Published online Mar 16, 2023. doi: 10.12998/wjcc.v11.i8.1702
Peer-review started: November 26, 2022
First decision: January 17, 2023
Revised: January 27, 2023
Accepted: February 21, 2023
Article in press: February 21, 2023
Published online: March 16, 2023
Processing time: 100 Days and 10.2 Hours
Thrombolytic therapy has been the mainstay for patients with pulmonary embolism (PE). Despite being linked to a higher risk of significant bleeding, clinical trials demonstrate that thrombolytic therapy should be used in patients with moderate to high-risk PE, in addition to hemodynamic instability symptoms. This prevents the progression of right heart failure and impending hemodynamic collapse. Diagnosing PE can be challenging due to the variety of presentations; therefore, guidelines and scoring systems have been established to guide physicians to correctly identify and manage the condition. Traditionally, systemic thrombolysis has been utilized to lyse the emboli in PE. However, newer techniques for thrombolysis have been developed, such as endovascular ultrasound-assisted catheter-directed thrombolysis for massive and intermediate-high submassive risk groups. Additional newer techniques explored are the use of extracorporeal membrane oxygenation, direct aspiration, or fragmentation with aspiration. Because of the constantly changing therapeutic options and the scarcity of randomized controlled trials, choosing the best course of treatment for a given patient may be difficult. To help, the Pulmonary Embolism Reaction Team is a multidisciplinary, rapid response team that has been developed and is used at many institutions. Hence to bridge the knowledge gap, our review highlights various indications of thrombolysis in addition to the recent advances and management guidelines
Core Tip: There are now many treatments to treat acute pulmonary embolism (PE). Patients are divided into low, moderate, and high-risk PE groups to identify those needing more advanced treatment. Unless contraindicated, systemic thrombolysis is advised for high-risk pulmonary embolism. Other than systemic thrombolysis, a number of treatment options for PE are being investigated, such as catheter-directed thrombolysis, extracorporeal membrane oxygenation, direct aspiration, or fragmentation with aspiration. Choosing the appropriate course of treatment for a certain patient may be challenging due to the plethora of therapeutic choices that are continually evolving and the paucity of randomized controlled trials. Therefore, the Pulmonary Embolism Reaction Team, is a multidisciplinary, rapid response team has been developed and is employed by various institutions to customize therapeutic options according to the need of the patient to address the ever-evolving therapeutic care.
- Citation: Ochani RK, Aibani R, Jatoi HN, Anwar M, Khan SA, Ratnani I, Surani S. Evolving paradigm of thrombolysis in pulmonary embolism: Comprehensive review of clinical manifestations, indications, recent advances and guideline. World J Clin Cases 2023; 11(8): 1702-1711
- URL: https://www.wjgnet.com/2307-8960/full/v11/i8/1702.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i8.1702
Pulmonary embolism (PE) is a common and potentially fatal form of venous thromboembolic disease with an estimated incidence of 1 case per 1000 persons per year in the United States. It is the third most common cause of cardiovascular death, with a diverse presentation from asymptomatic disease to death; diagnosis and sometimes challenging diagnosis[1]. Massive PEs necessitate immediate treatment to reestablish pulmonary circulation and blood flow.
Anticoagulation as a treatment for venous thromboembolism (VTE) dates back less than a century, and thrombolysis was only recently introduced, despite the fact that the thrombotic origin of PE has been extensively recognized for about two centuries. In patients with acute PE with hemodynamic compromise, thrombolytic therapy is used to lyse the emboli; however, new applications for thrombolysis emerge as the field of medicine advances[2].
In 1962, according to Browse and James, streptokinase was found to be able to lyse pulmonary emboli in both people and dogs. Four patients who received various dose regimens showed remarkable clinical improvement[3]. According to a randomized controlled trial conducted by Goldhaber and colleagues, recombinant tissue-type plasminogen activator (rtPA), introduced for the treatment of acute PE in the late 1980s, was found to act more quickly and be safer than urokinase[4].
rtPA (such as alteplase), streptokinase, and urokinase are three different thrombolytic agents that are effective in breaking up clots[5,6]. However, these agents came with considerable risks, including the potential for frequent severe bleeding and intracranial hemorrhage[7,8]. Although most of the research concurs that thrombolytic drugs accelerate the lysis of pulmonary thromboemboli better than heparin alone, their benefits for decreased PE death rates, influence on survival, and dangers of related hemorrhagic sequelae are still unclear. Therefore, this review article discusses the clinical manifestations, recent advances, and management guidelines to address the knowledge gap of the evolving treatment regimens for PE.
Understanding and acknowledging the variable clinical manifestations amongst patients with PE is key to their identification and prompt medical intervention. The inability to accurately diagnose the patients is considered an error that can potentially result in mortality; among patients with PE, 30% die due to a delay in their management[9]. Hence, devising an algorithm sensitive to the clinical picture of PE becomes essential to reach a diagnosis.
The clinical presentation of patients with PE can vary from an asymptomatic state to sudden death, and hence reaching a diagnosis can be challenging, limiting the physicians to mostly rely on history and physical examination[9]. Most of the signs and symptoms are broad-spectrum and typically non-specific; some of the non-specific signs may include tachycardia, dyspnea, chest pain, shock, and hypoxemia which are also found in patients presenting with other complaints such as myocardial infarction (MI), congestive heart failure and pneumonia[9]. Based on the Prospective Investigation of Pulmonary Embolism Diagnosis II (PIOPED II) trial, patients with PE frequently presented with some of these nonspecific signs; 54% of patients presented with tachypnea, 24% with tachycardia, 73% presented with dyspnea (at rest or on exertion), 44% with pleuritic pain, and 34% complained of cough[9]. However, since this trial did not include patients with serious risk factors such as recent MI and chronic kidney disease, the pertinence of these values is limited; hence, an evaluation of the patient's risk factors at the time of diagnosis becomes crucial[9]. It is researched that 80 to 90% of the patients have comorbidities that serve as risk factors for PE and that the absence of certain signs and symptoms, including dyspnea and tachypnea, indicates that the likelihood of the patient having PE is low[10].
Hamad et al[11] state that the clinical probability of PE relies on two elements. These include the risk factors and evidence of an alternative diagnosis. A low clinical probability is established in case of the absence of risk factors along with an alternative diagnosis, while a high clinical probability is established if there are no risk factors and there is no alternative diagnosis to explain the clinical presentation of the patient[11].
PIOPED study by Lee et al[10] describes that a correct diagnosis for PE was reached in 91% of cases where a low risk of PE was expected; however, in case of intermediate and high risk, a correct diagnosis was reached only 64%-68% of times, suggesting that it is less challenging to predict the diagnosis in the absence of PE and increasingly more difficult to predict the diagnosis when the underlying cause is PE. Miniati et al[12] report that according to their survey of 800 patients, who were divided into 2 groups based on their location, the frequently reported clinical symptoms included sudden-onset dyspnea (81% and 78%), chest pain (56% and 39%), syncope (26% and 22%), and hemoptysis (7% and 5%) while the reported asymptomatic patients were merely 1% of the study participants.
These results highlight the fact that transiently worsening dyspnea, especially sudden onset, is the most common symptom amongst patients with PE. It could be caused by bronchospasm, reduced effort of the diaphragm, or due to obstruction of the embolus, which results in reduced blood flow and hence a ventilatory/perfusion (V/Q) mismatch in the lungs[10,13,14]. This then results in reduced oxyge
With the challenging and varying set of features in each patient with PE, it is recommended that in the setting of patients presenting with at least one of these four symptoms (sudden-onset dyspnea, chest pain, syncope, and hemoptysis), the physicians should remain vigilant and must keep PE as one of the differentials followed by appropriate investigations to confirm the diagnosis[13].
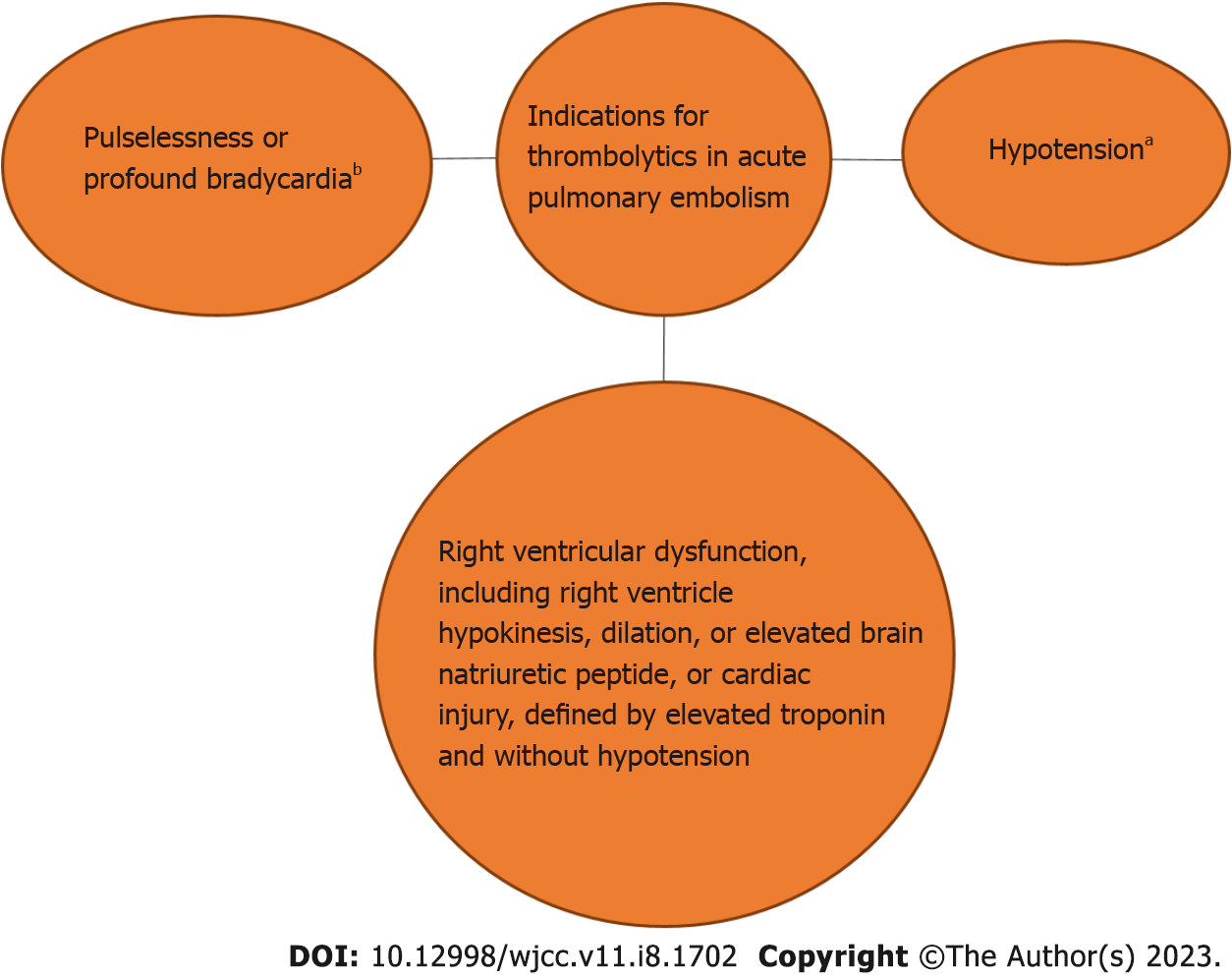
PE is categorized as high, intermediate, or low risk. According to European guidelines, massive PE, also known as high-risk PE, is acute PE with hypotension, which is defined as a systolic blood pressure (SBP) < 90 mmHg or a decrease of > 40 mmHg that cannot be attributed to another cause. Unless it is contraindicated, thrombolytic treatment should be given because this category of patients has a 30-d mortality risk that is more than 15%[15,16].
According to some recommendations, PE that results in profound bradycardia or pulselessness (heart rate < 40 bpm) should be regarded as massive[17]. Although according to the statement by the American Heart Association, thrombolytic therapy should be administered to patients with sub-massive or intermediate-risk PE, the utilization in such scenarios is debatable. These are defined by right ventricular dysfunction (RVD), including right ventricle (RV) hypokinesis, dilation, or elevated brain natriuretic peptide, or cardiac injury defined by elevated troponin and without hypotension. The use of thrombolytic, although, has a high efficacy, with a 30% reduction in mortality, however, the effect size on mortality of submassive PE patients is < 1%. Furthermore, in patients with intermediate risk, none of the patients treated adjunctively with alteplase showed an increase in right ventricular systolic pressure on the 6-mo followup. Such patients' 30-d mortality rates are lower than those of massive PE, ranging from 3% to 15%[17,18]. The risk of bleeding could lessen this group's overall benefit[17].
In low-risk PE without hypotension, RVD, or cardiac damage, systemic thrombolytic treatment is not advised because the 30-d mortality is often less than 2% when treated with conventional anticoagulation[17,19,20]. We have also illustrated the indications in Figure 1.
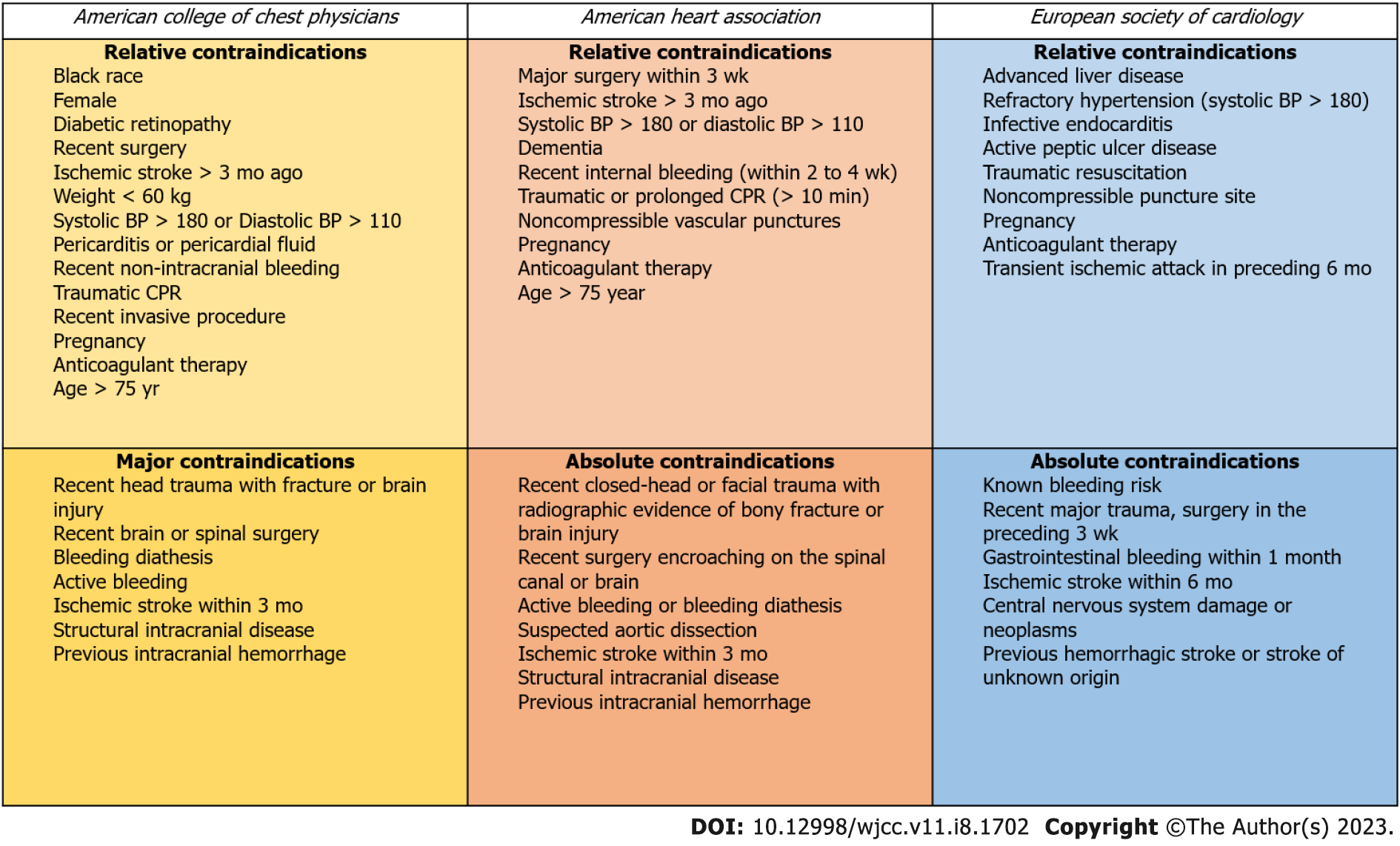
Absolute contraindications to thrombolytic therapy include an intracranial neoplasm, recent (within two months) intracranial or spinal surgery or trauma, a history of a hemorrhagic stroke, active bleeding or bleeding diathesis, or a non-hemorrhagic stroke within the previous three months. Pregnancy, non-hemorrhagic stroke older than three months, surgery within the last ten days, and severe uncontrolled hypertension (SBP > 200 mmHg or diastolic blood pressure > 110 mmHg) are all relative contraindications[21,22]. Contraindications to thrombolysis according to societal guidelines, have been demonstrated in Figure 2.
In an attempt to improve outcomes in patients with PE, the European Society of Cardiology, along with the European Respiratory Society, introduced certain changes to the 2014 guidelines for the diagnosis and management of patients with PE in August 2019[14]. These are key alterations that have ensured the appropriate management of this medical emergency. It has been noted in recent years that deaths due to PE have significantly declined[16].
According to the updated guidelines, patients presenting with PE are initially divided into different categories based on their clinical outcomes, symptoms, and risk factors for venous thromboembolism[14]. As stated earlier, clinical presentations of our demographic are often variable, and hence much of the diagnosis depends upon the knowledge of risk factors and the stability of the patient. Certain systems have been devised that aid in the determination of the likelihood of the patient having PE[9]. These diagnostic scoring systems include the Wells criteria and the Geneva score, which help clinicians determine the possibility of thrombosis[9].
The Wells criteria has a score assigned to specific clinical presentations. A score of greater than 4 indicates a higher likelihood of PE, while a score of less than or equal to 4 indicates a low likelihood of PE. Similarly, the Geneva score also assigns scores to patients based on the absence or presence of certain clinical indications. A score of greater than 5 would suggest a higher possibility of PE (Table 1).
| Features | Score |
| Suspected DVT | 3.0 |
| No alternative diagnosis | 3.0 |
| Heart rate > 100 bpm | 1.5 |
| Immobilization or surgery in the previous 4 weeks | 1.5 |
| Previous DVT or PE | 1.5 |
| Hemoptysis | 1.0 |
| Malignancy | 1.0 |
Additionally, PE Rule-Out Criteria (PERC) is used by clinicians to rule out PE amongst low-risk patients[9]. These criteria include eight clinical variables; if all of the PERC criteria are satisfied and the patient has a low likelihood of PE based on the Wells criteria, the physician can satisfactorily rule out PE, and no further tests are required[23]. These variables include age < 50 years, pulse < 100 bpm, SaO2 > 94%, absence of unilateral leg swelling, absence of hemoptysis, no history of recent trauma or surgery, absence of any VTE history, and no use of oral hormones[14]. However, the use of these systems amongst critically ill patients is limited as they merely serve as tools to assess the need for additional investigations in a stable patient[9,14].
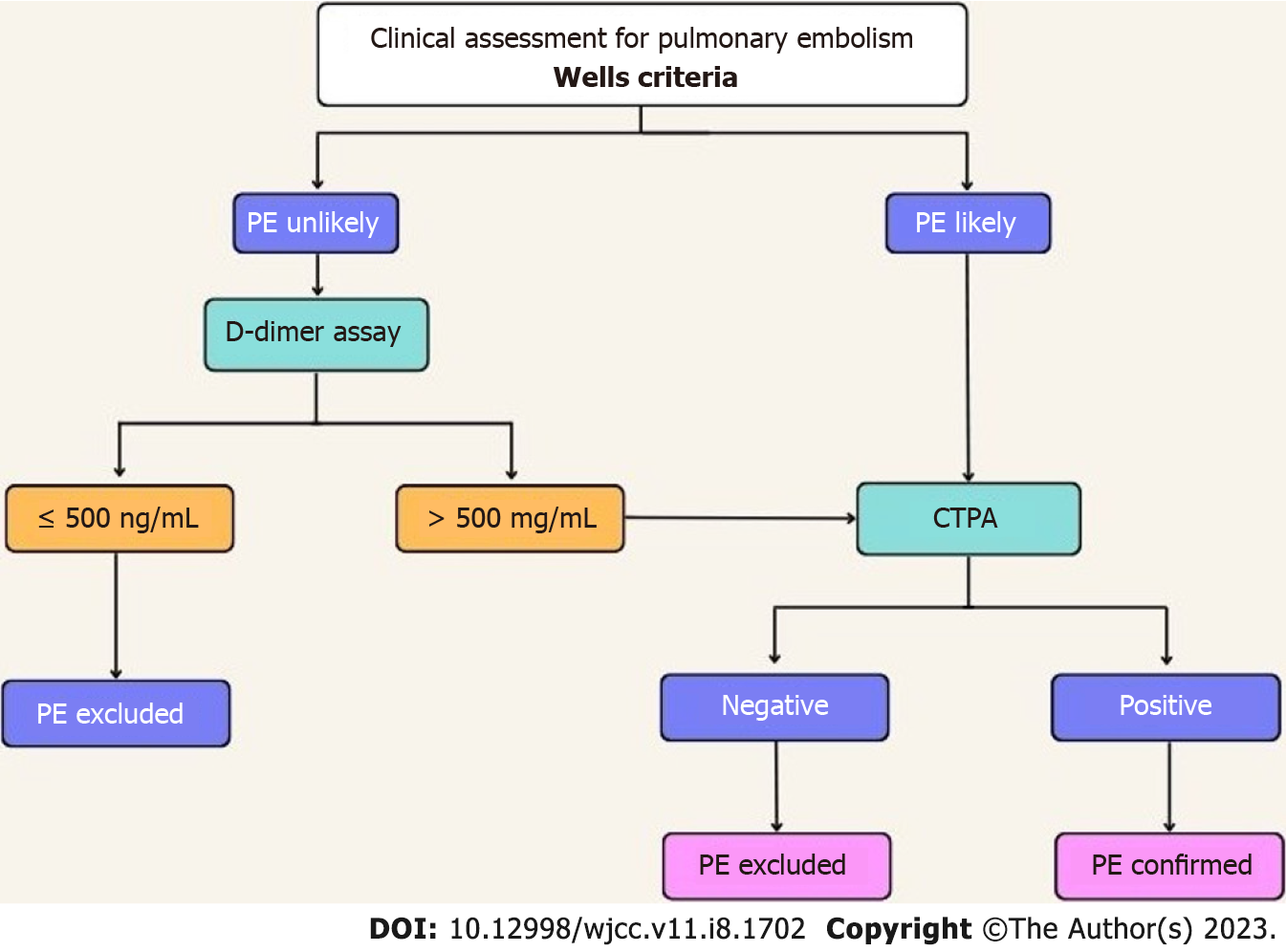
A D-dimer test is warranted among patients with low or intermediate risk of PE[24]. The elevated levels of D-dimer indicate the existence of acute thrombosis due to concurrent coagulation and fibrinolysis; however, in post-operative cases and critically ill patients such as those with cancer, D-dimer levels are normally high due to an increase in coagulation and fibrinolysis, rendering the test unproductive[14]. A normal level of D-dimer suggests there is no need for further testing as the possibility of PE or deep vein thrombosis (DVT) is significantly low[9,14,24]. According to the updated ESC guidelines, an age-adjusted cut-off value of D-dimers has replaced the fixed cut-off value of 500 ng/mL for excluding the diagnosis of PE in low or intermediate-risk patients[25]. An age-adjusted cut-off value can be calculated using the patient's age and multiplying it by 10 for those > 50 years of age. In patients < 50 years of age, the use of the fixed value of 500ng/mL is maintained[25].
If D-dimer levels are below 1000 ng/mL and clinical signs of Wells criteria (signs of DVT, Hemoptysis, or PE) are absent or if the D-dimer level is less than 500 ng/mL, in patients with a low or intermediate possibility of PE, the diagnosis of PE can be successfully excluded without chest imaging since research suggests that in this group of patients, the post-test likelihood of PE is less than 1.85%[24,25]. Patients with a high possibility of PE, however, must undergo chest imaging[24]. A multicenter study suggests that in patients presenting during pregnancy or the post-partum period, a diagnosis can be excluded after a thorough clinical assessment based on the likelihood of PE, D-dimer levels, compression ultrasonography (CUS), and computed pulmonary tomography angiography (CTPA). Clinical assessment for PE according to Well’s Criteria has been shown in Figure 3[26].
The 2019 guidelines by the European Society of Cardiology suggest that the management of a patient must be planned based on positive lower limb CUS findings along with PE risk assessment[25]. In order to identify DVT, CUS reduces the need for invasive procedures; however, it has some limitations in its use in case of obesity and edema as well as identification of pelvic thrombosis, and it must only be used in case of nondiagnostic V/Q scan or CTPA[9,11]. The updated guidelines also highlight the importance of V/Q SPECT for establishing a PE diagnosis[25]. In patients who are unable to undergo CUS due to the aforementioned limitations or due to intravenous contrast dye contraindications, a V/Q scan can be employed[9]. A normal scan excludes the diagnosis of PE effectively[9,11]. However, pulmonary angiography remains the gold standard in investigation regardless of its invasive nature. Additionally, in patients with low or intermediate clinical probability, anticoagulative therapy can be safely withheld if they have a negative CTPA and CUS; however, vigilance is required before stopping therapy, especially in cases of intermediate clinical probability[11]. A study by Hogg et al[27] concluded that computed tomography (CT) pulmonary angiography, when used in conjunction with investigations for DVT, provides substantial evidence to rule in or out the diagnosis of PE. Additionally, echocardiography or electrocardiogram can also be used to establish a diagnosis in cases of suspected PE. An increase in right-sided stress on the heart and McConnell’s sign suggests a high possibility of PE[9].
Once the diagnosis is established, hemodynamically stable patients are urgently administered anticoagulants parenterally in order to minimize the risk of deterioration, as the progression is rapid in severe cases[9]. In case of SBP of greater than 90 mmHg, direct anticoagulants, apixaban, rivaroxaban, or dabigatran are administered orally rather than heparin combined with a vitamin K antagonist as it has a 0.6% lower risk of bleeding[24]. In contrast, patients with SBP of less than 90 mmHg must be given systemic thrombolysis, as it has shown a mortality reduction of 1.6%[24].
Comprehending the clinical risks of thrombolytic therapy is vital for patient selection. The adverse events (AEs) due to use of thrombolysis can be divided into five broad categories: Intracranial hemorrhage, systemic hemorrhage, immunologic complications, hypotension, and myocardial rupture[28]. These AEs are more commonly observed with streptokinase or agents with a streptokinase moiety, including anistreplase (anisoylated plasminogen--streptokinase activator complex, APSAC), amongst all fibrinolytic agents due to its antigenic properties. The most common AE reported is intracranial hemorrhage. Furthermore, immunologic complications (anaphylaxis, immune complex diseases) are also observed, in addition to systemic hemorrhage, which is usually reported in patients who have had a major vascular puncture. Patients who have used streptokinase and anistreplase have also experienced manageable hypotension. Lastly, cardiac rupture is a potential delayed complication in patients taking thrombolysis.
Catheter-directed thrombolysis (CDT) is one of the evolving treatment options for PE. The American College of Chest Physicians currently recommends CDT as a class 2C treatment for acute PE patients with hypotension who have contraindications to thrombolysis, failed thrombolysis, or shock that is likely to result in death before systemic thrombolysis can take effect (e.g., within hours), provided that the necessary expertise and resources are available. This recommendation is for the massive and intermediate-high sub-massive risk groups[29]. CDT comprises a variety of endovascular procedures to eliminate or dissolve acute thrombus. It serves as an alternative to surgical embolectomy, transcatheter embolectomy, and systemic thrombolysis for revascularization. Because of the synergistic effects of higher local fibrinolytic medication concentrations and mechanical disruption with more exposed thrombus surface area and decreased hemorrhagic complications, this therapy has the advantage of improved thrombus-dissolving efficacy[30]. Theoretically, applying low-frequency sonic waves to the thrombus causes the fibrin monomers to break down, allowing thrombolytic penetration.
The use of ultrasound-assisted CDT in patients with intermediate or high-risk proximal PE has been studied in three recent trials (Table 2).
| Study | Ultima[32] | Seattle II[33] | Optalyse[34] |
| Aim of the study | The aim of the study was to investigate whether ultrasound-assisted catheter-directed thrombolysis (USAT) is superior to anticoagulation alone in the reversal of RV dilatation in intermediate-risk patients | To evaluate the safety and efficacy of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis | To determine the lowest optimal tissue plasminogen activator (tPA) dose and delivery duration using ultrasound-facilitated catheter-directed thrombolysis (USCDT) for the treatment of acute intermediate-risk (sub-massive) PE |
| Study design | Two arms | Single arm | Single arm |
| Single-center/multi-center | Multi-center | Multi-center | Multi-center |
| Completed/ongoing | Completed | Completed | Completed |
| Year of publication | 2014 | 2015 | 2018 |
| Primary endpoint | The difference in the RV/LV ratio at 24 h | The difference in RV/LV ratio at 48 h and bleeding at 72 h | reduction in RV/LV ratio on CT imaging at 48 h |
| Total number of patients | 59 | 150 | 101 |
| Mean age of patients | 63 +/- 14 | 59 | 60 |
| % of males | 47% | 48.7 | 53 |
| Drug used | UFH and USAT VS UFH alone | t-PA | t-PA and heparin |
| Results | The mean difference in RV/LV ratio from baseline to 24 h was 0.30 ± 0.20 vs 0.03 ± 0.16 (P < 0.001), respectively | The mean RV/LV diameter ratio decreased from baseline to 48 h post-procedure (1.55 vs 1.13; mean difference, -0.42; P < 0.0001) | Improvements in RV/LV ratio were as follows: Arm 1 (4 mg/lung/2 h), 0.40 (24%; P = 0.0001); arm 2 (4 mg/lung/4 h), 0.35 (22.6%; P = 0.0001); arm 3 (6 mg/lung/6 h), 0.42 (26.3%; P = 0.0001); and arm 4 (12 mg/lung/6 h), 0.48 (25.5%; P = 0.0001) |
| Adverse effects | 4 minor bleedings. No major bleeding | 17 major bleeding events within 30 d of the procedure observed in 15 patients (10%). One of these major bleeding events was a GUSTO severe/life-threatening hemorrhage (a right groin vascular access site hematoma with transient hypotension requiring vasopressor support). The remainder (94%) were GUSTO moderate bleeds, 3 of which were related to vascular access | 4 patients had a total of 5 major bleeding events |
The popularity of catheter-directed thrombolysis is increasing in the United States as more and more healthcare professionals are becoming trained in CDT. Overall, long-term data on right ventricular function, exercise tolerance, quality of life, and the likelihood of developing chronic thromboembolic pulmonary hypertension after CDT is still lacking. The optimal technique for catheter-based thrombus removal will ultimately be determined by trials concentrating on novel devices in the future[31].
When first-line therapy fails, and a patient has hemodynamic instability, mechanical hemodynamic support is an option to investigate. Extracorporeal membrane oxygenation (ECMO), venovenous and veno-arterial, and RV support devices are among the modalities.
Another alternative for patients with an unacceptably high risk of bleeding is mechanical disruption or removal of the thrombus without thrombolysis. Direct aspiration or fragmentations with aspiration are two methods of clot clearance. The FlowTriever (Inari Medical, Irivine, California, United States) and the Penumbra Indigo (Penumbra Inc, Alameda, California, United States) system are two examples of devices for this use that have received Food and Drug Administration (FDA) approval[32-34].
Selecting the best course of treatment for a given patient might be difficult because of the constantly changing therapeutic alternatives and the paucity of randomized controlled studies. To assist, the Pulmonary Embolism Reaction Team (PERT) type of multidisciplinary, fast response team has been created and is used at many institutions[32,33].
When caring for patients with PE, PERT aims to deliver a quick, suitable, multidisciplinary, team-based approach with the shared objective of customizing the best therapeutic decision-making while always placing the needs of the patient first. As soon as a clinician encounters a patient with complex acute PE, PERTs can be activated from anywhere in the hospital. A team member gathers pertinent clinical data about patients after the referring doctor activates the PERT and offers it to the other team members for quick consultation and decision-making. Along with managing and offering outpatient follow-up, PERT can also act as a research platform for patient registries or clinical studies.
Acute PE can now be treated in a variety of ways. Individuals who may need more advanced therapy are identified by risk stratifying patients into low, middle, and high-risk PE categories. In cases with a contraindication, systemic thrombolysis are recommended for high-risk and part intermediate risk pulmonary embolisms.
Endovascular treatment, including CDT, Medical Pulmonary Thromboendarterectomy, or catheter aspiration with or without fragmentation is a promising method for high-risk PE, especially under the support of ECMO. Further research is necessary to elucidate the significance of VA-ECMO in patients with major pulmonary emboli. PERT is a multidisciplinary, fast-response team which can assist in selecting the best treatment option for these patients and facilitate communication between various specialists for individualized care.
| 1. | Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 449] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 2. | Essien EO, Rali P, Mathai SC. Pulmonary Embolism. Med Clin North Am. 2019;103:549-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 3. | Browse NL, James DC. Streptokinase and Pulmonary Embolism. Lancet. 1964;2:1039-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Goldhaber SZ, Kessler CM, Heit J, Markis J, Sharma GV, Dawley D, Nagel JS, Meyerovitz M, Kim D, Vaughan DE. Randomised controlled trial of recombinant tissue plasminogen activator versus urokinase in the treatment of acute pulmonary embolism. Lancet. 1988;2:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 235] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Stambaugh RL, Alexander MR. Therapeutic use of thrombolytic agents. Am J Hosp Pharm. 1981;38:817-824. [PubMed] |
| 6. | Stewart LK, Kline JA. Fibrinolytics for the treatment of pulmonary embolism. Transl Res. 2020;225:82-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Chatterjee S, Chakraborty A, Weinberg I, Kadakia M, Wilensky RL, Sardar P, Kumbhani DJ, Mukherjee D, Jaff MR, Giri J. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA. 2014;311:2414-2421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 588] [Article Influence: 49.0] [Reference Citation Analysis (1)] |
| 8. | Gao GY, Yang P, Liu M, Ding M, Liu GH, Tong YL, Yang CY, Meng FB. Thrombolysis for acute intermediate-risk pulmonary embolism: A meta-analysis. Thromb Res. 2015;136:932-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Tarbox AK, Swaroop M. Pulmonary embolism. Int J Crit Illn Inj Sci. 2013;3:69-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Lee LC, Shah K. Clinical manifestation of pulmonary embolism. Emerg Med Clin North Am. 2001;19:925-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Hamad MM, Bhatia P, Ellidir E, Abdelaziz MM, Connolly V. Diagnostic approach to pulmonary embolism and lessons from a busy acute assessment unit in the UK. Breathe. 2011;7:315-323. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Miniati M, Cenci C, Monti S, Poli D. Clinical presentation of acute pulmonary embolism: survey of 800 cases. PLoS One. 2012;7:e30891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Morrone D, Morrone V. Acute Pulmonary Embolism: Focus on the Clinical Picture. Korean Circ J. 2018;48:365-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jiménez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Ní Áinle F, Prandoni P, Pruszczyk P, Righini M, Torbicki A, Van Belle E, Zamorano JL; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 2950] [Article Influence: 590.0] [Reference Citation Analysis (1)] |
| 15. | Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033-3069, 3069a. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1883] [Cited by in RCA: 1918] [Article Influence: 159.8] [Reference Citation Analysis (1)] |
| 16. | Jiménez D, de Miguel-Díez J, Guijarro R, Trujillo-Santos J, Otero R, Barba R, Muriel A, Meyer G, Yusen RD, Monreal M; RIETE Investigators. Trends in the Management and Outcomes of Acute Pulmonary Embolism: Analysis From the RIETE Registry. J Am Coll Cardiol. 2016;67:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 266] [Article Influence: 26.6] [Reference Citation Analysis (1)] |
| 17. | Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, Jenkins JS, Kline JA, Michaels AD, Thistlethwaite P, Vedantham S, White RJ, Zierler BK; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1460] [Cited by in RCA: 1608] [Article Influence: 107.2] [Reference Citation Analysis (0)] |
| 18. | Jiménez D, Kopecna D, Tapson V, Briese B, Schreiber D, Lobo JL, Monreal M, Aujesky D, Sanchez O, Meyer G, Konstantinides S, Yusen RD, On Behalf Of The Protect Investigators. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2014;189:718-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 19. | Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, Crowther M, Kahn SR. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e419S-e496S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2565] [Cited by in RCA: 2614] [Article Influence: 186.7] [Reference Citation Analysis (0)] |
| 20. | Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JRE, Wells P, Woller SC, Moores L. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3524] [Article Influence: 352.4] [Reference Citation Analysis (2)] |
| 21. | Zuo Z, Yue J, Dong BR, Wu T, Liu GJ, Hao Q. Thrombolytic therapy for pulmonary embolism. Cochrane Database Syst Rev. 2021;4:CD004437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Dieck JA, Ferguson JJ 3rd. Indications for thrombolytic therapy in acute pulmonary embolism. Tex Heart Inst J. 1989;16:19-26. [PubMed] |
| 23. | Kline JA, Courtney DM, Kabrhel C, Moore CL, Smithline HA, Plewa MC, Richman PB, O'Neil BJ, Nordenholz K. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6:772-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 295] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 24. | Freund Y, Cohen-Aubart F, Bloom B. Acute Pulmonary Embolism: A Review. JAMA. 2022;328:1336-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 154] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 25. | Erythropoulou-Kaltsidou A, Alkagiet S, Tziomalos K. New guidelines for the diagnosis and management of pulmonary embolism: Key changes. World J Cardiol. 2020;12:161-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 26. | Righini M, Robert-Ebadi H, Elias A, Sanchez O, Le Moigne E, Schmidt J, Le Gall C, Cornuz J, Aujesky D, Roy PM, Chauleur C, Rutschmann OT, Poletti PA, Le Gal G; CT-PE-Pregnancy Group. Diagnosis of Pulmonary Embolism During Pregnancy: A Multicenter Prospective Management Outcome Study. Ann Intern Med. 2018;169:766-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 27. | Hogg K, Brown G, Dunning J, Wright J, Carley S, Foex B, Mackway-Jones K. Diagnosis of pulmonary embolism with CT pulmonary angiography: a systematic review. Emerg Med J. 2006;23:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Califf RM, Fortin DF, Tenaglia AN, Sane DC. Clinical risks of thrombolytic therapy. Am J Cardiol. 1992;69:12A-20A. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Brown KN, Devarapally SR, Lee LS, Gupta N. Catheter Directed Thrombolysis Of Pulmonary Embolism. 2022 May 5. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. [PubMed] |
| 30. | Piazza G. Advanced Management of Intermediate- and High-Risk Pulmonary Embolism: JACC Focus Seminar. J Am Coll Cardiol. 2020;76:2117-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 31. | Xue X, Sista AK. Catheter-Directed Thrombolysis for Pulmonary Embolism: The State of Practice. Tech Vasc Interv Radiol. 2018;21:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Kucher N, Boekstegers P, Müller OJ, Kupatt C, Beyer-Westendorf J, Heitzer T, Tebbe U, Horstkotte J, Müller R, Blessing E, Greif M, Lange P, Hoffmann RT, Werth S, Barmeyer A, Härtel D, Grünwald H, Empen K, Baumgartner I. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 778] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 33. | Piazza G, Hohlfelder B, Jaff MR, Ouriel K, Engelhardt TC, Sterling KM, Jones NJ, Gurley JC, Bhatheja R, Kennedy RJ, Goswami N, Natarajan K, Rundback J, Sadiq IR, Liu SK, Bhalla N, Raja ML, Weinstock BS, Cynamon J, Elmasri FF, Garcia MJ, Kumar M, Ayerdi J, Soukas P, Kuo W, Liu PY, Goldhaber SZ; SEATTLE II Investigators. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study. JACC Cardiovasc Interv. 2015;8:1382-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 692] [Article Influence: 69.2] [Reference Citation Analysis (1)] |
| 34. | Sista AK. The OPTALYSE PE Trial: Another Step Toward Understanding the Truth About Catheter-Directed Thrombolysis for Submassive Pulmonary Embolism. JACC Cardiovasc Interv. 2018;11:1411-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ding X, China; Maslennikov R, Russia S-Editor: Liu JH L-Editor: A P-Editor: Liu JH