Published online Mar 26, 2023. doi: 10.12998/wjcc.v11.i9.1974
Peer-review started: November 19, 2022
First decision: January 30, 2023
Revised: February 7, 2023
Accepted: March 3, 2023
Article in press: March 3, 2023
Published online: March 26, 2023
Processing time: 118 Days and 4.9 Hours
Celecoxib has been used to treat hip discomfort and functional difficulties associated with osteonecrosis of the femoral head (ONFH), although significant adverse reactions often follow long-term use. Extracorporeal shock wave therapy (ESWT) can delay the progression of ONFH, alleviate the pain and functional limitations it causes, and avoid the adverse effects of celecoxib.
To investigate the effects of individual ESWT, a treatment alternative to the use of celecoxib, in alleviating pain and dysfunction caused by ONFH.
This was a randomized, controlled, double-blinded, non-inferiority trial. We examined 80 patients for eligibility in this study; 8 patients were excluded based on inclusion and exclusion criteria. A total of 72 subjects with ONFH were randomly assigned to group A (n = 36; celecoxib + alendronate + sham-placebo shock wave) or group B (n = 36; individual focused shock wave [ESWT based on magnetic resonance imaging three-dimensional (MRI-3D) reconstruction] + alendronate). The outcomes were assessed at baseline, at the end of treatment, and at an 8-wk follow-up. The primary outcome measure was treatment efficiency after 2 wk of intervention using the Harris hip score (HHS) (improvement of 10 points or more from the baseline was deemed sufficient). Secondary outcome measures were post-treatment HHS, visual analog scale (VAS), and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores.
After treatment, the pain treatment efficiency of group B was greater than that of group A (69% vs 51%; 95%CI: 4.56% to 40.56%), with non-inferiority thresholds of -4.56% and -10%, respectively. Furthermore, the HHS, WOMAC, and VAS scores in group B dramatically improved during the follow-up period as compared to those in group A (P < 0.001). After therapy, the VAS and WOMAC in group A were significantly improved from the 2nd to 8th wk (P < 0.001), although HHS was only significantly altered at the 2 wk point (P < 0.001). On the 1st d and 2nd wk after treatment, HHS and VAS scores were different between groups, with the difference in HHS lasting until week 4. Neither group had severe complications such as skin ulcer infection or lower limb motor-sensory disturbance.
Individual shock wave therapy (ESWT) based on MRI-3D reconstruction was not inferior to celecoxib in managing hip pain and restrictions associated with ONFH.
Core Tip: This is a randomized, controlled, and non-inferiority trial. To the best of our knowledge, the present study is the first to investigate the short-term effectiveness of extracorporeal shock wave therapy (ESWT) in the management of osteonecrosis of the femoral head (ONFH). Traditional ESWT was innovated by magnetic resonance imaging three-dimensional (MRI-3D) reconstruction technology. The final results demonstrate that ESWT based on MRI-3D reconstruction is not inferior to celecoxib in treating hip discomfort and restrictions associated with ONFH.
- Citation: Zhu JY, Yan J, Xiao J, Jia HG, Liang HJ, Xing GY. Effects of individual shock wave therapy vs celecoxib on hip pain caused by femoral head necrosis. World J Clin Cases 2023; 11(9): 1974-1984
- URL: https://www.wjgnet.com/2307-8960/full/v11/i9/1974.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i9.1974
Osteonecrosis of the femoral head (ONFH) is a prevalent condition in orthopedic clinics, characterized by the gradual loss of bone cellularity and structure. Ischemia, necrosis, and collapse are characteristic pathological processes associated with ONFH[1,2]. The hip is one of the most important weight-bearing joints in the human body[3], and structural and pathological changes of this joint often lead to pain, dysfunction, and even disability[4]. Those suffering from ONFH are mostly young or middle-aged[5]. In the United States, more than 20000 individuals are afflicted by ONFH annually, and the prevalence continues to rise[6,7]. In China, it is estimated that more than 8 million individuals have suffered from ONFH[2].
Currently, the treatment of ONFH mainly includes the following: (1) Surgical treatment (artificial hip arthroplasty and drilling decompression); (2) Drug therapy [nonsteroidal anti-inflammatory drugs (NSAIDs) and alendronate]; (3) Physical therapies [extracorporeal shock wave therapy (ESWT) or high-pressure oxygen therapy]; and (4) Lifestyle changes (weight control and reduction of weight-bearing activities)[2,8,9]. Because of the limited life of the artificial joint in hip arthroplasty, the difficulties of secondary revision, the inadequate impact of hip preservation surgery, and considerable trauma, most patients prefer oral pharmacotherapy to alleviate symptoms[10]. The main goal of pharmacotherapy is to relieve hip pain and improve joint function. It has achieved some success in the management of ONFH, but long-term use of certain drugs is often accompanied by severe side effects[11-13]. Therefore, exploring a noninvasive treatment that can reduce side effects and replace traditional drug therapy is urgently needed. In treating ONFH, the biological effects of ESWT include analgesia, improvement of microcirculation, and promotion of osteocyte proliferation and differentiation[14-16]. A clinical study by Wang et al[17] in 2008 demonstrated that ESWT delays the progression of necrosis, alleviates hip joint pain, and promotes recovery of hip joint function.
In terms of analgesia and improving activity, ESWT avoids the side effects of drugs and dramatically decreases the trauma and economic burden associated with surgery[18,19]. However, the effects of ESWT often cannot be maximized due to acetabular obstruction during the transmission of shock wave energy and its attenuation in bone conduction[20-22]. Magnetic resonance imaging three-dimensional (MRI-3D) reconstruction to strengthen extracorporeal shock wave (ESW) targeting may be a reasonable solution for this issue[23]. Thus, the primary aim of this prospective, randomized study was to determine whether ESWT based on MRI-3D reconstruction was as effective as NSAIDs in improving pain and dysfunction associated with ONFH.
This study was a prospective, double-blinded, non-inferiority randomized controlled trial designed per the principles of the Declaration of Helsinki. It was approved by the Ethics Committee of The Third Medical Center of Chinese People’s Liberation Army General Hospital (ID: 001-R1) and registered on the Chinese Clinical Trial Registry (ChiCTR2100047844).
The study was conducted at the outpatient rehabilitation medicine department of The Third Medical Center of Chinese People’s Liberation Army General Hospital. All consecutive subjects affected by ONFH associated with hip pain and dysfunction referred to the hospital from June 2021 to October 2021 were screened for inclusion in an outpatient rehabilitative setting. The recruitment procedure was performed by 2 specialists and included clinical examination of the affected hip, an MRI of the pelvis, an X-ray of the pelvis, and assessment of patient disease history and general condition.
Eligibility criteria were as follows: (1) Adult age (18-75 years); (2) Diagnosed as ONFH and Association Research Circulation Osseous staging I-IV, confirmed with plain radiograph and MRI; (3) Patient unwillingness to accept surgical treatment and agreement not to use any other non-study treatment for hip pain during the study period; and (4) Voluntary participation in the clinical trial, compliance with the requirements of the trial, and signing of an informed consent form.
General contraindications to shock wave therapy were considered, including use of a pacemaker, pregnancy, bleeding disorder, anticoagulant drug use, or cancer in the focal area, and use of immunosuppressive agents. Patients with any of these contraindications were excluded.
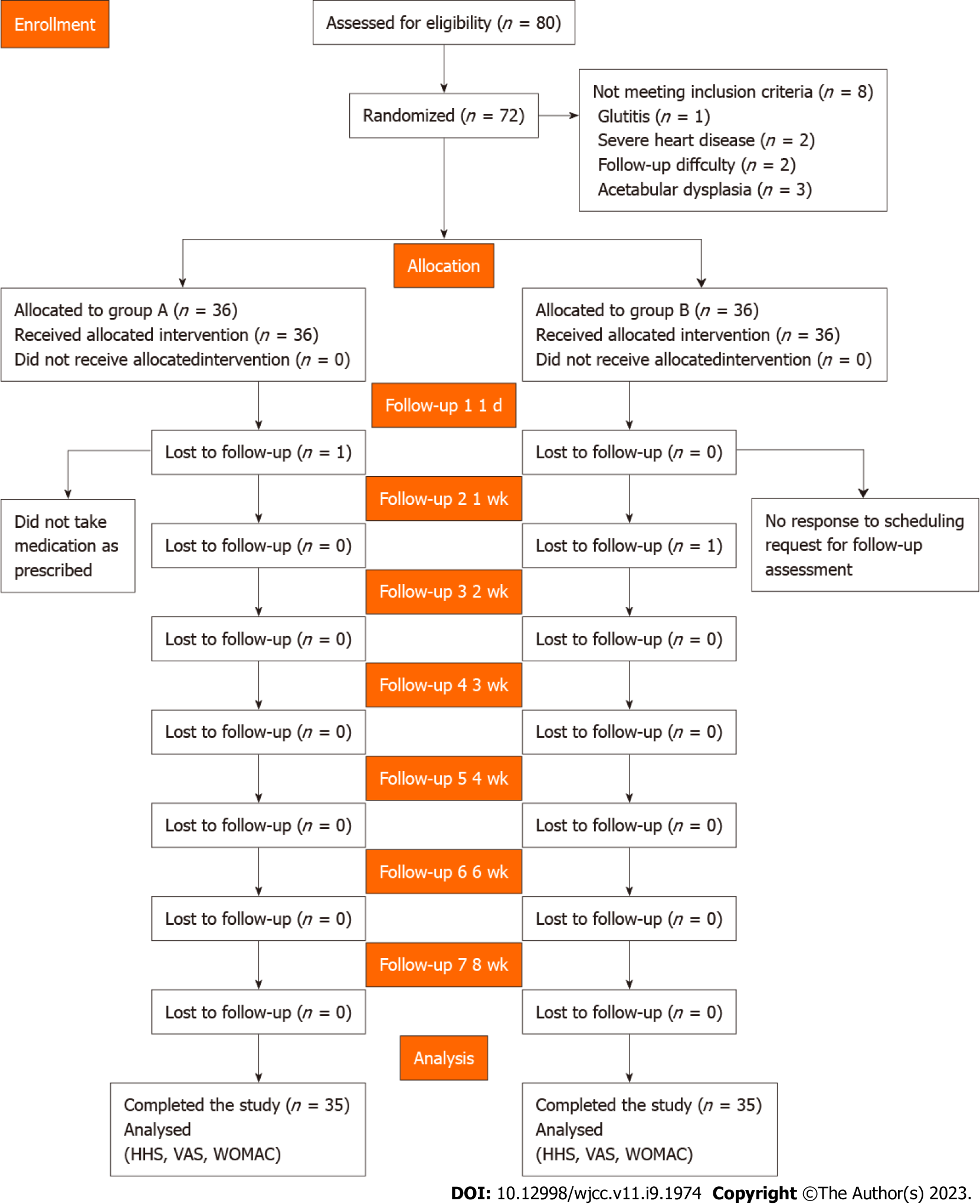
Seventy-two patients were recruited and randomly divided into two groups. All participants provided written informed consent after a detailed understanding of the objectives and procedures of the study. The randomization was performed using the IWRS central random system (https://iwrs.ymedical.net/#/projects/157/dashboard), generating group A and group B. A flow diagram of the trial profile is shown in Figure 1.
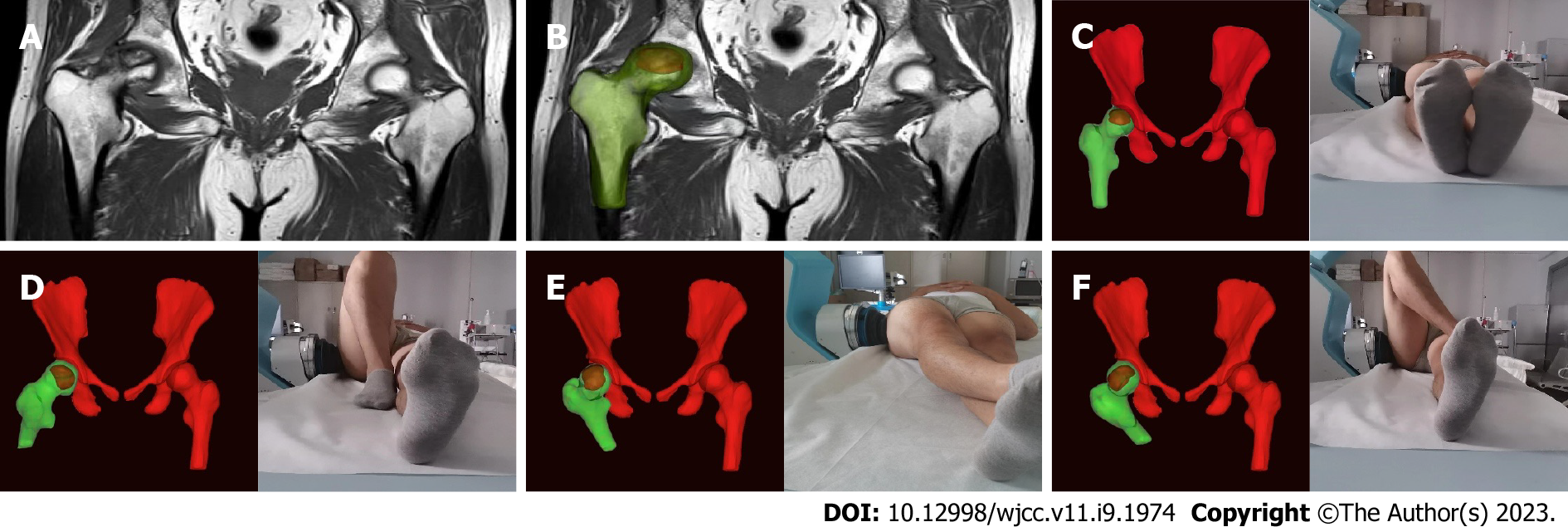
In group A, 200 mg celecoxib was used once daily for 9 consecutive days. Using sham focused ESWT, the protocol was the same as that used in group B, except that the energy level was a single grade (0.07 mJ/mm2) and no coupling gel was used on the treatment site. Instead, thick gauze was placed between the skin and the transmitter, with no energy applied. In group B, using personalized focused extracorporeal shockwave therapy (fESWT), the shock wave was generated by a focused shock wave generator (HK.SWT-007; Huikan AG, China) with a penetration depth between 0 and 70 mm and a focus diameter of 7.5 mm. All ESWT procedures were performed without general or regional anesthesia. First, raw MRI data from imaging of both hip joints (Digital Imaging and Communication in Medicine coronal T1-weighted MRI) were imported to the interactive medical imaging control system software (Mimics; Materialise Company, Laueven, Belgium). A three-dimensional (3D) view of the femoral head and its necrotic area was obtained by image segmentation, visualization, registration, and other functions. The size and spatial location of the necrotic area were determined using the 3D perspective of the reconstructed necrotic area. The junctional zone between normal and necrotic bone within the femoral head was delineated under MRI-3D image guidance. Within the junctional zone, 2 or 3 points approximately 1.0 cm apart were chosen under MRI-3D imaging guidance and the corresponding locations were marked on the skin in the groin area. Next, physicians adjusted the treatment points according to personalized posture with the subjects in different lying positions. Furthermore, they placed the treatment head at the surface of the hip corresponding to the necrotic area (with avoidance of keep essential blood vessels and nerves) (Figure 2). Next, the water sac was adjusted to the appropriate position, and the proper amount of medical coupling agent was applied to the surface of the water in contact with the human body. Finally, when using 5-10 grade energy therapy (energy flow density: 0.20-0.6 mJ/mm2), the energy was increased from low to high according to the subject’s sensitivity to pain. Simultaneously, to ensure exact targeting, the position was monitored throughout treatment, and the drift of the treatment point was adjusted in real-time.
The clinicians administering ESWT had extensive experience using this treatment to treat various musculoskeletal disorders. Each treatment cycle included 5 sessions with 1000 impulses per point, each administered at a frequency of 60 times per minute, at 48-72 h intervals.
During the trial, all the subjects were treated with basic treatment (70 mg oral alendronate sodium once per wk).
The primary outcome measure was treatment efficacy after the 2nd wk of treatment. Harris hip score (HHS) improved by 10 points after therapy, which was deemed sufficient. The secondary outcome measures [HHS, visual analog scale (VAS), and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores] were improved on the 1st d and the 2nd, 4th, and 8th wk after treatment as compared to baseline. After completing therapy, pain recurrence was evaluated if the subject's VAS pain score was equal to or higher than baseline during the follow-up period. Each subject was instructed to record his or her VAS score at the following timepoints following therapy: day 1, week 1, week 2, week 3, week 4, week 6, and week 8.
A sample size of 64 patients was computed given an alpha error of 5% (two-sided) and a power of 80%, assuming that the pain improvement rate of group A and group B was 65% and 85%, respectively, with a non-inferiority limit of -10%. Thus, non-inferiority would be demonstrated if the lower boundary of the 95%CI for the difference was higher than -10%. After considering the potential for patient dropout (estimated at 10%), the final sample was 72 patients (36 per group).
Patients were randomized to group A or group B after providing written informed consent. Randomization was performed by a person not involved in the study, and a computer-generated list of random numbers was used. Patients and investigators were blinded to allocation. After intervention, results were recorded by a specialized physician. To maintain blinding, statistical analyses were conducted by independent statisticians, and results were not shared with the patients or other participants before the end of the study.
This experiment was a non-inferiority test. Statistical analysis was performed using Graphpad5.0 software (La Jolla, CA, United States). All statistical tests were bilateral, and P < 0.05 was considered statistically significant. Quantitative data were normally distributed by mean ± SD, and the skewed distribution was described by median or interquartile range. A paired sample t-test was used to compare the mean change of each evaluation indicator between the pre-treatment and scheduled follow-up time points. Two-sample independent t-test or Wilcoxon rank-sum test was used to compare the groups. The classified data distribution was described by rate or composition ratio. The main curative effect used the normal approximation method, and the 95%CI of the rate difference between the two groups was compared with the non-inferiority boundary value of -10%.
The datasets are available from the corresponding author upon reasonable request.
We examined 80 patients for study eligibility and 8 patients were excluded per inclusion and exclusion criteria. Seventy-two patients were randomized into two groups, which received treatment predefined for each group. All patients completed the designated interventions, which was monitored and reported at the end of the treatment period by physiotherapists. Two participants were lost to follow-up. All others were analyzed at each of the assessment points (Figure 1). There were no differences in age, sex, height, weight, BMI, or symptom duration between the groups (P > 0.05) (Table 1).
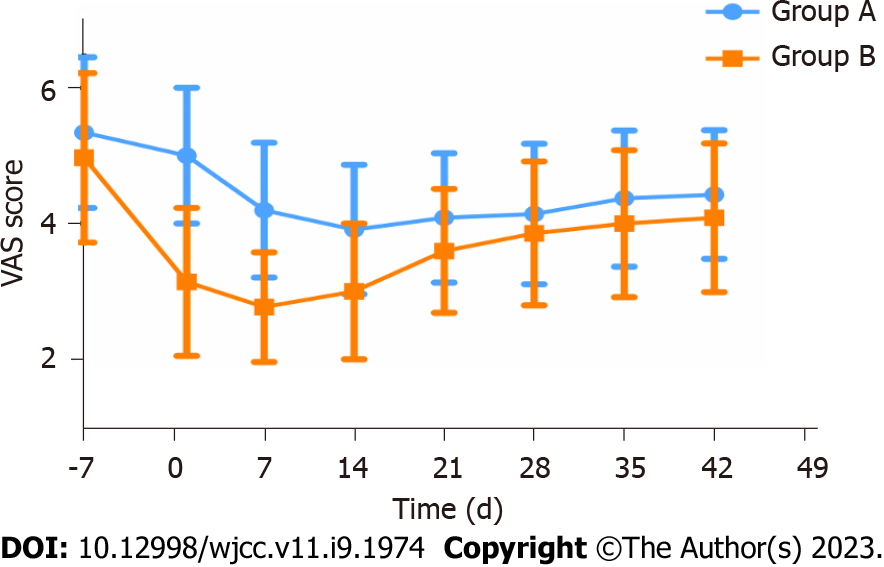
The findings of the Harris, VAS, and WOMAC score analyses were documented over the follow-up period (Table 2). The analysis found no statistical significance in the baseline data of the two groups of patients. With respect to the continuous changes in VAS, the downward trend in group B was faster than in group A (Figure 3).
| Parameter | Baseline | 1 d | 2 wk | 4 wk | 8 wk |
| Group A | |||||
| Patients, n | 35 | 35 | 35 | 35 | 35 |
| HHS range 0-100, mean ± SD | 51.8 ± 11.8 | 51.8 ± 11.6 | 57.9 ± 12.5 | 52.9 ± 11.5 | 52.7 ± 11.2 |
| Difference vs baseline | 0.9621 | < 0.0011 | 0.2181 | 0.3151 | |
| Difference vs group B | < 0.0012 | 0.0042 | 0.0472 | 0.1472 | |
| VAS (0-10), mean ± SD | 5.3 ± 1.1 | 5.0 ± 1.0 | 3.9 ± 1.0 | 4.1 ± 1.0 | 4.4 ± 1.0 |
| Difference vs baseline | 0.0031 | < 0.0011 | < 0.0011 | < 0.0011 | |
| Difference vs group B | < 0.0012 | < 0.0012 | 0.2572 | 0.1662 | |
| WOMAC range 0-240, mean ± SD | 93.7 ± 32.4 | 92.3 ± 31.8 | 87.6 ± 31.5 | 89.3 ± 31.4 | 90.2 ± 31.2 |
| Difference vs baseline | 0.0221 | < 0.0011 | < 0.0011 | < 0.0011 | |
| Difference vs group B | 0.1962 | 0.4472 | 0.5962 | 0.6512 | |
| Group B | |||||
| Patients, n | 35 | 35 | 35 | 35 | 35 |
| HHS range 0-100, mean ± SD | 53.9 ± 13.3 | 62.4 ± 11.6 | 62.9 ± 12.1 | 58.5 ± 12.0 | 56.9 ± 12.9 |
| Difference vs baseline | < 0.0011 | < 0.0011 | < 0.0011 | < 0.0011 | |
| VAS range 0-10, mean ± SD | 5.0 ± 1.2 | 3.1 ± 1.1 | 3.0 ± 1.0 | 3.9 ± 1.1 | 4.1 ± 1.1 |
| Difference vs baseline | < 0.0011 | < 0.0011 | < 0.0011 | < 0.0011 | |
| WOMAC range 0-240, mean ± SD | 91.0 ± 25.1 | 83.5 ± 23.9 | 82.5 ± 24.0 | 86.0 ± 24.3 | 87.1 ± 24.7 |
| Difference vs baseline | < 0.0011 | < 0.0011 | < 0.0011 | < 0.0011 |
The treatment efficacy observed in group A (18/35) and group B (24/35) were 51% and 69%, respectively (95%CI: -4.56% to 40.56%. The non-inferiority thresholds according to the primary outcome measure for group A and group B were -4.56% and -10%, respectively. In addition, the Harris, WOMAC, and VAS scores in group B were significantly improved at day 1, week 2, week 4, and week 8 following treatment (Table 2). In group A, the VAS and WOMAC scores were significantly improved at day 1, week 2, week 4, and week 8 following treatment (P < 0.001), while changes in HHS significant only at week 2 following treatment (P < 0.001). On day 1 and week 2 following treatment, HHS and VAS scores were significantly different between groups, with the difference in HHS lasting until week 4 (Table 2).
Short-term side effects such as discomfort, edema, and bruising were similar in both groups. Heart rate, blood pressure, body temperature, and routine blood tests were unaffected by treatment. Patients from either group experienced no serious complication such as skin ulceration infection or lower extremity motor-sensory impairment.
This study demonstrated that fESWT can effectively and safely treat hip pain and functional restrictions associated with ONFH. The treatment efficacy observed in group B was significantly higher than that of group A. Moreover, most secondary outcomes, including composite scores and response criteria, showed improvements favoring the use of fESWT.
ONFH causes the collapse of the femoral head with severe pain and limited mobility[24]. Hip pain and functional limitations are the typical clinical manifestations of this condition[25]. Therefore, symptom alleviation and recovery of hip joint function are essential in determining the effectiveness of short-term treatment1. In this study, we proposed that individualized fESWT could be used for short-term symptomatic treatment of ONFH and it is as effective as NSAIDs (e.g., celecoxib) with respect to analgesia and improvement of joint function[26]. After an 8-wk follow-up, we found that individualized fESWT was effective in alleviating hip joint pain and promoting recovery of hip joint function during short-term applications. The therapeutic effect peaked 1 wk after the intervention and was superior to celecoxib in both therapeutic and analgesic efficacy.
ONFH is more likely to occur in young and active patients as compared to osteoarthritis[4,27]. Therefore, most patients choose non-surgical treatments. In the short term, many non-operative treatments for ONFH are designed to relieve hip pain and improve joint function, while long-term treatments largely aim to prevent the progression of necrosis[28]. Recent studies recommend the use of osteoclast inhibitors and NSAIDs for symptomatic treatment[2,29]. In 2009, a nationwide cohort study conducted in Denmark showed that NSAIDs were associated with increased relative risks of cardiovascular events and death, even in the low-risk population[30]. At the same time, Singh et al[31] showed that NSAIDs had severe gastrointestinal toxicity, highlighting the significant adverse reactions associated with pharmacotherapy.
ESWT represents mechanical stimulation of the joint and has great potential in treating ONFH as it avoids the adverse effects of drug therapy. In 2 clinical studies, Wang et al[32] and Vulpiani et al[33] separately explored the long-term dose effects of ESWT in the treatment of ONFH. These studies demonstrated that ESWT could stimulate the growth of new bone at the site of necrosis and delay the progression of ONFH with long-term application[33]. Other studies have also indicated that ESWT might relieve pain by suppressing the release of inflammatory factors and downregulating the expression of pain-related, calcitonin gene-related peptides in dorsal root ganglion[34,35]. Additionally, it can directly act on peripheral sensory nerve endings, improving the pain threshold and preventing the production and propagation of pain signals[36]. These data suggest that ESWT may be useful as short-term, symptomatic supportive treatment in the management of ONFH.
To the best of our knowledge, this is the first investigation of the efficacy of ESWT in the short-term management of ONFH. Our results showed that the Harris, WOMAC, and VAS scores of group B improved significantly at day 1, week 2, week 4, and week 8 following treatment (P < 0.001). In group A, the VAS and WOMAC scores were significantly improved at week 2, week 4, and week 8 following treatment (P < 0.001), while Harris scores differed significantly only at week 2 (P < 0.001). These data suggest that both fESWT and celecoxib are effective in improving hip pain and promoting joint function in patients with ONFH. However, fESWT was superior to celecoxib in speed of onset and analgesic effect (a comparison of the two groups found significant differences in HHS and VAS at day 1 and week 2 following treatment, with the difference in HHS lasting until week 4). This finding suggests that, as compared to pharmacotherapy, ESWT avoids the lack of targeted treatment and limits bone marrow edema induced by early ONFH. During treatment, fESWT promotes blood circulation and reduces inflammation, thereby limiting pain associated with ONFH[14,37,38]. Treatment with traditional NSAIDs does not exhibit these features.
Additionally, based on our prior experience with ESWT, we noted that the acetabulum frequently blocks the incoming shock wave and reduces the therapeutic impact as a result of the uniqueness of hip joint anatomy. In a study of the energy decay of shock waves utilizing the femoral heads of pigs, ESW attenuated by 50% for every 10 mm of penetration into the femoral head[20]. Coupled with acetabular blocking, traditional ESWT often fails to maximize function[21]. Therefore, to reduce unnecessary energy attenuation in ESW transmission, our team designed an individual fESWT treatment for each patient according to MRI-3D reconstruction combined with posture adjustment (Figure 1). The 3D picture of the MRI, as opposed to the conventional X-ray localization, better reflected the spatial location and size of the necrotic area, increasing the accuracy of ESWT targeting and improving the curative impact. This supports the idea that MRI is the gold standard for diagnosing early stage ONFH, whereas X-ray and computed tomography less effective in diagnosing this condition[1,2,39].
Our study does have some limitations. Because the trial used a new treatment method (ESWT) based on MRI-3D reconstruction, we performed only a conservative non-inferiority test. Furthermore, due to ethical concerns and a lack of relevant pathology and other objective markers, the study could not investigate the exact causes of pain induced by ONFH. As a result, the lack of this objective evaluation may have led to bias in interpretation of the experimental results. Therefore, our goals in the next phase of study are optimization of the treatment regimen and exploration of the mechanism of ESWT to improve treatment of pain associated with ONFH. Finally, this study only included patients from a single medical center, and recruitment of subjects was somewhat limited.
Short-term ESWT based on MRI-3D reconstruction can relieve hip pain caused by ONFH, and is not inferior to celecoxib in terms of treatment efficacy and continuous analgesic effect. It also shows significant efficacy in improving the function of the hip joint, indicating that ESWT is a promising alternative for the short-term management of ONFH.
Hip pain and functional joint limitations are the major symptoms associated with osteonecrosis of the femoral head (ONFH). The long-term use of nonsteroidal anti-inflammatory drugs (NSAIDs) such as celecoxib are often associated with severe adverse effects.
The motivation of this study was to explore a new treatment, extracorporeal shock wave therapy (ESWT) to replace pharmacotherapy in the treatment of ONFH.
This study investigated the efficacy of ESWT in improving pain and joint dysfunction associated with ONFH as compared to that of NSAID therapy.
The eligible 72 ONFH patients were randomly assigned to two groups: the experiment group and the control group. All patients underwent clinical assessment and analysis during pre- and post-treatment periods.
The Harris hip score, Western Ontario and McMaster Universities Arthritis Index, and visual analog scale scores in both the experiment and control groups were significantly improved, but those of the experimental group improved to a greater degree.
ESWT based on magnetic resonance imaging three-dimensional reconstruction was not inferior to celecoxib in controlling hip pain and function associated with ONFH.
The findings of this study indicate that individual shock wave therapy represents an alternative to pharmacotherapy in effectively alleviating pain associated with ONFH.
We are grateful to the patients who participated in our study. We thank our research team, Geng-Yan Xing, Jun Yan, Hao-Jun Liang, Hai-Guan Jia, Li Tong, Jian Xiao, Fan Hu, Hao Li, and Fu-Jin Li, for their assistance with data collection and management, and the occupational therapists who provided the intervention.
| 1. | Microsurgery Department of the Orthopedics Branch of the Chinese Medical Doctor Association; Group from the Osteonecrosis and Bone Defect Branch of the Chinese Association of Reparative and Reconstructive Surgery; Microsurgery and Reconstructive Surgery Group of the Orthopedics Branch of the Chinese Medical Association. Chinese Guideline for the Diagnosis and Treatment of Osteonecrosis of the Femoral Head in Adults. Orthop Surg. 2017;9:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 2. | Zhao D, Zhang F, Wang B, Liu B, Li L, Kim SY, Goodman SB, Hernigou P, Cui Q, Lineaweaver WC, Xu J, Drescher WR, Qin L. Guidelines for clinical diagnosis and treatment of osteonecrosis of the femoral head in adults (2019 version). J Orthop Translat. 2020;21:100-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 278] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 3. | Li Z, Zhuang Z, Hong Z, Chen L, He W, Wei Q. Avascular necrosis after femoral neck fracture in children and adolescents: poor prognosis and risk factors. Int Orthop. 2021;45:2899-2907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 4. | Hauzeur JP, Malaise M, de Maertelaer V. A prospective cohort study of the clinical presentation of non-traumatic osteonecrosis of the femoral head: spine and knee symptoms as clinical presentation of hip osteonecrosis. Int Orthop. 2016;40:1347-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Yoon BH, Jones LC, Chen CH, Cheng EY, Cui Q, Drescher W, Fukushima W, Gangji V, Goodman SB, Ha YC, Hernigou P, Hungerford M, Iorio R, Jo WL, Khanduja V, Kim H, Kim SY, Kim TY, Lee HY, Lee MS, Lee YK, Lee YJ, Mont MA, Sakai T, Sugano N, Takao M, Yamamoto T, Koo KH. Etiologic Classification Criteria of ARCO on Femoral Head Osteonecrosis Part 1: Glucocorticoid-Associated Osteonecrosis. J Arthroplasty. 2019;34:163-168.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 6. | Hungerford DS. Osteonecrosis: avoiding total hip arthroplasty. J Arthroplasty. 2002;17:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Mont MA, Salem HS, Piuzzi NS, Goodman SB, Jones LC. Nontraumatic Osteonecrosis of the Femoral Head: Where Do We Stand Today? J Bone Joint Surg Am. 2020;102:1084-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 262] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 8. | Puljak L, Marin A, Vrdoljak D, Markotic F, Utrobicic A, Tugwell P. Celecoxib for osteoarthritis. Cochrane Database Syst Rev. 2017;5:CD009865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Lai KA, Shen WJ, Yang CY, Shao CJ, Hsu JT, Lin RM. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. A randomized clinical study. J Bone Joint Surg Am. 2005;87:2155-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Tsukanaka M, Halvorsen V, Nordsletten L, EngesæTer IØ, EngesæTer LB, Marie Fenstad A, Röhrl SM. Implant survival and radiographic outcome of total hip replacement in patients less than 20 years old. Acta Orthop. 2016;87:479-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Dahlberg LE, Holme I, Høye K, Ringertz B. A randomized, multicentre, double-blind, parallel-group study to assess the adverse event-related discontinuation rate with celecoxib and diclofenac in elderly patients with osteoarthritis. Scand J Rheumatol. 2009;38:133-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Mallen SR, Essex MN, Zhang R. Gastrointestinal tolerability of NSAIDs in elderly patients: a pooled analysis of 21 randomized clinical trials with celecoxib and nonselective NSAIDs. Curr Med Res Opin. 2011;27:1359-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Moore RA, Derry S, Makinson GT, McQuay HJ. Tolerability and adverse events in clinical trials of celecoxib in osteoarthritis and rheumatoid arthritis: systematic review and meta-analysis of information from company clinical trial reports. Arthritis Res Ther. 2005;7:R644-R665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 14. | Sung PH, Yin TC, Chai HT, Chiang JY, Chen CH, Huang CR, Yip HK. Extracorporeal Shock Wave Therapy Salvages Critical Limb Ischemia in B6 Mice through Upregulating Cell Proliferation Signaling and Angiogenesis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Li B, Wang R, Huang X, Ou Y, Jia Z, Lin S, Zhang Y, Xia H, Chen B. Extracorporeal Shock Wave Therapy Promotes Osteogenic Differentiation in a Rabbit Osteoporosis Model. Front Endocrinol (Lausanne). 2021;12:627718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Alshihri A, Niu W, Kämmerer PW, Al-Askar M, Yamashita A, Kurisawa M, Spector M. The effects of shock wave stimulation of mesenchymal stem cells on proliferation, migration, and differentiation in an injectable gelatin matrix for osteogenic regeneration. J Tissue Eng Regen Med. 2020;14:1630-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Wang CJ, Wang FS, Yang KD, Huang CC, Lee MS, Chan YS, Wang JW, Ko JY. Treatment of osteonecrosis of the hip: comparison of extracorporeal shockwave with shockwave and alendronate. Arch Orthop Trauma Surg. 2008;128:901-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Wang CJ, Huang CC, Wang JW, Wong T, Yang YJ. Long-term results of extracorporeal shockwave therapy and core decompression in osteonecrosis of the femoral head with eight- to nine-year follow-up. Biomed J. 2012;35:481-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Ludwig J, Lauber S, Lauber HJ, Dreisilker U, Raedel R, Hotzinger H. High-energy shock wave treatment of femoral head necrosis in adults. Clin Orthop Relat Res. 2001;119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Hausdorf J, Lutz A, Mayer-Wagner S, Birkenmaier C, Jansson V, Maier M. Shock wave therapy for femoral head necrosis-Pressure measurements inside the femoral head. J Biomech. 2010;43:2065-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Tsutsumi M, Nimura A, Akita K. New insight into the iliofemoral ligament based on the anatomical study of the hip joint capsule. J Anat. 2020;236:946-953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Tsutsumi M, Yamaguchi I, Nimura A, Utsunomiya H, Akita K, Kudo S. In vivo magnetic resonance imaging study of the hip joint capsule in the flexion abduction external rotation position. Sci Rep. 2022;12:6656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Derry S, Moore RA. Single dose oral celecoxib for acute postoperative pain in adults. Cochrane Database Syst Rev. 2013;2013:CD004233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Ikeuchi K, Hasegawa Y, Seki T, Takegami Y, Amano T, Ishiguro N. Epidemiology of nontraumatic osteonecrosis of the femoral head in Japan. Mod Rheumatol. 2015;25:278-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Wang P, Wang C, Meng H, Liu G, Li H, Gao J, Tian H, Peng J. The Role of Structural Deterioration and Biomechanical Changes of the Necrotic Lesion in Collapse Mechanism of Osteonecrosis of the Femoral Head. Orthop Surg. 2022;14:831-839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 26. | Luan S, Wang S, Lin C, Fan S, Liu C, Ma C, Wu S. Comparisons of Ultrasound-Guided Platelet-Rich Plasma Intra-Articular Injection and Extracorporeal Shock Wave Therapy in Treating ARCO I-III Symptomatic Non-Traumatic Femoral Head Necrosis: A Randomized Controlled Clinical Trial. J Pain Res. 2022;15:341-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Seki T, Hasegawa Y, Masui T, Yamaguchi J, Kanoh T, Ishiguro N, Kawabe K. Quality of life following femoral osteotomy and total hip arthroplasty for nontraumatic osteonecrosis of the femoral head. J Orthop Sci. 2008;13:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Petek D, Hannouche D, Suva D. Osteonecrosis of the femoral head: pathophysiology and current concepts of treatment. EFORT Open Rev. 2019;4:85-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 234] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 29. | Luyten FP, Geusens P, Malaise M, De Clerck L, Westhovens R, Raeman F, Vander Mijnsbrugge D, Mathy L, Hauzeur JP, De Keyser F, Van den Bosch F. A prospective randomised multicentre study comparing continuous and intermittent treatment with celecoxib in patients with osteoarthritis of the knee or hip. Ann Rheum Dis. 2007;66:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Fosbøl EL, Gislason GH, Jacobsen S, Folke F, Hansen ML, Schramm TK, Sørensen R, Rasmussen JN, Andersen SS, Abildstrom SZ, Traerup J, Poulsen HE, Rasmussen S, Køber L, Torp-Pedersen C. Risk of myocardial infarction and death associated with the use of nonsteroidal anti-inflammatory drugs (NSAIDs) among healthy individuals: a nationwide cohort study. Clin Pharmacol Ther. 2009;85:190-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Singh G. Recent considerations in nonsteroidal anti-inflammatory drug gastropathy. Am J Med. 1998;105:31S-38S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 286] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 32. | Wang CJ, Huang CC, Yip HK, Yang YJ. Dosage effects of extracorporeal shockwave therapy in early hip necrosis. Int J Surg. 2016;35:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Vulpiani MC, Vetrano M, Trischitta D, Scarcello L, Chizzi F, Argento G, Saraceni VM, Maffulli N, Ferretti A. Extracorporeal shock wave therapy in early osteonecrosis of the femoral head: prospective clinical study with long-term follow-up. Arch Orthop Trauma Surg. 2012;132:499-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Murata R, Nakagawa K, Ohtori S, Ochiai N, Arai M, Saisu T, Sasho T, Takahashi K, Moriya H. The effects of radial shock waves on gene transfer in rabbit chondrocytes in vitro. Osteoarthritis Cartilage. 2007;15:1275-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Hausdorf J, Lemmens MA, Kaplan S, Marangoz C, Milz S, Odaci E, Korr H, Schmitz C, Maier M. Extracorporeal shockwave application to the distal femur of rabbits diminishes the number of neurons immunoreactive for substance P in dorsal root ganglia L5. Brain Res. 2008;1207:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Takahashi N, Ohtori S, Saisu T, Moriya H, Wada Y. Second application of low-energy shock waves has a cumulative effect on free nerve endings. Clin Orthop Relat Res. 2006;443:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Zhao W, Gao Y, Zhang S, Liu Z, He L, Zhang D, Li W, Meng Q. Extracorporeal shock wave therapy for bone marrow edema syndrome in patients with osteonecrosis of the femoral head: a retrospective cohort study. J Orthop Surg Res. 2021;16:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Sukubo NG, Tibalt E, Respizzi S, Locati M, d'Agostino MC. Effect of shock waves on macrophages: A possible role in tissue regeneration and remodeling. Int J Surg. 2015;24:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 39. | Hopkins C, Genant HK. Editorial for Guidelines for clinical diagnosis and treatment of osteonecrosis of the femoral head in adults (2019 version). J Orthop Translat. 2020;21:A1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bianchi J, United States; Verlaan JJ, Netherlands S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang H