Published online Mar 6, 2023. doi: 10.12998/wjcc.v11.i7.1569
Peer-review started: October 24, 2022
First decision: January 3, 2023
Revised: January 13, 2023
Accepted: February 15, 2023
Article in press: February 15, 2023
Published online: March 6, 2023
Processing time: 129 Days and 2.6 Hours
A large percentage of patients with ectopic pancreas are asymptomatic. When present, the symptoms are typically non-specific. These lesions are predominantly located in the stomach and benign in nature. Synchronous multiple early gastric cancer (SMEGC) (two or more simultaneous malignant lesions with early gastric cancer) is relatively rare and particularly easy to overlook during endoscopic examination. The prognosis of SMEGC is generally poor. We report a rare case of ectopic pancreas with concomitant SMEGC.
A 74-year-old woman presented with paroxysmal upper abdominal pain. On initial investigations, she tested positive for Helicobacter pylori (H. pylori). She underwent esophagogastroduodenoscopy which revealed a 1.5 cm × 2 cm major lesion at the greater curvature and a 1 cm minor lesion at the lesser curvature of the stomach. On endoscopic ultrasound, the major lesion showed hypoechoic changes, uneven internal echoes and unclear boundaries between some areas and the muscularis propria. Endoscopic submucosal dissection was performed to excise the minor lesion. A laparoscopic resection was chosen for the major lesion. On histopathological examination, the major lesion contained high grade intraepithelial neoplasia with a small focus of cancer. A separate underlying ectopic pancreas was found under this lesion. The minor lesion contained high grade intraepithelial neoplasia. In this case, the patient was diagnosed with SMEGC with concomitant ectopic pancreas in the stomach.
Patients with atrophy, H. pylori, and other risk factors should be carefully investigated to avoid missing other lesions including SMEGC and ectopic pancreas.
Core Tip: This case demonstrates that ectopic pancreas can be found in combination with synchronous multiple early gastric cancer (SMEGC). Careful endoscopic inspection of the mucosal surface is mandatory to avoid overlooking SMEGC. It is important to be mindful of the possible presence of ectopic pancreas, perform an in-depth evaluation and select the correct surgical option for excision or resection. This case also reminds us that in the presence of atrophy, intestinal metaplasia and Helicobacter pylori infection combined with ectopic pancreas, it is necessary to evaluate the stomach very carefully, so as not to miss the diagnosis of early gastric cancer.
- Citation: Zhao ZY, Lai YX, Xu P. Gastric ectopic pancreas combined with synchronous multiple early gastric cancer: A rare case report. World J Clin Cases 2023; 11(7): 1569-1575
- URL: https://www.wjgnet.com/2307-8960/full/v11/i7/1569.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i7.1569
Ectopic pancreas is usually asymptomatic, is situated in the stomach, and is usually benign. Inflammation, ulceration, bleeding, luminal obstruction, and, very rarely, malignant transformation are all complications related to ectopic pancreas. There are some papers of concomitant early gastric cancer (EGC) and ectopic pancreas. Murabayashi et al[1] reported the first case of EGC located just above an ectopic pancreas. Similarly, Yoon et al[2] reported an EGC combined with underlying ectopic pancreas. The majority of reports in the literature describe ectopic pancreas with gastric carcinoma. Ectopic pancreas combined with synchronous multiple EGC (SMEGC) has rarely been reported.
SMEGC accounts for 3%-15% of all gastric cancer cases. It is defined as two or more simultaneous, malignant lesions associated with EGC detected during postoperative pathological examination[3]. Overall, the prognosis is poor. Importantly, it is particularly easy to overlook during preoperative endoscopic examination. Risk factors include severe mucosal atrophy, intestinal metaplasia and Helicobacter pylori (H. pylori) infection.
SMEGC combined with ectopic pancreas is rare. Here, we report the case of SMEGC combined with an underlying ectopic pancreas in a female in her 70’s. In our case, two EGC lesions were found in the patient’s stomach. The major lesion was located just above an ectopic pancreas. Synchronously, another lesion, an EGC, was found in the gastric body.
Paroxysmal upper abdominal pain for 1 mo.
A 74-year-old female complaining of paroxysmal upper abdominal pain for 1 mo was admitted to our hospital for investigation.
She had a history of hypertension for more than 10 years and was treated with one pill of irbesartan 1 d.
She denied a history of abdominal disease and/or surgery and had no family history of cancer.
Her admission vital signs were normal. No abnormality was found in the physical examination.
Serum laboratory results and tumor markers were within normal limits. Her urea breath test was positive for H. pylori.
Computed tomography did not demonstrate a tumor or lymphadenopathy.
SMEGC combined with ectopic pancreas.
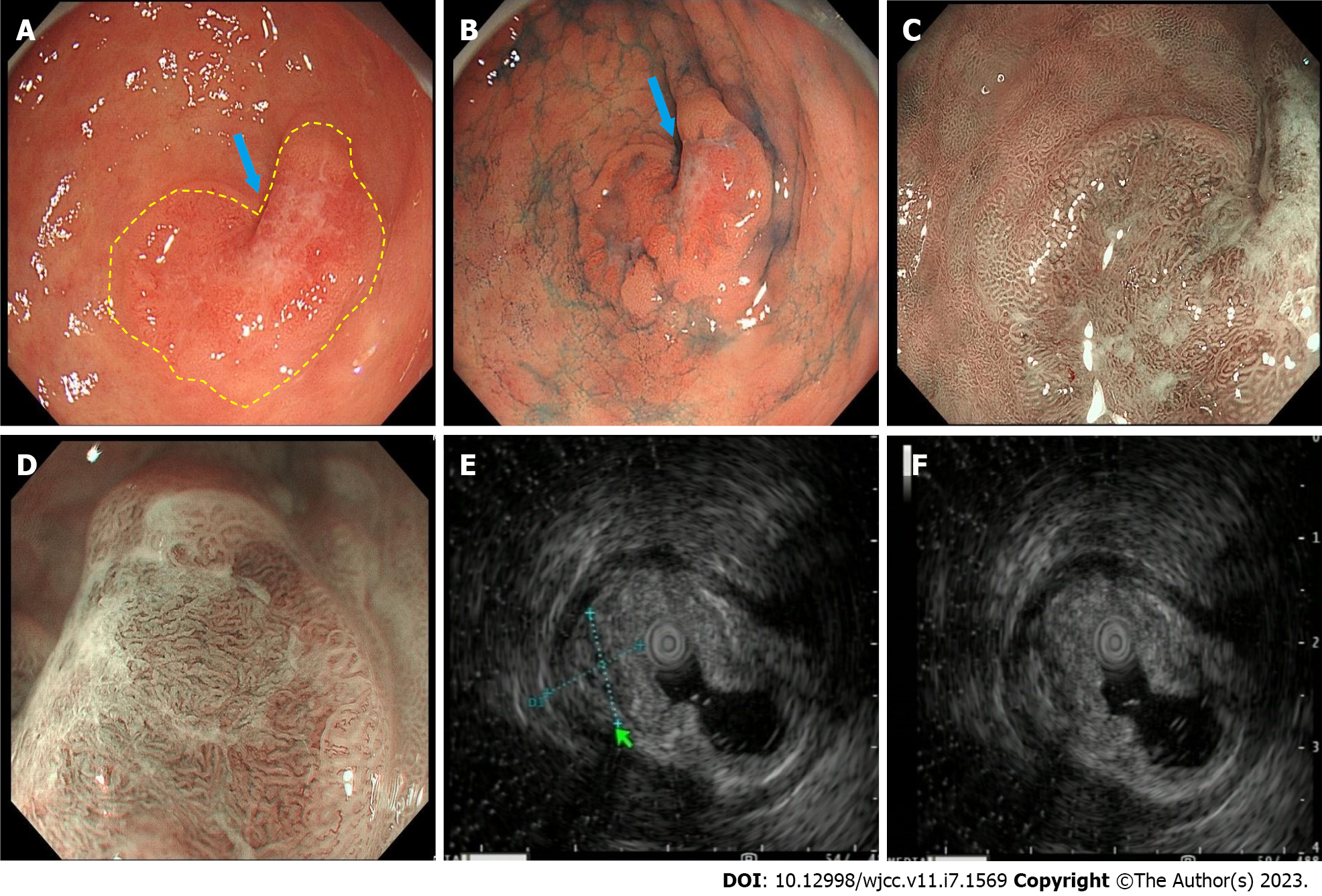
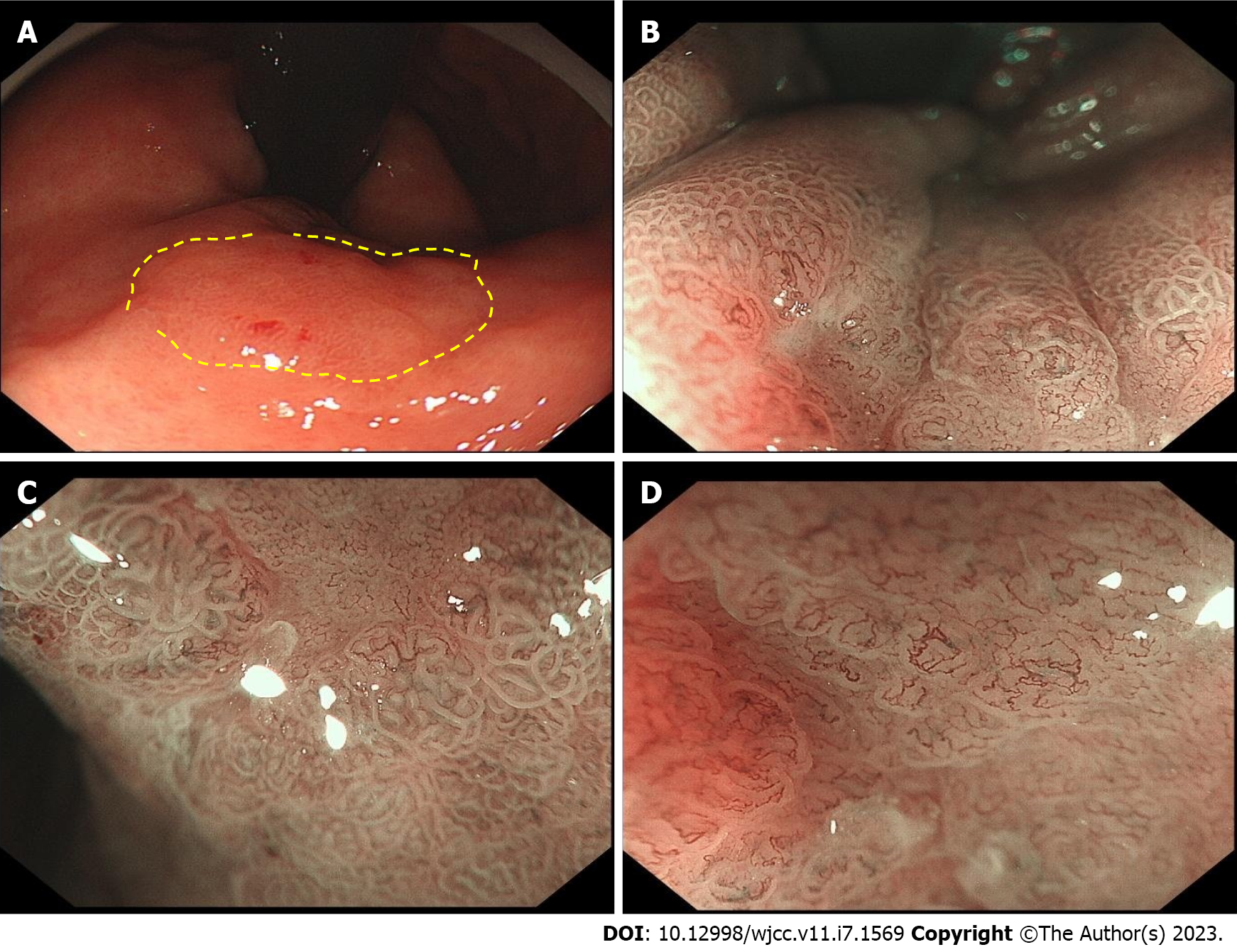
She underwent esophagogastroduodenoscopy, which revealed two lesions. The larger curvature of the stomach antrum was where the major lesion was found (Figure 1). It had a raised erythematous surface with heart-shaped ulceration. The smaller curvature of the upper stomach body was where the minor lesion was found (Figure 2). Its surface was also erythematous. There was a slight depression in its center. The major and minor lesions measured 1.5 cm × 2 cm and 1.0 cm respectively. Narrow band imaging (NBI) combined with magnifying endoscopy (ME) was utilized to further classify the lesions and suggested that they were all type IIc (superficial depressive type per the Japanese Classification of Gastric Cancer classification system). ME-NBI exhibited uneven microvascular and micro surface patterns with a demarcation line of the stomach antrum. On endoscopic ultrasonography, the major lesion showed hypoechoic changes, uneven internal echoes and unclear boundaries between some areas and the muscularis propria. Biopsies were taken of both lesions. Histopathological examination revealed that the major lesion contained high grade intraepithelial neoplasia and the minor lesion contained low grade intraepithelial neoplasia. Initially, the patient was going to be treated exclusively with endoscopic submucosal dissection (ESD); however, a laparoscopic resection was chosen for the major lesion (Billroth II combining Braun), Because of the liquid is injected into the submucosa, there is no upward movement of the muscle layer in the lesion; while ESD was performed on the minor lesion (Figure 3).
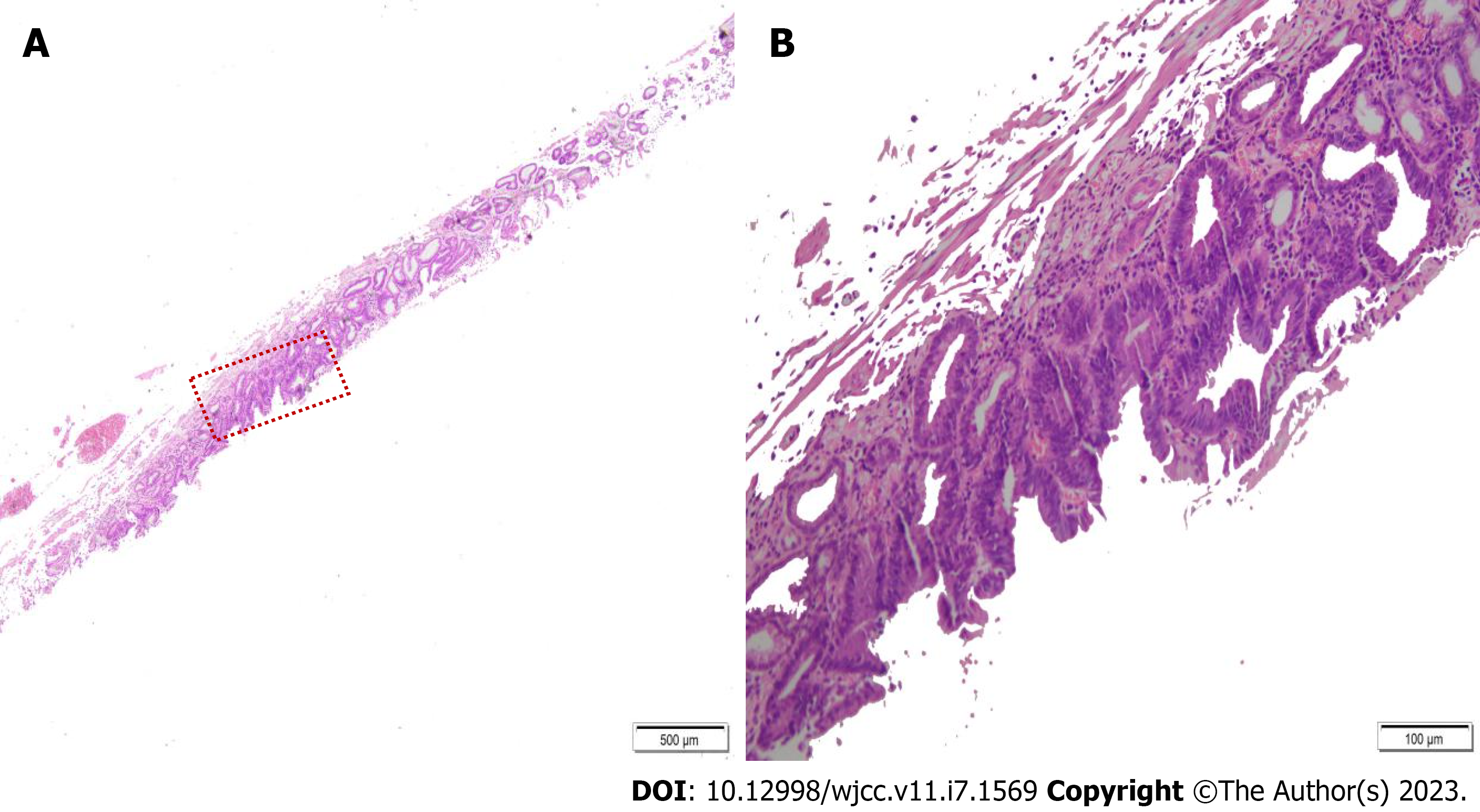
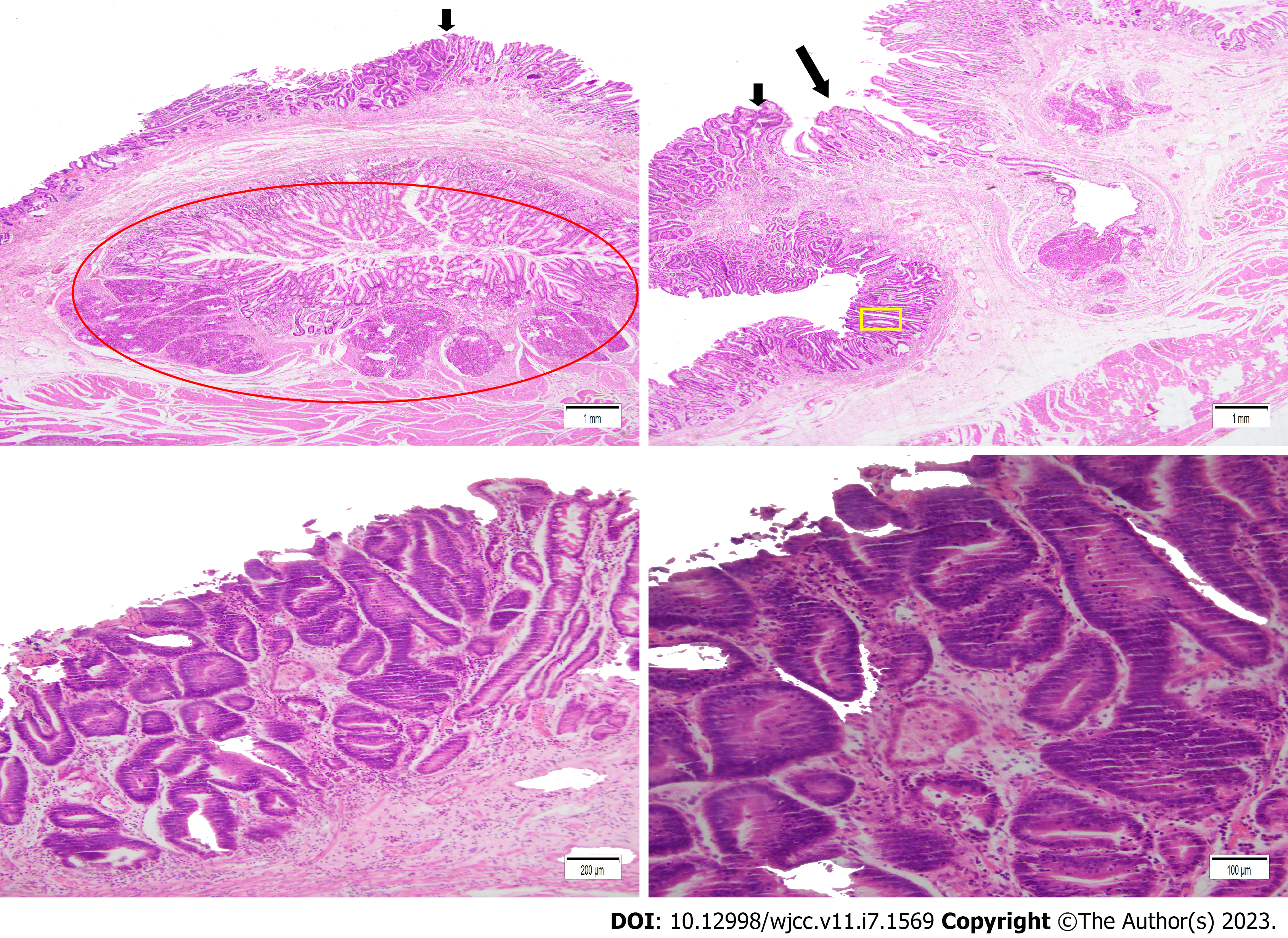
On histopathological examination of the resected specimens, the gastric antrum lesion contained high grade intraepithelial neoplasia with a small focus of cancer. The lesion was limited to the mucosa. A separate underlying ectopic pancreas was found under this lesion (Figure 4). The minor lesion specimen showed high grade intraepithelial neoplasia. The patient improved and discharged.
EGC is defined as gastric cancer that is confined to mucosa or submucosa, without considering lymph node metastasis. The prognosis for stomach cancer is generally poor; however, early detection increases the 5-year disease survival rate (99.3% for mucosal cancer and 97.2% for submucosal cancer)[4]. According to Moertel et al’s criteria, SMEGC is defined as multiple EGC lesions[5]. SMEGC contributes for 3%-15% of all incidences of gastric cancer. As a result, it's critical to recognize other potential lesions while making an endoscopic diagnosis of EGC. These individuals may miss the chance for early therapy and their early cancer might progress if synchronous cancer lesions are disregarded. The detection rate of SMEGC has greatly risen recently as a result of continual improvements in diagnostic endoscopy, pathologic examination, and therapeutic approaches; nonetheless, SMEGC is still particularly simple to miss during endoscopic inspection.
In previous studies, advanced age, male sex, well-differentiated type, raised morphology, smaller tumor size and less depth of invasion have been associated with SMEGC[6]. The “field carcinogenesis” hypothesis suggests that a chronic carcinogenic background may result in two or more lesions with similar clinicopathological characteristics[7,8]; however, little is known about the pathogenesis of the development of multiple gastric cancers. In our case, two lesions with early cancer were simultaneously detected. Advanced age, H. pylori infection, severe mucosal atrophy and intestinal metaplasia were risk factors for gastric cancer. Our case had the very rare finding of a major lesion located above the ectopic pancreas.
Ectopic pancreas is a birth defect in which pancreatic tissue splits from the primary gland and survives without vascular or ductal integrity. There are four distinct varieties of ectopic pancreas according to histology: type I (full ectopic), which is composed of all three types of pancreatic cells (acini, ducts, and islet cells); type II (partial ectopic); and type III (partial ectopic). Type II, canalicular ectopic, just ductal components; The only cells present in Type III (exocrine ectopic) are acinar cells, while Type IV (endocrine ectopic) are islet cells[9]. Ectopic pancreas can occur anywhere in the gastrointestinal tract; however, in 20%-40% of cases, the ectopic pancreas is found in the stomach[10]. Most patients are asymptomatic. When present, the main clinical manifestations are abdominal pain, abdominal distention and gastrointestinal bleeding.
Although its radiological and endoscopic characteristics can be helpful, determining a preoperative diagnosis of ectopic pancreas is difficult. Therefore, pathological examination is the gold standard for the diagnosis. Ectopic pancreas functions are similar to a normal pancreas. Hence, it is easily affected by the diseases, such as pseudocyst development and pancreatitis. Possible complications caused by mass effect These include Ulcer, bleeding, obstruction and intussusception[2]. Malignant transformation of ectopic pancreas is rare. The three criteria listed below must be satisfied for the diagnosis of a cancer originating from an ectopic pancreas: A direct transition between the pancreatic structures and the carcinoma must be seen, the carcinoma must be within or close to the heterotopic pancreatic tissue, and the non-neoplastic pancreatic tissue must have at least completely developed acini and ductal structures[11].
In our case, ectopic pancreas was composed of ductal components and acinar cells. There were no dysplastic or malignant changes in the ectopic pancreas. Our case was characterized by a SMEGC combined with an underlying ectopic pancreas. Some literature reports that ectopic pancreas may secrete pancreatic juice or enzymes which may cause damage to the gastrointestinal mucosa and cause abdominal pain, abdominal distension and even gastrointestinal bleeding[12]. It is also reported that a small number of ectopic pancreas secrete mucus, which is discharged through the duct and forms a foveal like change. The characteristic manifestation of “central foveal sign” may appear[13]. Some case reports demonstrate the existence of ectopic pancreas combined with EGC; however, there are few reports of ectopic pancreas combined with SMEGC[1,2]. There may be a certain correlation between ectopic pancreas and the occurrence of multiple gastric cancers. According to the JCGC classification, the macroscopic type of gastric lesions are divided into the following: elevated (0-I), superficial elevated (0-IIa), flat (0-IIb), superficial depressive (0-IIc), and depressive types (0-III). The macroscopic type of the lesions in our case were all the flat type (IIc). A depression may be seen at the top of any of these gastric lesions. This is where the opening of the ectopic pancreas may be located. The ectopic pancreas can then secrete mucus through the opening affecting inflammation of the local gastric mucosa and changing the internal environment of the stomach. This may further be associated with the occurrence of multiple gastric cancers. Additionally, it may be that longstanding inflammation or erosion or mucosal protrusion itself promotes gastric carcinogenesis.
In conclusion, we report a rare case of SMEGC combined with ectopic pancreas which may have important clinical implications. It may be that the ectopic pancreas secreted pancreatic enzymes further causing alteration of the gastric environment and promoting SMEGC. Careful endoscopic inspection of the mucosal surface is mandatory to avoid overlooking SMEGC. It is important to be mindful of the possible presence of ectopic pancreas, perform an in-depth evaluation and select the correct surgical option for excision or resection. Finally, this case also reminds us that in the presence of atrophy, intestinal metaplasia and H. pylori infection combined with ectopic pancreas, it is necessary to evaluate the stomach very carefully, so as not to miss the diagnosis of EGC.
| 1. | Murabayashi T, Kawaguchi S, Okuda N, Oyamada J, Yabana T. Early Gastric Cancer Just above a Heterotopic Pancreas. Case Rep Gastroenterol. 2016;10:308-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Yoon JB, Lee BE, Kim DH, Park DY, Jeon HK, Baek DH, Kim GH, Song GA. A Rare Case of Early Gastric Cancer Combined with Underlying Heterotopic Pancreas. Clin Endosc. 2018;51:192-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Jeong SH, An J, Kwon KA, Lee WK, Kim KO, Chung JW, Kim YJ, Park DK, Kim JH. Predictive risk factors associated with synchronous multiple early gastric cancer. Medicine (Baltimore). 2017;96:e7088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Yoshida N, Doyama H, Yano T, Horimatsu T, Uedo N, Yamamoto Y, Kakushima N, Kanzaki H, Hori S, Yao K, Oda I, Katada C, Yokoi C, Ohata K, Yoshimura K, Ishikawa H, Muto M. Early gastric cancer detection in high-risk patients: a multicentre randomised controlled trial on the effect of second-generation narrow band imaging. Gut. 2021;70:67-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 5. | Moertel CG, Bargen JA, Soule EH. Multiple gastric cancers; review of the literature and study of 42 cases. Gastroenterology. 1957;32:1095-1103. [PubMed] |
| 6. | Zhao B, Mei D, Luo R, Lu H, Bao S, Xu H, Huang B. Clinicopathological features, risk of lymph node metastasis and survival outcome of synchronous multiple early gastric cancer. Clin Res Hepatol Gastroenterol. 2020;44:939-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Kim JH, Jeong SH, Yeo J, Lee WK, Chung DH, Kim KO, Chung JW, Kim YJ, Kwon KA, Park DK. Clinicopathologic Similarities of the Main and Minor Lesions of Synchronous Multiple Early Gastric Cancer. J Korean Med Sci. 2016;31:873-878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Yasuda M, Kuwano H, Watanabe M, Toh Y, Ohno S, Sugimachi K. p53 expression in squamous dysplasia associated with carcinoma of the oesophagus: evidence for field carcinogenesis. Br J Cancer. 2000;83:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Hammock L, Jorda M. Gastric endocrine pancreatic heterotopia. Arch Pathol Lab Med. 2002;126:464-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Hickman DM, Frey CF, Carson JW. Adenocarcinoma arising in gastric heterotopic pancreas. West J Med. 1981;135:57-62. [PubMed] |
| 11. | Guillou L, Nordback P, Gerber C, Schneider RP. Ductal adenocarcinoma arising in a heterotopic pancreas situated in a hiatal hernia. Arch Pathol Lab Med. 1994;118:568-571. [PubMed] |
| 12. | Iwahashi S, Nishi M, Yoshimoto T, Kashihara H, Takasu C, Tokunaga T, Miyatani T, Higashijima J, Yoshikawa K, Wada Y, Bando Y, Shimada M. A case of gastric heterotopic pancreas with gastroduodenal invagination. Surg Case Rep. 2019;5:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Liu TZ, Peng ZP, Huang LS, Zhang WC, Tang JH. [Imaging diagnosis of suspected heterotopic pancreatic lesions in the gastrointestinal tract by multi-slice spiral CT]. Zhongguo Yixue Wulixue ZaZhi. 2020;37:317-321. [DOI] [Full Text] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: García-Compeán D, Mexico; Shen TC, Taiwan S-Editor: Chang KL L-Editor: A P-Editor: Chang KL