Published online Mar 26, 2023. doi: 10.12998/wjcc.v11.i9.2084
Peer-review started: December 28, 2022
First decision: February 8, 2023
Revised: February 20, 2023
Accepted: March 3, 2023
Article in press: March 3, 2023
Published online: March 26, 2023
Processing time: 79 Days and 9.2 Hours
Left-sided accessory pathways (APs) can be accessed with either a transaortic (TA) or transseptal approach (TS). For children with Marfan syndrome (MFS) who have aortic disease, the use of TA can aggravate the disease, making TS the best choice for these patients.
A 10-year-old girl was hospitalized because of intermittent heart palpitations and chest tightness. She was diagnosed with MFS, supraventricular tachycardia, Wolff-Parkinson-White syndrome, and left-sided AP was detected by cardiac electrophysiological. Catheter ablation was successfully performed via TS under the guidance of the Ensite system. During the follow-up, no recurrence or complications occurred.
The TS for catheter ablation of left-sided APs can be considered in children with MFS. Adequate evaluation and selection of the appropriate puncture site are particularly important.
Core Tip: Left-sided accessory pathways (APs) can be accessed with either a transseptal (TS) or transaortic approach (TA). For children with Marfan syndrome (MFS) who have aortic disease, however, the use of TA can aggravate the disease. We report a case of successful catheter ablation of the left-sided accessory pathway by TS in one such child. The TS approach for catheter ablation of left-sided APs appears to be a safe and effective therapeutic option applicable in older children with MFS, though it requires a certain level of training and experience to perform.
- Citation: Dong ZY, Shao W, Yuan Y, Lin L, Yu X, Cui L, Zhen Z, Gao L. Transseptal approach for catheter ablation of left-sided accessory pathways in children with Marfan syndrome: A case report. World J Clin Cases 2023; 11(9): 2084-2090
- URL: https://www.wjgnet.com/2307-8960/full/v11/i9/2084.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i9.2084
Catheter ablation is the main method for eradicating supraventricular tachycardia (SVT) in children, and left-sided accessory pathways (APs) can be accessed by using a transaortic approach (TA) or a transseptal approach (TS). Reports show that children with Marfan syndrome (MFS) have a higher risk of developing SVT than healthy children[1] and that they also often have aortic lesions. When left-sided APs are accessed in these children, TA for catheter ablation may result in aggravated aortic disease. Hence, TS may be a better method. However, due to the combination of mitral valve disease, the difficulty of catheter ablation in MFS, and the small amount of experience most providers have with atrial septal puncture in children, there are currently no relevant reports on this technique. We therefore report a case of successful ablation of the left-sided AP by TS in a child with MFS and review the current literature, providing new ideas for the treatment of MFS.
A 10-year-old girl was admitted to Beijing Children's Hospital in May, 2021 for a 4-year history of intermittent heart palpitations and chest tightness.
Symptoms started 4 years before the child was admitted to the hospital. She presented with intermittent episodes of heart palpitations and chest tightness with pallor. Each episode lasted from 15 min to 3 h, with a frequency of about twice a year. Paroxysmal SVT was diagnosed at a local hospital, and could be converted to sinus rhythm after application of propafenone. Four months before admission to Beijing Children’s Hospital, her palpitations increased in frequency and had occurred 4 times in the last month. Antiarrhythmic drugs were needed to convert.
Pectus excavatum was found at birth. In April, 2014, Nuss pectus excavatum correction was performed, and congenital short esophagus and congenital diaphragmatic hernia were diagnosed. In April 2016, the corrective plate was removed.
The child was born full-term to nonconsanguineous, healthy parents by normal vaginal delivery after an uneventful pregnancy.
The weight of the patient was 27.5 kg, and her height was 153 cm. A surgical scar of about 6 cm was observed under her right armpit, and she was noted to have dysmorphic features including long and thin extremities with hyperflexible joints, prior pectus excavatum, and scoliosis. Both wrist sign and thumb sign were positive. Marfan syndrome was suspected and additional tests were carried out.
No abnormalities were found in routine blood or urine analyses.
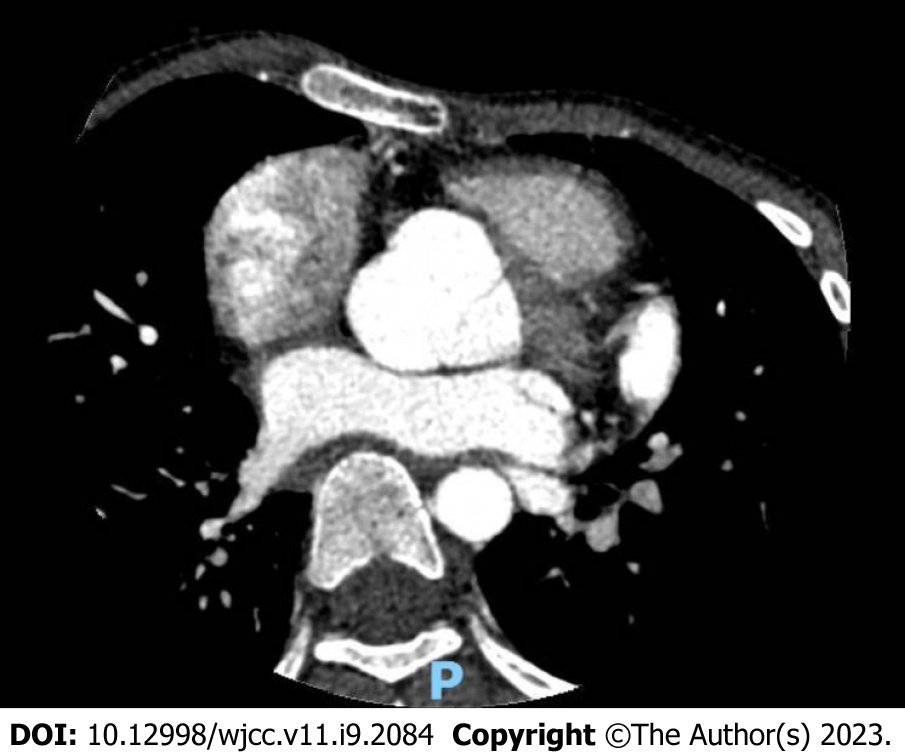
Chest X-ray revealed a right diaphragmatic hernia and scoliosis. Echocardiography showed mild mitral and tricuspid prolapse and regurgitation, and the aortic sinus and ascending aorta were widened. Cardiac computed tomography (CT) scan and reconstruction demonstrated severe aortic root dilatation, with left-right diameters of 3.56 cm (z-score of +6.41), anteroposterior diameters of 3.32 cm (z-score of +5.33), and perforating septum thickness of approximately 13.9 mm (Figure 1). Ophthalmic examination revealed high myopia in both eyes and mild opacification of the posterior lens capsule in the right eye. Finally, genetic testing revealed a heterozygous mutation in the FBN1 gene, c.5841delC (p.C1947). Because she had an aortic root Z-score ≥ 2, a pathogenic FBN1 gene mutation, and a systemic score of 7, MFS was diagnosed according to the 2010 revised Ghent Criteria[2] (Table 1).
| Wrist and thumb sign | Pectus excavatum | Myopia > 3 diopters | Scoliosis | Mitral valve prolapse | |
| Score | 3 | 1 | 1 | 1 | 1 |
During hospitalization, the child still suffered from palpitation attacks. Electrocardiogram (ECG) during an episode revealed SVT with a heart rate of 214/min. After a few minutes, the episode terminated spontaneously. The ECG showed sinus rhythm with a ventricular pre-excitation pattern. Paroxysmal SVT and Wolff-Parkinson-White syndrome type A were subsequently diagnosed.
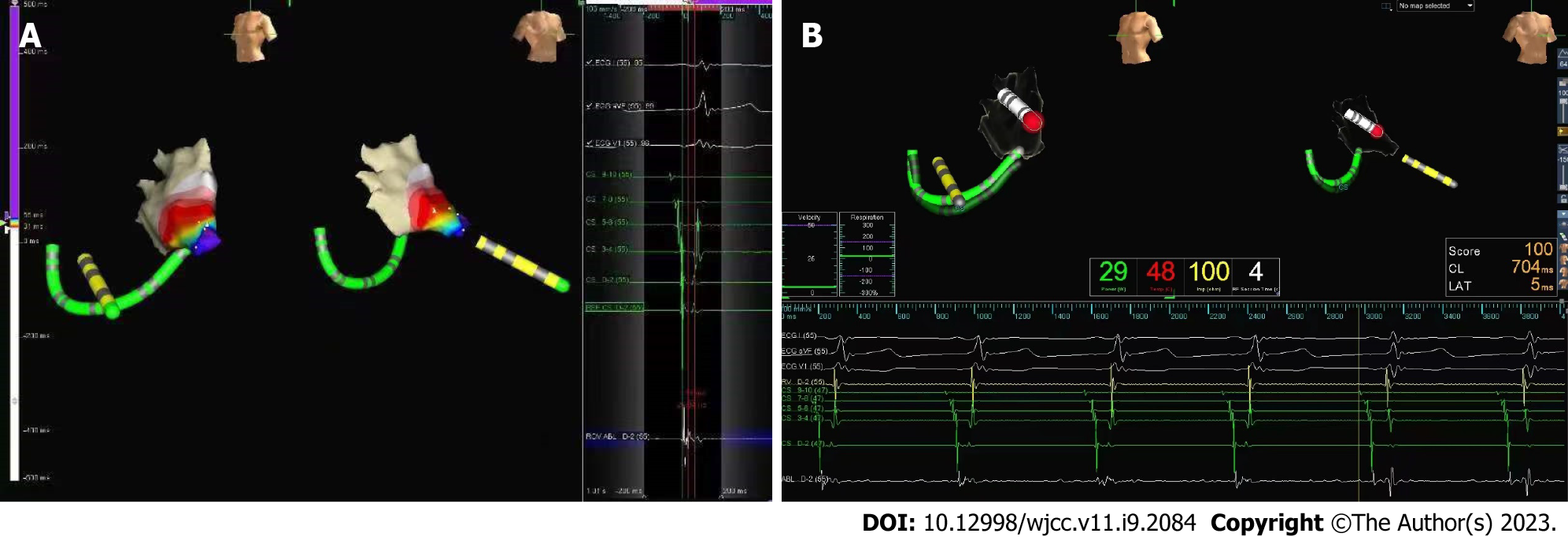
After general anesthesia, cardiac electrophysiological examination was performed under the guidance of Ensite NavX mapping system (Figure 2). Electrode catheters were placed in the coronary sinus (CS) and the right ventricle. Atrioventricular nodal jump phenomenon was not found during atrial program stimulation. Stable narrow QRS tachycardia could be induced by stimulation at 270 ms, with a ventricular rate of 200 bpm and an A/V ratio of 1. The A wave of the CS catheter was eccentrically distributed, and was the most advanced at 1-2 electrodes. Atrioventricular reentrant tachycardia (AVRT) with AP located on the left side was diagnosed.
Based on the child’s medical history, her final diagnosis was MFS, SVT, Wolff-Parkinson-White syndrome, and left-sided AP.
Considering that the child had aortic sinus disease, there was a greater risk of retrograde delivery of ablation catheter through the aorta, so we delivered the ablation catheter to the left side of the heart by transseptal puncture performed under X-ray fluoroscopy. The introducer sheath and dilator, followed with the guide wire, were introduced into right femoral vein and placed to the superior vena cava. After that, we pushed the transseptal needle (Brockenbrough BRK, 71 cm, St. Jude Medical/Abbott) to about 1 cm from the end of the dilator. The needle and sheath were rotated clockwise until the tip pointed toward 4 o'clock, and then were slowly retracted. After two "jumps" the tip of the sheath fell into the fossa ovalis. We then adjusted the direction of the introducer sheath tip in the right anterior oblique view, advanced the puncture needle, and injected contrast agent to confirm that the needle tip was in the left atrium. In the left anterior oblique view, the outer sheath had been guided to the left atrium.
The ablation catheter (Safire, 7-F, 115 cm, St. Jude Medical/Abbott) was then advanced into the left atrium along with the sheath, and the target was subsequently mapped. A satisfactory target was marked on the atrial side at a location of 3:30 (o’clock) of the mitral annulus. During sinus rhythm, the ablation was performed at 52°C and 30 W. After 2 s of discharge, the body surface pre-excitation pattern disappeared, and the V-A wave separated. Under ventricular pacing, the V-A wave of the ablation catheter separated, and the reverse A wave of the CS catheter changed to a centripetal distribution. Consolidation discharges were performed at the target for a total of 120 s, and repeated cardiac electrophysiological examination and intravenous isoproterenol failed to induce AVRT or other arrhythmias during the 48-h observation period after ablation. Ventricular pre-excitation was not restored, and echocardiography showed no aggravation of valve injury.
Regular follow-up was performed at 1, 3, 6, and 12 mo, during which ECG, echocardiography, and dynamic ECG were re-examined. No complication or recurrence of SVT were detected at any of these follow-ups.
MFS is an autosomal dominant disorder of the connective tissue that usually occurs due to mutations in the FBN1 gene and has an incidence of 1.5–17.2 per 100000[3]. About 50% of MFS patients have aortic dilatation in early childhood that gradually progresses. Aortic root disease, which was initially considered to be the main cause of complications and death in patients with MFS, became the target of most treatments and clinical studies. However, the German Marfan Organization believes that some manifestations of MFS may seriously threaten the health of patients, including SVT, ventricular tachycardia, and repolarization abnormalities[4]. Wafa et al[5] showed that the burden of inpatient arrhythmia in MFS is higher than the general non-Marfan population and that SVTs were significant predictors of higher adjusted health care–associated cost. In addition, early aortic repair is recommended by the Ghent guidelines[2], and surgical treatment should also be considered in patients with aortic aneurysm or valvular insufficiency. Postoperative scarring both increases the incidence of arrhythmias and poses difficulties for catheter ablation, however. Demolder et al[6] demonstrated that heart failure and arrhythmia have become the primary causes of death for patients with MFS after aortic surgery. Therefore, eradicating arrhythmias in patients with MFS in childhood who have not undergone intracardiac surgery has the potential to save lives. Furthermore, with modern the improvements in aortic repair, the treatment of arrhythmias in children with MFS should be the focus of future research.
The TS is preferred for catheter ablation to avoid aggravated aortic injury when there is a left-sided AP. The TS does not enter the left side of the heart through the aorta, which effectively avoids the risks of aortic valve injury and ascending aortic aneurysm rupture, among others. Moreover, for children with APs located in the left posterior lateral or left posterior septum, the operation of the ablation catheter is more flexible by a TS[7]. In adults, a TS is associated with a higher success rate and lower recurrence and complication rates, and the safety and feasibility of this method have also been demonstrated by recent studies. Yoshida, Rami, and Koca showed that TS for ablation was safe and effective even in children weighing less than 30 kg[8-10]. However, considering of the need for special training and the risk of serious potential complications (cardiac tamponade and pericardial effusion), TS is still not recommended by most cardiovascular pediatricians at present[11].
Our center at Beijing Children’s Hospital is a national children's medical center, and our staff are experienced in ablating left-sided AP in children using a TS. The child in this report was the only one with left-sided AP who had confirmed MFS, however. She had mitral and tricuspid regurgitation, pectus excavatum, previous history of thoracic surgery, and may also have had a potential delay in vascular healing and repair that presents in most patients with MFS. These factors not only increase the risk of arrhythmia but also pose difficult challenges for atrial septal puncture and radiofrequency ablation[12,13].
The complications of atrial septal puncture include pericardial effusion and cardiac tamponade, as well as others. Cardiac ultrasound, cardiac CT scan and reconstruction must be performed to understand fully the shape and degree of aortic dilatation and cardiac chamber size. Although the child had mitral and tricuspid regurgitation, fortunately the atrial size was normal and had little change at the puncture site. However, her noncoronary sinus, located in the anterosuperior aspect of the interatrial septum, was significantly dilated and pushed against the septum, resulting in a narrow septum. In a case like this the needle tip should therefore be slightly oriented away from the aortic root during puncture (moved backward and downward compared to normal children) to avoid perforating the aorta. In addition, the child weighed < 30 kg, leading to a small heart size. To reduce the risk of penetrating the heart chamber we increased the curvature of the puncture needle.
For safety, a small amount of radiation is required during the puncture. Transseptal puncture under fluoroscopy provides real-time images of surrounding organs (trachea and spine) and CS electrodes, which is helpful in locating the puncture site. However, zero-radiation transseptal puncture can be achieved with the assistance of other imaging tools, such as transthoracic echocardiography, transesophageal echocardiography, or intracardiac echocardiography, but these methods have rarely been used in children[14].
Children are more sensitive to radiation, which may increase their risk of developing hematologic or intracranial tumors[15]. Thus, our subsequent ablation procedure was guided by 3D mapping to minimize the radiation dose while ensuring safety and efficacy. Radiofrequency ablation complications can include valve injury, atrioventricular block, cardiac tamponade, and thromboembolism. Furthermore, valve disease associated with MFS can increase the risk of thromboembolism. Because of the patient’s tricuspid regurgitation, to avoid aggravation of injury, gentle operation low movement amplitude of the ablation catheter at the valve site were necessary. Additionally, in the presences of mitral regurgitation, careful mapping should be performed to determine the ablation site in order to reduce the potential damage of repeated discharges to the valve. For the patient in this case report, echocardiography was performed after catheter ablation and showed that valve damages were not aggravated.
Since the operation, there has been no recurrence of SVT, and no complication have been found during the 1-year follow-up period. The child has gone to school and lived a normal life. For children with aortic diseases such as MFS, who cannot undergo catheter ablation by TA, a TS can be attempted in experienced medical centers. However, infantile MFS is more severe than classical MFS[16]. Severe mitral valve disease and small heart size make ablation much more difficult, and atrial enlargement also causes changes in the puncture site, increasing the risk of accidental atrial or aortic penetration. Weight < 15 kg and age < 4/5 years have also been used as criteria for high risk of catheter ablation in previous studies and guidelines[17]. The weight of this child was large enough so that our conclusions are not applicable to infantile MFS; subsequent studies are needed for using a TS in infantile MFS patients specifically.
In conclusion, it is necessary to perform catheter ablation for MFS patients in order to eliminate SVT in childhood before intracardiac surgery. TS for catheter ablation of left-sided APs, appears to be safe, effective and applicable in older children with MFS, can certainly be attempted in experienced medical centers. Increasing the curvature of the puncture needle and moving the tip posteriorly and inferiorly may be safer for children weighing < 30 kg who have significantly dilated aorta. Gentle manipulation is needed to avoid damaging the valve during ablation.
| 1. | Andersen NH, Groth KA, Berglund A, Hove H, Gravholt CH, Stochholm K. Non-aortic cardiovascular disease in Marfan syndrome: a nationwide epidemiological study. Clin Res Cardiol. 2021;110:1106-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, Devereux RB, Hilhorst-Hofstee Y, Jondeau G, Faivre L, Milewicz DM, Pyeritz RE, Sponseller PD, Wordsworth P, De Paepe AM. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47:476-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1234] [Cited by in RCA: 1408] [Article Influence: 88.0] [Reference Citation Analysis (1)] |
| 3. | von Kodolitsch Y, De Backer J, Schüler H, Bannas P, Behzadi C, Bernhardt AM, Hillebrand M, Fuisting B, Sheikhzadeh S, Rybczynski M, Kölbel T, Püschel K, Blankenberg S, Robinson PN. Perspectives on the revised Ghent criteria for the diagnosis of Marfan syndrome. Appl Clin Genet. 2015;8:137-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 4. | von Kodolitsch Y, Demolder A, Girdauskas E, Kaemmerer H, Kornhuber K, Muino Mosquera L, Morris S, Neptune E, Pyeritz R, Rand-Hendriksen S, Rahman A, Riise N, Robert L, Staufenbiel I, Szöcs K, Vanem TT, Linke SJ, Vogler M, Yetman A, De Backer J. Features of Marfan syndrome not listed in the Ghent nosology - the dark side of the disease. Expert Rev Cardiovasc Ther. 2019;17:883-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 5. | Wafa SEI, Chahal CAA, Sawatari H, Khanji MY, Khan H, Asatryan B, Ahmed R, Deshpande S, Providencia R, Deshmukh A, Owens AT, Somers VK, Padmanabhan D, Connolly H. Frequency of Arrhythmias and Postural Orthostatic Tachycardia Syndrome in Patients With Marfan Syndrome: A Nationwide Inpatient Study. J Am Heart Assoc. 2022;11:e024939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 6. | Demolder A, von Kodolitsch Y, Muiño-Mosquera L, De Backer J. Myocardial Function, Heart Failure and Arrhythmia in Marfan Syndrome: A Systematic Literature Review. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Yu X, Dong Z, Gao L, Lin L, Cui L, Shao W, Yu W, Zhen Z, Yuan Y. Transseptal Approach Versus Transaortic Approach for Catheter Ablation of Left-Sided Accessory Pathways in Children. Front Pediatr. 2022;10:888029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Yoshida S, Suzuki T, Yoshida Y, Watanabe S, Nakamura K, Sasaki T, Kawasaki Y, Ehara E, Murakami Y, Kato T, Nakamura Y. Feasibility and safety of transseptal puncture procedures for radiofrequency catheter ablation in small children weighing below 30 kg: single-centre experience. Europace. 2016;18:1581-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Fogelman R, Birk E, Dagan T, Fogelman M, Einbinder T, Bruckheimer E, Swissa M. Catheter ablation of left-sided accessory pathways in small children. J Arrhythm. 2019;35:742-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Koca S, Akdeniz C, Tuzcu V. Transseptal Puncture for Catheter Ablation in Children. Pediatr Cardiol. 2019;40:799-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Ayabakan C, Şahin M, Çeliker A. Radiofrequency catheter ablation of left-sided accessory pathways via retrograde aortic approach in children. J Arrhythm. 2016;32:176-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Goumans MJ, Ten Dijke P. TGF-β Signaling in Control of Cardiovascular Function. Cold Spring Harb Perspect Biol. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 277] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 13. | Lazea C, Bucerzan S, Crisan M, Al-Khzouz C, Miclea D, Şufană C, Cismaru G, Grigorescu-Sido P. Cardiovascular manifestations in Marfan syndrome. Med Pharm Rep. 2021;94:S25-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 14. | Sharma SP, Nalamasu R, Gopinathannair R, Vasamreddy C, Lakkireddy D. Transseptal Puncture: Devices, Techniques, and Considerations for Specific Interventions. Curr Cardiol Rep. 2019;21:52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | von Graffenried T, Rizzi M, Russo M, Nydegger A, Kayemba-Kay's S. Atypical hematological manifestation of celiac disease: A case report of aplastic anemia in a 2-year-old child and review of the literature. Pediatr Investig. 2021;5:159-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Ardhanari M, Barbouth D, Swaminathan S. Early-Onset Marfan Syndrome: A Case Series. J Pediatr Genet. 2019;8:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Brugada J, Blom N, Sarquella-Brugada G, Blomstrom-Lundqvist C, Deanfield J, Janousek J, Abrams D, Bauersfeld U, Brugada R, Drago F, de Groot N, Happonen JM, Hebe J, Yen Ho S, Marijon E, Paul T, Pfammatter JP, Rosenthal E; European Heart Rhythm Association; Association for European Paediatric and Congenital Cardiology. Pharmacological and non-pharmacological therapy for arrhythmias in the pediatric population: EHRA and AEPC-Arrhythmia Working Group joint consensus statement. Europace. 2013;15:1337-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 250] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Atanasova EG, Bulgaria; Gupta P, United States S-Editor: Liu JH L-Editor: A P-Editor: Liu JH