Published online Mar 26, 2023. doi: 10.12998/wjcc.v11.i9.2091
Peer-review started: December 24, 2022
First decision: January 30, 2023
Revised: January 31, 2023
Accepted: February 27, 2023
Article in press: February 27, 2023
Published online: March 26, 2023
Processing time: 83 Days and 0.7 Hours
Superficial temporal artery-middle cerebral artery (STA-MCA) bypass helps treat cerebral ischemia. However, the STA is not available for bypass in some conditions. Therefore, with some technical tips, the authors introduced a bypass technique using the occipital artery (OA).
Two female patients complained of hemiparesis. Brain magnetic resonance imaging (MRI) indicated contralateral infarction from the MCA steno-occlusion. On Diamox single photon emission computed tomography or perfusion MRI, the contralateral front parietotemporal reserve was diminished. On transfemoral cerebral angiography, the STA was thin with a weak flow; however, the OA was prominent. Direct OA-MCA end-to-side extracranial-intracranial bypass surgery was implemented instead of STA because the caliber was too narrow. The postoperative course was uneventful in both cases, with well-maintained bypass patency and neurological stability during follow-up.
OA might be an acceptable alternative for MCA cerebral ischemic cases with an unsuitable STA.
Core Tip: In cases of cerebral ischemic disease or progressive ischemia where the Superficial temporal artery is unsuitable in the middle cerebral artery territory, occipital artery might be an acceptable donor artery for a less invasive EC-IC bypass surgery.
- Citation: Hong JH, Jung SC, Ryu HS, Kim TS, Joo SP. Occipital artery bypass importance in unsuitable superficial temporal artery: Two case reports. World J Clin Cases 2023; 11(9): 2091-2097
- URL: https://www.wjgnet.com/2307-8960/full/v11/i9/2091.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i9.2091
Superficial temporal artery-middle cerebral artery (STA-MCA) bypass helps treat cerebral ischemia, complex cerebral aneurysms, and tumors[1,2]. If the STA is inaccessible after preceding bypass operations, injuries, or atrophy, graft bypass can be attempted through the radial artery, great saphenous vein, or occipital artery (OA). However, the graft would anastomose two sites, increasing bypass occlusion and surgical wound complication risks. In this study, the authors introduced a technique to relieve ischemia via direct bypassing of the OA to the MCA.
Case 1: The patient complained of motor weakness in the right extremities and intermittent headaches.
Case 2: The patient complained of motor weakness in the left extremities.
Case 1: In August 2019, a 55-year-old female patient visited this institution’s neurology department, reporting intermittent weakness in the right extremities and headaches.
Case 2: In August 2021, a 68-year-old male patient visited this institution’s emergency room for left extremities weakness.
Case 1: There was no significant past illness.
Case 2: There was no previous complaint of left-side motor weakness.
Case 1: There was no significant past or family history.
Case 2: He had right-sided hemiparesis due to the sequelae of traumatic brain injury 15 years ago.
Case 1: There was no significant motor weakness at the outpatient clinic.
Case 2: The left motor grade was IV/IV in the manual muscle test.
There were no significant laboratory test findings in both cases.
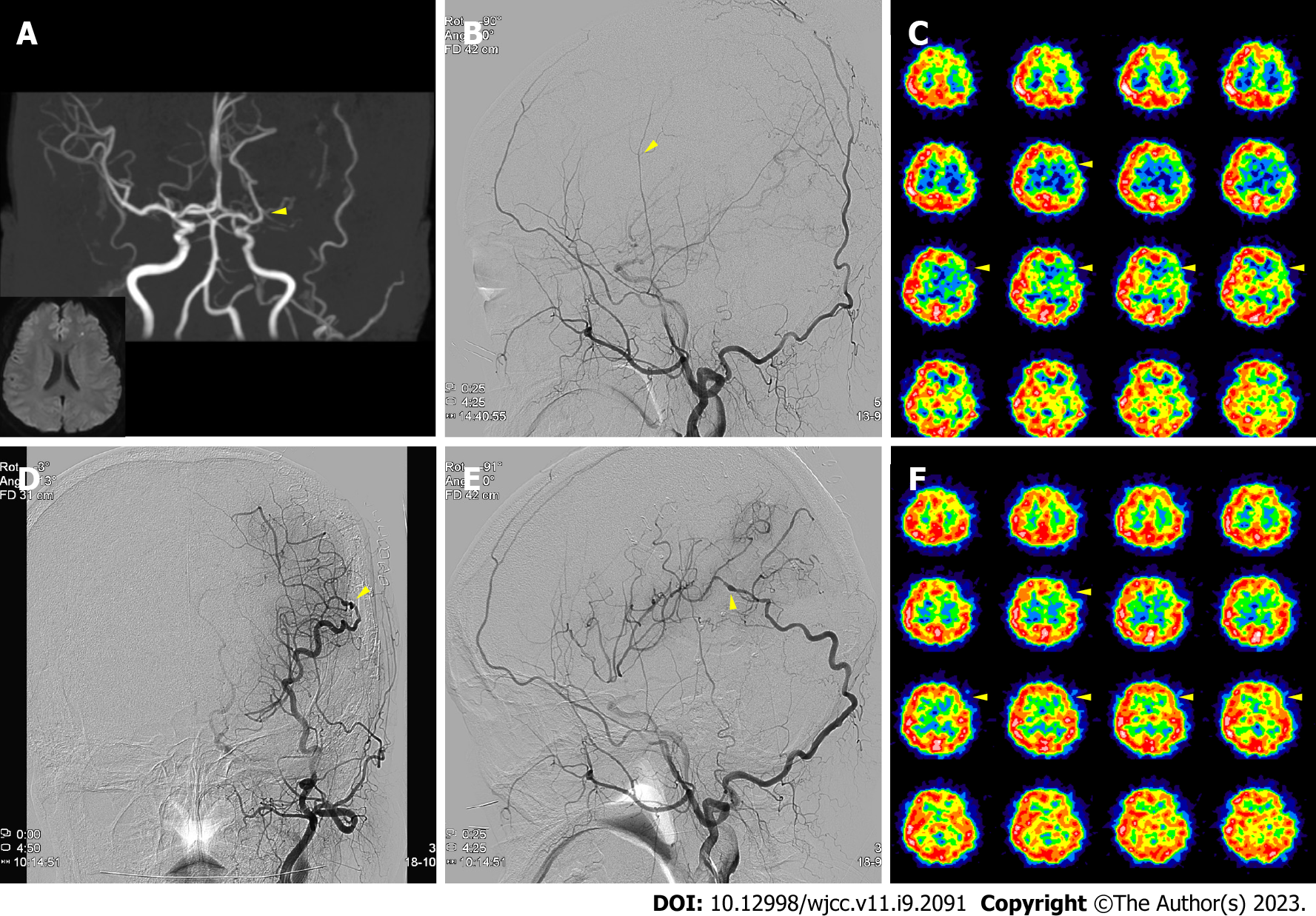
Case 1: Magnetic resonance imaging (MRI) showed left frontal infarction from the left MCA stenosis (Figure 1A). A follow-up brain MRI on July 2021 indicated that the left MCA stenotic lesion had progressed to occlusion (Figure 1A). Therefore, the patient was referred to this department for surgery. Upon acetazolamide-loading single-photon-emission computed tomography (Diamox SPECT), the left front parietotemporal reserve was diminished (Figure 1C). In addition, the transfemoral cerebral angiography (TFCA) revealed a thin left STA with a weak flow but a prominent OA (Figure 1B).
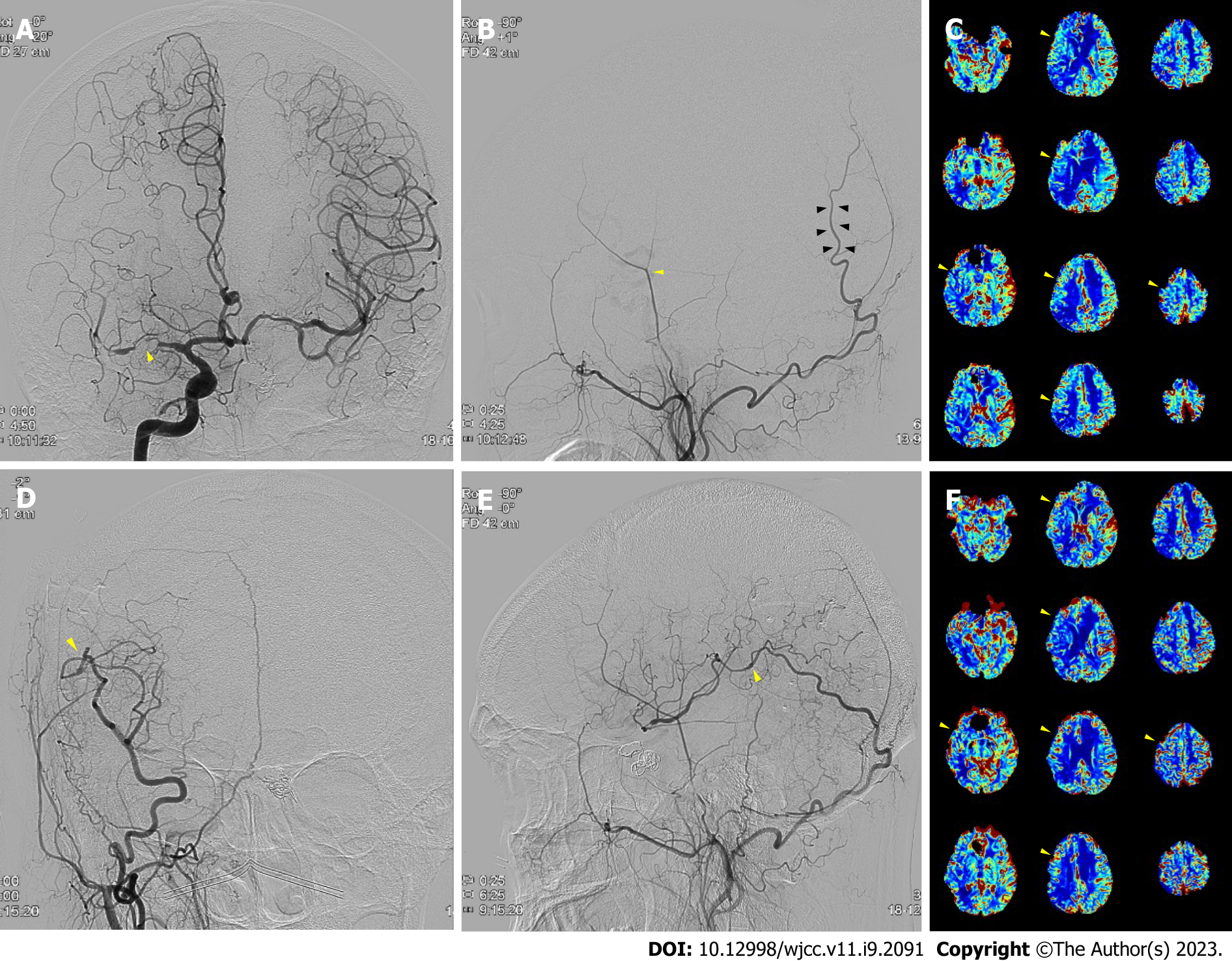
Case 2: An MRI showed a right MCA territory infarction due to right MCA occlusion. After three months of conservative treatment, a follow-up perfusion MRI exhibited worsening cerebral perfusion throughout the MCA territory (Figure 2C). On TFCA, the right STA was noticeably thin with a weak flow, yet the OA was prominent (Figure 2A and B).
The authors diagnosed the patients with symptomatic steno-occlusive MCA infarction by combining imaging and clinical findings.
Thus, OA-MCA bypass surgery was decided in both cases. The OA and MCA were end-to-side anastomosed.
After bypass, blood flow at the surgical site was intact (Figure 1D and E), with perfusion defect improvement on the postoperative Diamox SPECT (Figure 1F). The patient was discharged without symptoms in a weak and has not experienced any follow-up care complications one year after surgery.
After bypass, blood flow at the surgical site was intact (Figure 2D and E), with perfusion defect improvement on the postoperative perfusion MRI (Figure 2F). The patient was discharged without any symptom aggravations in a weak and has not experienced any follow-up care complications one year after surgery.
The cerebrovascular steno-occlusive disease can cause acute cerebral infarction or transient ischemic accident (TIA) symptoms. An unstable TIA can originate from decreased cerebral perfusion; therefore, immediate and adequate blood supply is the most crucial consideration in bypass surgery. STA-MCA bypass is a relatively simple and efficient surgical modality, especially for cerebrovascular occlusive diseases with a high patency rate during a short period. However, the STA is inaccessible for bypass in cases of previous operations, injuries, or atrophy. In this condition, interposition graft surgery is considered. The graft technique is more complex and requires additional anastomosis, increasing operation difficulty and requiring more time. However, there are disadvantages to this technique, such as donor site complications and size discrepancy-related graft failure. Moreover, there is a hyperperfusion syndrome risk if the donor artery is large[3].
Direct OA bypass can be an alternative to the interposition graft with the advantage of arterial graft use. In cases 1 and 2 presented here, the OA was selected as an alternative because the STA was too thin. Direct OA-MCA bypass for ischemic stroke has rarely been reported in literature[4-7]. OA is a frequently used donor artery for trapping and bypassing aneurysm surgeries arising from posterior circulation. Meanwhile, bypass from the OA to the MCA is not commonly performed because of its technical difficulties. The OA is more onerous to harvest than the STA because it passes through several tortuous layers[8].
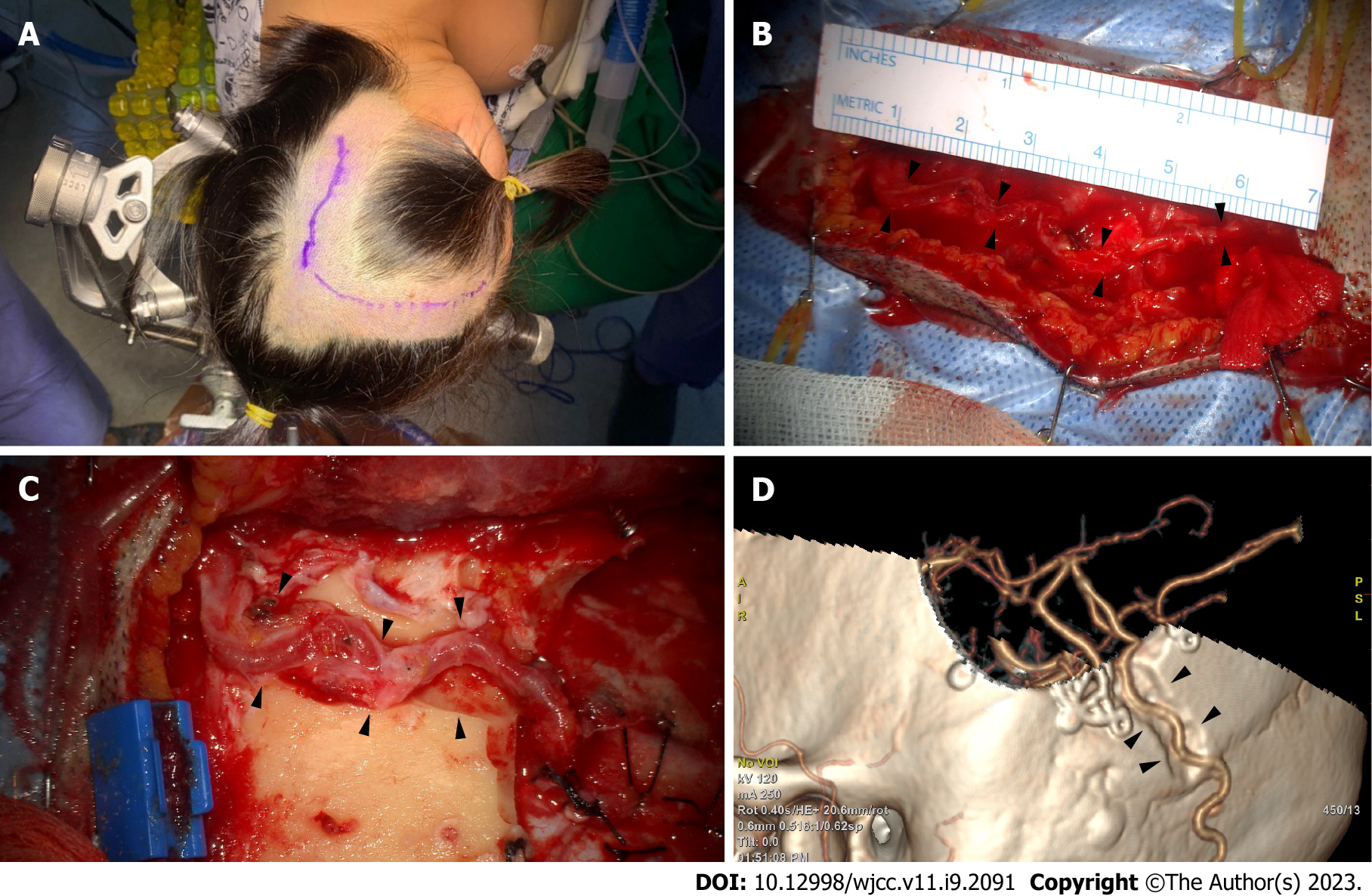
In the three-quarter position, the retrosigmoid area was at the top, and the vertex was slightly raised to reduce venous pressure (Figure 3A). After marking the OA path, as confirmed through angiography and fingertip palpation, a microscopy-guided incision was made on the OA course (Figure 3A). The artery was located, and the soft tissue was bluntly dissected. Small OA branches were identified and cut after meticulous cauterization. The proximal part was secured through dissection to where the OA penetrated the splenius capitis muscle. The large branch in the main artery’s subgaleal portion was likely to be used for a second anastomosis and could be cleaned of the donor artery to ensure its preservation to the greatest extent[9]. The splenius capitis muscle was incised along the OA. Free space was secured by dissecting and retracting the muscle, creating a vascular groove. The descending branch that supplies blood flow to the suboccipital muscle was preserved as much as possible; however, if OA’s full length was not long enough, it was selectively cut after ligation. A length sufficient for MCA bypass is approximately 7 cm above the proximal stump, which is just below the superior nuchal line (Figure 3B). However, this varies depending on OA functionality and condition.
The posterior Sylvian fissure area should be exposed to access the donor artery for MCA bypass[5]. The incision was extended according to the recipient’s target craniotomy location. To achieve an MCA bypass, the skull was exposed enough to incise the skin by anteriorly turning it vertically along the superior temporal line. If the OA was bent superomedially, a separate incision was conducted in the temporal region for craniotomy of the recipient artery. Following this, the OA can be passed through the subcutaneous tunnel for an OA-MCA bypass[5]. To prevent donor artery compression in the supine position, a route between the craniotomy site and the OA’s proximal part was cut to create a sough (Figure 3C and D). Particular caution was exercised on the secured OA when making the furrow. Small fabrics were not typically placed around the drill. An end-to-side anastomosis was performed with nylon 10-0 in the recipient artery, such as the angular artery. As the OA had a tortuous path, the surgeon did not point the anastomotic end in a direction that twisted the blood vessel. The recipient artery was a peripheral artery and thinner than the M4 used for STA-MCA bypass. Therefore, the arteriotomy must be slightly longer than the STA-MCA bypass to match the anastomosis length accurately. With these points in mind, the authors successfully performed a less invasive bypass surgery.
A particular postoperative wound necrosis risk is possible due to insufficient collateral superficial blood circulation if an ipsilateral bypass surgery has been performed previously[10]. In patients who have undergone the indirect bypass alone, additional surgery might worsen the overlying scalp’s blood circulation, resulting in ischemia in the existing bypass area. Therefore, accurate confirmation is necessary for the donor artery course based on preoperative imaging tests, including angiography, and to minimize collateral blood vessel damage during skin incision and dissection.
The recipient artery choice is controversial. Bypassing at the best location is vital for improving the patient's symptoms; however, it might be preferred to select a nearby artery considering the OA’s available length. Hirano et al. have proposed two incision methods to approach the recipient artery regarding the OA’s travel direction and distance to the recipient artery: an extension of the OA incision and an additional incision, respectively[5]. The recipient artery was accessed through a single incision in the cases presented here. When an OA of sufficient length is harvested, bypass surgery can be performed less invasively without additional incision. This is also advantageous from a cosmetic point of view.
In cases where the STA is unsuitable in cerebral ischemic disease or progressive ischemia of the MCA territory, OA may be an acceptable donor artery for less invasive extracranial-intracranial bypass surgery.
| 1. | Hanakita S, Lenck S, Labidi M, Watanabe K, Bresson D, Froelich S. The Occipital Artery as an Alternative Donor for Low-Flow Bypass to Anterior Circulation After Internal Carotid Artery Occlusion Failure prior to Exenteration for an Atypical Cavernous Sinus Meningioma. World Neurosurg. 2018;109:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Yasargil MG, Yonekawa Y. Results of microsurgical extra-intracranial arterial bypass in the treatment of cerebral ischemia. Neurosurgery. 1977;1:22-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 134] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Stiver SI, Ogilvy CS. Acute hyperperfusion syndrome complicating EC-IC bypass. J Neurol Neurosurg Psychiatry. 2002;73:88-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Kimura T, Morita A. Occipital Artery to Middle Cerebral Artery Bypass: Operative Nuances. World Neurosurg. 2017;108:201-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Hirano T, Mikami T, Suzuki H, Hirano T, Kimura Y, Komatsu K, Akiyama Y, Wanibuchi M, Mikuni N. Occipital Artery to Middle Cerebral Artery Bypass in Cases of Unavailable Superficial Temporal Artery. World Neurosurg. 2018;112:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Zenonos GA, Morcos JJ. Occipital to Angular Artery Bypass for Post-irradiation Ischemia: 3-Dimensional Operative Video. Oper Neurosurg (Hagerstown). 2020;18:E78. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Chung Y, Lee SH, Ryu J, Kim J, Chung SB, Choi SK. Tailored Double-Barrel Bypass Surgery Using an Occipital Artery Graft for Unstable Intracranial Vascular Occlusive Disease. World Neurosurg. 2017;101:813.e5-813.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Alvernia JE, Fraser K, Lanzino G. The occipital artery: a microanatomical study. Neurosurgery. 2006;58:ONS114-22; discussion ONS114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Funaki T, Takahashi JC, Takagi Y, Yoshida K, Araki Y, Kikuchi T, Kataoka H, Iihara K, Miyamoto S. Impact of posterior cerebral artery involvement on long-term clinical and social outcome of pediatric moyamoya disease. J Neurosurg Pediatr. 2013;12:626-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Takanari K, Araki Y, Okamoto S, Sato H, Yagi S, Toriyama K, Yokoyama K, Murotani K, Matsui S, Wakabayashi T, Kamei Y. Operative wound-related complications after cranial revascularization surgeries. J Neurosurg. 2015;123:1145-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
Open-Access: This is an open-access article selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution-Noncommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer-reviewed.
Peer-review model: Single-blind
Specialty type: Surgery
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Showkat HI, India; Tan JK, Malaysia S-Editor: Yan JP L-Editor: A P-Editor: Yan JP