injury in three patients with diffuse proliferative lupus nephritis (LN) who un
Clinical data of three diffuse proliferative LN patients with different pathological characteristics (case 1 was LN IV-G (A), case 2 was LN IV-G (A) + V, and case 3 was LN IV-G (A) + thrombotic microangiopathy) were reviewed. All patients underwent repeated renal biopsies 6 mo later, and renal biopsy specimens were studied. Macrophage infiltration was assessed by CD68 expression detected by immunohistochemical staining, and an immunofluorescence assay was used to detect podocin expression to assess podocyte damage. After treatment, Case 1 changed to LN III-(A), Case 2 remained as type V LN lesions, and Case 3, which changed to LN IV-S (A), had the worst prognosis. We observed reduced macro
It may be possible to reverse podocyte damage and decrease the infiltrating ma
Core Tip: In this study, we retrospectively analysed the clinical and pathological data of three patients who underwent repeated renal biopsies after treatment for diffuse proliferative lupus nephritis (LN) with different pathological characteristics. Immunohistochemistry and immunofluorescence tests were used to assess the podocyte damage and infiltration of macrophages. Diffuse proliferative LN with different pathological features has different prognoses, and the prognosis of LN with thrombotic microangiopathy is relatively poor. Podocyte injury and macrophage infiltration may be involved in the pathogenesis of LN. It may be possible to reverse podocyte damage and decrease the infiltrating macrophages in LN patients through effective treatment.
- Citation: Liu SY, Chen H, He LJ, Huang CK, Wang P, Rui ZR, Wu J, Yuan Y, Zhang Y, Wang WJ, Wang XD. Changes in macrophage infiltration and podocyte injury in lupus nephritis patients with repeated renal biopsy: Report of three cases. World J Clin Cases 2024; 12(1): 188-195
- URL: https://www.wjgnet.com/2307-8960/full/v12/i1/188.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i1.188
Diffuse proliferative lupus nephritis (LN) is the most common pathological type of LN, accounting for approximately 50% of all LN cases. Type IV LN is often severe, and pathological examination of renal biopsy often shows obvious proliferative lesions and a large amount of immune complex deposition[1]. Many studies have found that renal resident cells and infiltrating inflammatory cells are involved in the tissue damage process of LN[2]. The injured podocytes activate inflammatory cells through the expression of Tolllike receptors and activate T cells through major histocompatibility complexes and CD86, thereby participating in the local immune response and the formation of crescents in coordination with parietal epithelial cells in LN[3,4]. The accumulation of infiltrating macrophages in the kidney with a proinflammatory phenotype correlated with the degree of glomerulosclerosis and interstitial fibrosis by producing inflammatory cytokines and chemokines in LN[5]. However, few studies have compared macrophage infiltration and podocyte injury in repeated LN renal biopsy specimens before and after treatment. In this study, we retrospectively analysed the clinical and pathological data of three patients who underwent repeated renal biopsies after treatment for diffuse proliferative LN with different pathological characteristics [case 1 was LN IV-G (A), case 2 was LN IV-G (A) + V, and case 3 was LN IV-G (A) + thrombotic microangiopathy (TMA)]. Immunohistochemistry and immunofluorescence tests were used to assess the podocyte damage and infiltration of macrophages to provide guidance for clinical practice.
Case 1: A 35-year-old woman was hospitalized because of oedema.
Case 2: A 34-year-old woman was hospitalized because of oedema.
Case 3: A 22-year-old woman was hospitalized because of oedema, with accompanying oliguria.
Case 1: Facial erythema for nearly 2 mo and oedema for 5 d.
Case 2: Oedema for 20 d.
Case 3: Diarrhea and oedema for 5 d accompanied by reduced urine volume.
Cases 1 and 2: The patients had no history of past illness.
Case 3: The patient had a history of kidney disease.
All the three cases had no remarkable personal or family history.
Case 1: The patient had erythema of the cheeks, and mild oedema of the face and both lower limbs.
Case 2: The patient had mild ankle oedema of both lower limbs.
Case 3: The patient had oedema of both lower limbs.
Case 1: Routine blood test showed white blood cells 2.76 × 109/L, hemoglobin 100 g/L, and albumin 29.3 g/L, and routine urine test showed urine protein (++++).
Case 2: Routine blood test showed albumin 23.6 g/L, and routine urine test showed urine protein (++++) and urine red blood cells 15/high power field.
Case 3: Routine blood test showed albumin 15.5 g/L, low-value platelets 51 × 109/L, hemoglobin 61 g/L, and serum creatinine 3.57 mg/dL, and routine urine test showed urine protein (++++).
No abnormalities were detected in the imaging examination in all the three cases.
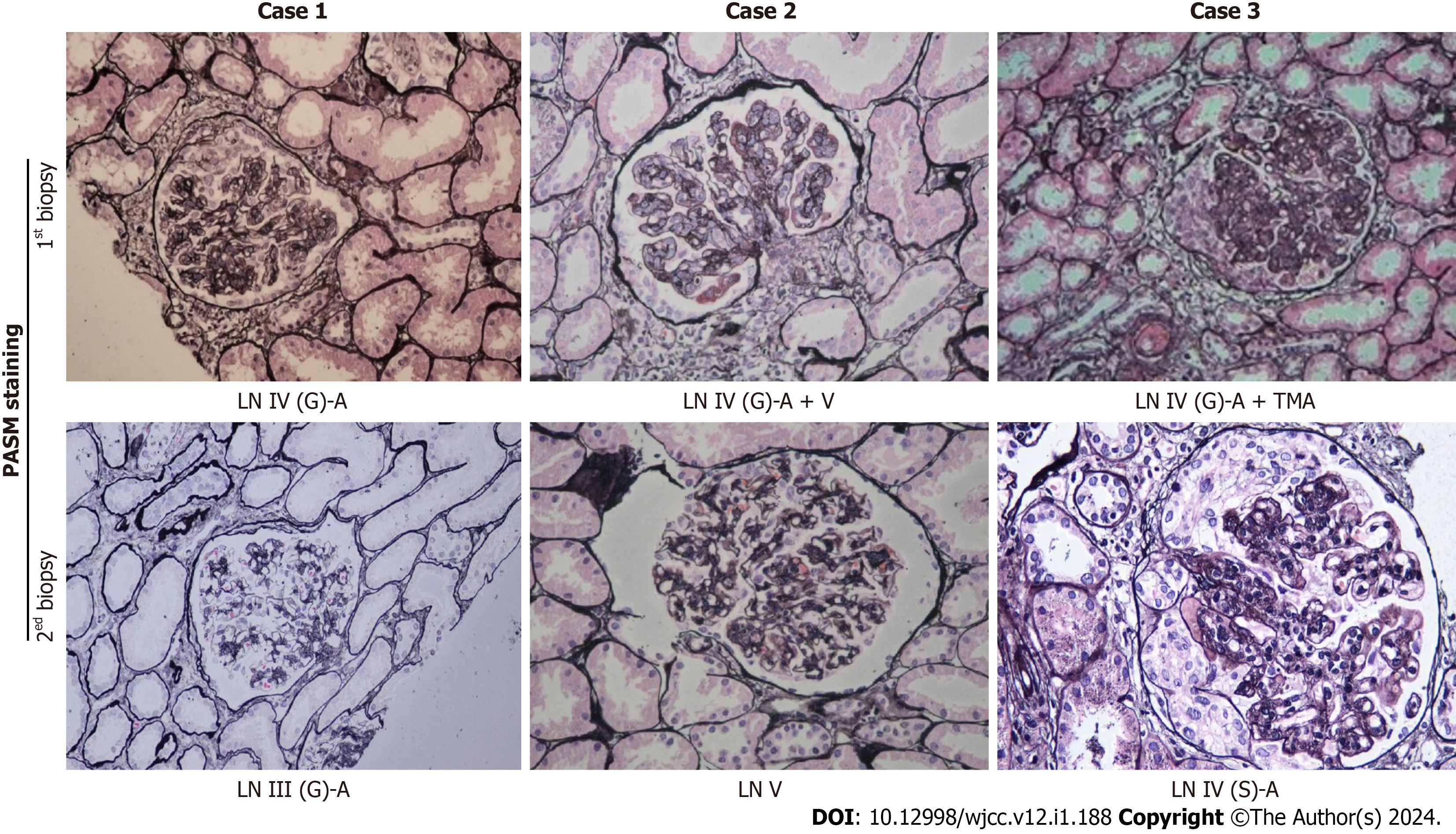
Pathological images of renal biopsies from the three patients are shown in Figure 1 (periodic acid-silver methenamine staining, 200 × magnification). The clinical and pathological characteristics of the three patients are summarized in Table 1.
| Item | Case 1 | Case 2 | Case 3 | |||
| First renal biopsy | Repeated renal biopsy | First renal biopsy | Repeated renal biopsy | First renal biopsy | Repeated renal biopsy | |
| Pathological type | LN IV-G (A) | LN III (A) | LN IV-G (A) + V | LN V | LN IV-G (A) + TMA | LN IV-S (A) |
| AI | 12 | 7 | 13 | 4 | 16 | 13 |
| CI | 1 | 4 | 0 | 2 | 2 | 2 |
| SLEDAI | 18 | 6 | 18 | 4 | 24 | 14 |
| Change of treatment | MP and CTX pulse therapy | MP pulse therapy | MP and CTX pulse therapy | Reduce glucocorticoids | MP and CTX pulse therapy, plasma exchange | MP pulse therapy, hemodialysis |
| Prognosis | - | Well | - | Well | - | Maintenance haemodialysis |
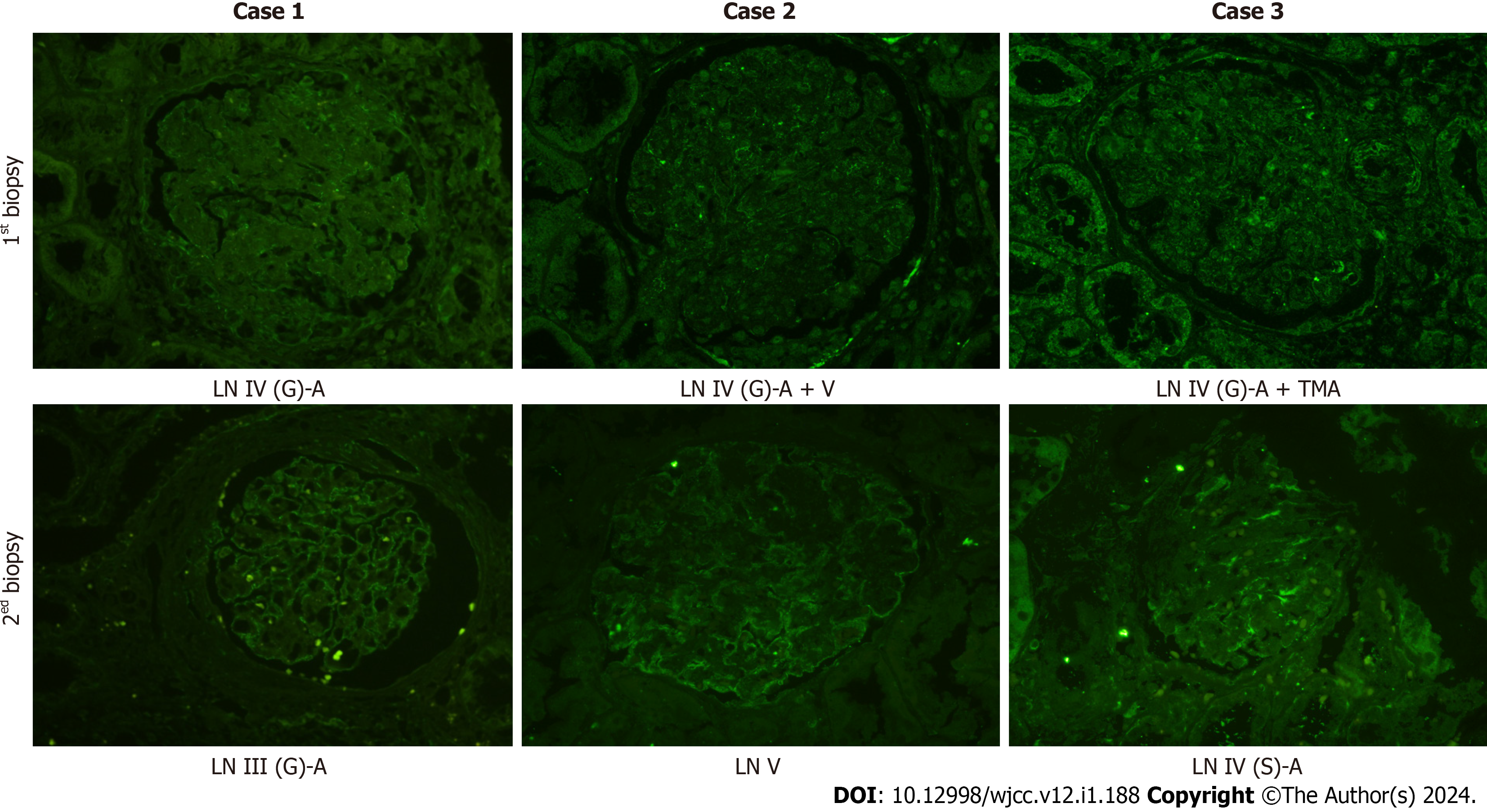
| Podocin expression | Visual reduction | Complete and continuous | Visual reduction | Increased expression | Visual reduction | Visual reduction |
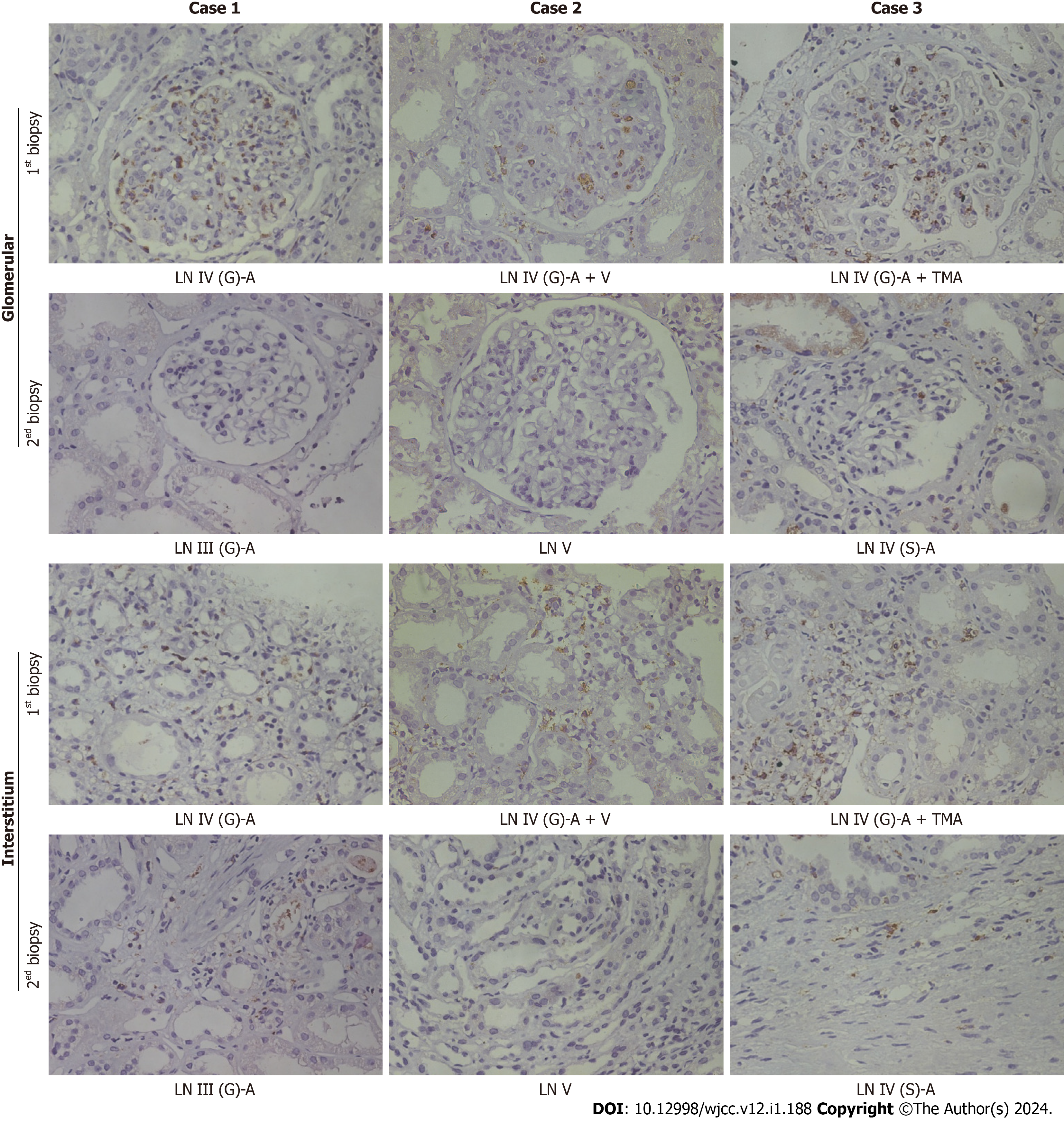
| Infiltrating monocytes | Many monocytes infiltrated in the glomerulus and renal interstitium | Monocytes infiltrated in the renal interstitium | Many monocytes infiltrated in the glomerulus and renal interstitium | No obvious infiltration | Many monocytes infiltrated in the glomerulus and renal interstitium | Monocytes infiltrated in the renal interstitium |
Immunohistochemical staining for CD68 was used to evaluate macrophage infiltration in the glomeruli and renal interstitium (Figure 2). Before therapy, a large number of CD68-positive cells were observed in the glomeruli and renal interstitium. In the second renal biopsy specimen, the number of CD68-positive macrophages in the glomeruli of the three patients were significantly reduced; notably, Case 2 had no obvious active disease after treatment. However, some CD68-positive cells could still be observed in the renal interstitium in Case 1 and Case 3 with active disease. It is suggested that infiltrated macrophages in the kidney may be associated with disease activity in LN.
Immunofluorescence staining was used to determine the expression of podocin, which is a specific protein in podocytes (Figure 3). Before treatment, all the three patients showed discontinuous and reduced expression of podocin. However, after treatment, the expression of podocin in Case 1 became significantly continuous and complete, which suggests that podocyte injury was reversible. Although increased podocin expression was noticed in Case 2, there was still discontinuous expression, which was indicative of podocyte injury in membranous LN. In Case 3, the expression of podocin was not significantly improved.
The patient was diagnosed with LN IV-G (A) by the first renal biopsy.
The patient was diagnosed with LN IV-G (A) + V by the first renal biopsy.
The patient was diagnosed with LN IV-G (A) + TMA by the first renal biopsy.
The patient was given 0.5 g intravenous methylprednisolone (MP) for 3 d, and the treatment was repeated after 1 mo. During this period, 48 mg of MP tablets and 0.2 g of hydroxychloroquine were orally administered once a day, and 0.8 g of cyclophosphamide (CTX) was intravenously administered every month (total CTX ≤ 12 g). In the sixth month, the patient underwent repeated renal biopsy, which revealed that the pathological type had changed to LN III (A). However, it still showed active lesions and six small fibrocellular crescents (a total of 20 glomeruli). The treatment was switched to intravenous infusion of MP 0.5 mg for 3 d.
The patient was given 0.5 g intravenous MP for 3 d, and the treatment was repeated after 1 mo. During this period, 55 mg of prednisone tablets and 0.3 g of hydroxychloroquine were administered orally once a day, and 0.5 g CTX was intravenously administered once a month (total CTX ≤ 12 g). In the sixth month, repeated renal biopsy revealed that the pathological type had changed to LN V type, and no obvious active disease was observed. Prednisone tablets were tapered gradually.
The patient was immediately given intravenous MP 0.25 g for 3 d. After 1 wk, 0.5 g of MP was intravenously admi
The patient achieved complete remission and no recurrence was observed after 6 years of follow-up.
The patient achieved complete remission and she had no recurrence after 5 years of follow-up.
The patient had been on haemodialysis for 4 years.
The severity of clinical manifestations of LN is not parallel to pathological changes in the kidney. Therefore, renal biopsy plays an indispensable role in the diagnosis and management of LN patients. In this study, repeated renal biopsy after 6 mo revealed that Case 1 had changed to LN III (A), and there were still active lesions and crescents. The ratio of crescent cells to fibroblasts is an independent risk factor for poor renal survival[6]. Thus, MP pulse therapy was continued. In Case 2, the active LN lesions were significantly improved, and the pathological type remained type V LN. In Case 3, improvement in TMA was found, but proliferative active lesions of LN were still present. Therefore, MP pulse therapy was continued. The relevance of repeated kidney biopsy in patients with LN is controversial. However, there are no doubts that repeated renal biopsy represents a useful tool in difficult cases to evaluate the response to therapy to modulate the intensity of treatment[7,8].
There have been reports that infiltration of macrophages plays an important role in the pathogenesis of LN[9]. In this study, we observed reduced macrophage infiltration after therapy. Especially, the infiltrating macrophages in the glomerulus disappeared sooner than those in the renal interstitium. Two patients with active lesions after treatment still showed macrophage infiltration in the renal interstitium, which suggested that macrophage infiltration in the kidney was related to disease activity. Some studies have also found that interstitial macrophages may be the major effectors of chronic injury that correlated with proteinuria and poor renal prognosis in patients with LN[10].
In recent years, a large amount of renal biopsy data has revealed glomerular podocyte damage in LN[11]. It was also observed in this study that the integrity of podocin expression was restored after treatment in Case 1, which was not mentioned in other reports. In addition, in Case 3, there was no significant improvement in immune damage, while the expression of podocin was still deficient. We suspect that podocyte damage in type IV LN may be mainly due to immune damage.
Diffuse proliferative LN with different pathological features has different prognoses, and the prognosis of LN with TMA is relatively poor. Podocyte injury and macrophage infiltration may be involved in the pathogenesis of LN. By comparing pathological changes before and after treatment, we found that it may be possible to reverse podocyte damage and decrease the infiltrating macrophages in diffuse proliferative LN patients through effective treatment.
| 1. | Yu C, Li P, Dang X, Zhang X, Mao Y, Chen X. Lupus nephritis: new progress in diagnosis and treatment. J Autoimmun. 2022;132:102871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 111] [Reference Citation Analysis (0)] |
| 2. | Richoz N, Tuong ZK, Loudon KW, Patiño-Martínez E, Ferdinand JR, Portet A, Bashant KR, Thevenon E, Rucci F, Hoyler T, Junt T, Kaplan MJ, Siegel RM, Clatworthy MR. Distinct pathogenic roles for resident and monocyte-derived macrophages in lupus nephritis. JCI Insight. 2022;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 3. | Bhargava R, Li H, Tsokos GC. Pathogenesis of lupus nephritis: the contribution of immune and kidney resident cells. Curr Opin Rheumatol. 2023;35:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 4. | Liu R, Wen X, Peng X, Zhao M, Mi L, Lei J, Xu K. Immune podocytes in the immune microenvironment of lupus nephritis (Review). Mol Med Rep. 2023;28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 5. | Kwant LE, Vegting Y, Tsang-A-Sjoe MWP, Kwakernaak AJ, Vogt L, Voskuyl AE, van Vollenhoven RF, de Winther MPJ, Bemelman FJ, Anders HJ, Hilhorst ML. Macrophages in Lupus Nephritis: Exploring a potential new therapeutic avenue. Autoimmun Rev. 2022;21:103211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 6. | Cai F, Han F, Wang H, Han H, Le J, Lan L, Xu Y, Chen J. The Crescentic Implication of Renal Outcomes in Proliferative Lupus Nephritis. J Rheumatol. 2018;45:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Haładyj E, Cervera R. Repeat renal biopsy in lupus nephritis - unnecessary harm and risk of complications or important diagnostic tool with clinical consequences? Reumatologia. 2016;54:49-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Moroni G, Depetri F, Ponticelli C. Lupus nephritis: When and how often to biopsy and what does it mean? J Autoimmun. 2016;74:27-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Dias CB, Malafronte P, Lee J, Resende A, Jorge L, Pinheiro CC, Malheiros D, Woronik V. Role of renal expression of CD68 in the long-term prognosis of proliferative lupus nephritis. J Nephrol. 2017;30:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Tsai CY, Li KJ, Shen CY, Lu CH, Lee HT, Wu TH, Ng YY, Tsao YP, Hsieh SC, Yu CL. Decipher the Immunopathological Mechanisms and Set Up Potential Therapeutic Strategies for Patients with Lupus Nephritis. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Zhou XJ, Klionsky DJ, Zhang H. Podocytes and autophagy: a potential therapeutic target in lupus nephritis. Autophagy. 2019;15:908-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pathology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Salvadori M, Italy S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Xu ZH