Published online Mar 6, 2023. doi: 10.12998/wjcc.v11.i7.1528
Peer-review started: October 10, 2022
First decision: November 18, 2022
Revised: December 17, 2022
Accepted: February 10, 2023
Article in press: February 10, 2023
Published online: March 6, 2023
Processing time: 143 Days and 0.9 Hours
Hospitalized and severely ill coronavirus disease 2019 (COVID-19) patients necessitate prophylactic or therapeutic anticoagulation to minimize the risk of thrombosis at different sites. Life-threatening bleeding complications include spontaneous iliopsoas hematoma, peritoneal bleeding, and extra-abdominal manifestations such as intracranial hemorrhage.
Bleeding in the abdominal wall results in less severe complications than seen with iliopsoas hematoma or peritoneal bleeding. In our case series of 9 patients, we present retroperitoneal and abdominal bleeding complications following anticoagulation in hospitalized COVID-19 patients with severe acute respiratory syndrome coronavirus 2 pneumonia. Contrast-enhanced computed tomography (CE-CT) is the best imaging modality for assessing hematoma secondary to anticoagulation and determines the therapeutic approach, whether interventional, surgical, or conservative management.
We present the role of CE-CT for rapid and precise localization of the bleeding site and prognostic counseling. Finally, we provide a brief review of the literature.
Core Tip: Hospitalized patients with coronavirus disease 2019 (COVID-19) possess a higher risk for both hypercoagulable state and thrombotic complications due to an imbalance in platelet production and destruction and coagulation system disorders that accompany the pathophysiology of this disease. In line with this, the indication of performing contrast-enhanced multidetector computed tomography may help to identify active hemorrhage as a complication of anticoagulant therapy in hospitalized patients with COVID-19 pneumonia. In addition, pre-contrast scans may define the size and the precise location of suspected hematoma, especially when ultrasound findings are inconclusive.
- Citation: Evrev D, Sekulovski M, Gulinac M, Dobrev H, Velikova T, Hadjidekov G. Retroperitoneal and abdominal bleeding in anticoagulated COVID-19 hospitalized patients: Case series and brief literature review. World J Clin Cases 2023; 11(7): 1528-1548
- URL: https://www.wjgnet.com/2307-8960/full/v11/i7/1528.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i7.1528
As of the beginning of October 2022, 615 million cases of coronavirus disease 2019 (COVID-19) have been reported globally, with 6.5 million fatalities[1]. Hospitalized patients with COVID-19 possess a higher risk for hypercoagulable state and thrombotic complications. The pathophysiology behind this has been suspected to result from increased proinflammatory cytokines such as interleukin-6 (IL-6), IL-1 beta and tumor necrosis factor alpha[2]. The possible causes for this phenomenon are due to hemostatic changes as a direct effect of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or as a result of cytokine storm that changes (remodels) the onset of systemic inflammatory response syndrome, as seen in other viral diseases[3-5].
Moreover, the prolonged hospital stays with immobilization, corticosteroid use, and dehydration in severely ill patients also contribute to thrombotic events at different sites. Therefore, according to different guidelines[6] and recommendations from accredited institutions and randomized controlled trials, prophylactic or therapeutic anticoagulation is recommended or mandatory in moderate and severely affected patients with COVID-19, respectively[7-11].
Although hypercoagulability and thrombotic events are common, various bleeding complications can occur due to predisposing factors such as the use of anticoagulants and antiplatelets, thrombocytopenia, hyperfibrinolytic state, and consumption of coagulation factors[8,9]. Additional risk factors include age, high body mass index, critically ill status, dialysis, etc[10]. From the pathological and critical care medicine points of view, severe bleeding complications, which can be life-threatening, include spontaneous retroperitoneal hematoma (SRH), intra- or extraperitoneal bleeding, and gastrointestinal or intracranial hemorrhage. These complications are also thought to be related to the effects of SARS-CoV-2 on mucosae[12]. Rectus sheath hematomas (RSHs) are less dramatic, but have potential for major blood loss.
Unfortunately, the mechanisms by which coronavirus causes severe damage to organs and fatal complications are still unclear. According to literature and statistics, there are currently about 1.86 million deaths reported as being caused by complications of coronavirus infection, and the leading cause of death in these cases remains acute lung damage leading to respiratory failure[13]. However, SARS-CoV-2 infection may also be associated with coagulopathy, suggested by findings consistent with infection-induced inflammatory changes in blood vessels. To this end, there are frequently described cases of thromboembolism in COVID-19 patients, with more than half exhibiting deep thrombosis of the lower extremities at autopsy[14-16].
COVID-19 patients are prone to both hemostasis alterations and risk of thrombosis or bleeding, as well as complications associated with anticoagulation. However, retroperitoneal and intraabdominal hemorrhages have also occurred, and bleeding manifestations clearly connected to COVID-19, even in those without disseminated intravascular coagulation, have been reported in several studies[17,18]. Therefore, it is critical to highlight potential problems connected with COVID-19 therapy. Furthermore, based on the research available, we should retain a heightened concern for spontaneous bleeding in COVID-19 patients with nonspecific abdominal discomfort or are taking therapeutic dosages of anticoagulation[19]. As such, it is crucial to have a potent tool to diagnose and manage these clinical situations.
Thrombocytopenia and low fibrinogen, common in patients with COVID-19, appear to be associated with an increased risk of hemorrhage and in-hospital mortality. According to our research and other recently published reports, the most likely major cause of these bleeding complications in patients with severe SARS-CoV-19 infection is cytokine storm syndrome associated with immune disorders[20,21].
In other words, COVID-19 patients are at increased risk of both thrombosis and bleeding due to an imbalance in platelet production and destruction along with other coagulopathies[22-25]. These are possibly affected by the systemic inflammatory response, which causes an imbalance between procoagulant and anticoagulant homeostatic mechanisms. Unfortunately, as of now we have insufficient data on the underlying pathophysiology and do not have any protocol for managing hemostatic alterations in COVID-19 patients.
Recently, some authors have found evidence that the virus can directly infect endothelial cells and promote inflammation. Furthermore, angiotensin converting enzyme -2 receptor is not only expressed by pneumocytes, but also by endothelial cells, activation of which may favor endothelial damage and changes in vascular permeability[17]. Thus, it follows that endothelial damage can be caused either by direct viral infection of the endothelium or an immune-mediated response that ultimately leads to endothelial dysfunction associated with apoptosis and cell death[26]. In addition, endothelial inflammation can cause vasoconstriction with subsequent organ ischemia and a change in homeostatic mechanisms.
Such inflammation of the vascular wall could lead to impaired vascular integrity and the potential development of aneurysms, which can be complicated by rupture. Although the underlying mechanism is unclear, active monitoring and balanced therapy in these patients are highly recommended to manage the risks of thromboembolism and bleeding and help clinicians make a personalized decision in each case. Importantly, retroperitoneal hematoma is not a common radiologically detected consequence of COVID-19, with only a few case studies reported, but may occur in COVID-19 patients on anticoagulation[19,24,27-29]. Additionally, some conditions related to defective hemostasis are challenging to diagnose, and treatment management may be complicated by any comorbidities present.
The indication for performing contrast-enhanced multidetector computer tomography (CE-CT) is concern for active hemorrhage as a complication of anticoagulant therapy in hospitalized patients with COVID-19 pneumonia, as we showed previously[30]. In addition, pre-contrast scans may define the size and precise location of the suspected hematoma, especially when ultrasound (US) findings are inconclusive.
Since hemostatic alterations in COVID-19 patients are common, and the diagnostic process is often challenging, we present 11 cases of abdominal bleeding in COVID-19 patients. The role of imaging in the diagnosis and management of abdominal and retroperitoneal bleeding in COVID-19 on anticoagulation patients is crucial; the present case series and review aims to shed light on these important complications.
We present a series of 11 patients with abdominal bleeding during their hospitalization at University Hospital "Lozenetz" for COVID-19 bilateral pneumonia. These patients had SARS-CoV-2 infection confirmed with real-time reverse transcription polymerase chain reaction testing. All patients provided informed consent, and the study was conducted following the Declaration of Helsinki and the ethics standards of University Hospital "Lozenetz".
In total, 6 of the patients were diagnosed with retroperitoneal hematoma, 4 with RSH (1 bilateral and 2 with extraperitoneal propagation), and 1 with intraperitoneal bleeding. These patients were among 914 COVID-19 patients admitted to our institution from October 1, 2020, to January 1, 2021; the incidence of bleeding in patients hospitalized for COVID-19 pneumonia therefore is 12 cases per 1000 hospitalizations. The male-to-female ratio was 6 to 5, with age ranging between 51 years and 92 years (mean of 74.27 years).
Patient demographic and clinical characteristics, treatments, and outcomes are shown in Tables 1 and 2.
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
| Age in yr | 63 | 51 | 74 | 86 | 80 | 92 |
| Sex | Female | Female | Male | Female | Female | Female |
| Comorbidities | AH, Asthma | AH, renal transplant, CKD, history of abdominal tumor surgery | AH, IHD, Tonsil carcinoma (status post radiation) | AH, COPD, CKD | AH, ischemic stroke | AH |
| Chronic anticoagulant/antiplatelet use | ASA | No | No | ASA | ASA | ASA |
| Clinical presentationat admission | Fever, asthenia, myalgia | Asthenia, dyspnea, cough | Fever, asthenia, loss ofappetite | Fever, asthenia, dyspnea | Fever, asthenia, diarrhea | Fever, asthenia, dyspnea, cough |
| Duration of illness before admission in days | 5 | 20 | 11 | 7 | 7 | 7 |
| SpO2 at admission, % | 92 | 94 | 94 | 78 | 87 | 97 |
| Time from admission to bleeding in days | 9 | 2 | 5 | 10 | 10 | 6 |
| Type of bleed | Intraperitoneal | RSH, Extra-peritoneal | SRH | RSH | SRH | RSH |
| Signs and symptoms of bleed | Abdominal pain | Leg edema, asthenia, low hemoglobin | Abdominal pain | Abdominal pain, hypotension, abdominal guarding | Abdominal Pain (lateral) | Low hemoglobin, abdominal swelling |
| Hemodynamic instability | No | No | No | Yes | No | No |
| Maximum O2 therapy prior to bleed | 15 L, mask | 3-4 L, mask | 9 L, mask | 4 L, mask | 7 L, nasal | OTI |
| Pulmonary changes | Moderate | Severe | Mild | Mild | Moderate | Moderate |
| Length of hospitalization (days) | 20 | 15 | 20 | 11 | 25 | 10 |
| Anticoagulation (prophylactic) | LMWH 4000 IU, BID | No | LMWH 4000 IU, BID | LMWH 4000 IU, BID | No | LMWH 4000 IU, BID |
| Anticoagulation (therapeutic) | No | LMWH 4000 IU, BID | No | No | LMWH 6000 IU, BID | No |
| Antiplatelet therapy | ASA | No | ASA | ASA | No | No |
| Steroids | Methyl-prednisolone | Methyl-prednisolone | Dexa-methasone | Methyl-prednisolone | Methyl-prednisolone | Methyl-prednisolone |
| Bioproduct units (pRBC/FFP) | 2/3 | 4/4 | 3/2 | 2/2 | No | No |
| Surgery | No | Yes | No | No | Yes | No |
| Outcome | Discharged | Discharged | Discharged | Died | Discharged | Died |
| Characteristic | Patient 7 | Patient 8 | Patient 9 | Patient 10 | Patient 11 |
| Age in yr | 86 | 74 | 65 | 72 | 74 |
| Sex | Male | Male | Male | Male | Male |
| Comorbidities | AH, IHD, PE, AF, PM, CKD | AH, IHD, AF | AH | AH, asthma | AH, IHD, diabetes mellitus, AF |
| Chronic anticoagulant/antiplatelet use | Acenocoumarol | Acenocoumarol | No | Rivaroxaban | Rivaroxaban |
| Clinical presentationat admission | Fever, asthenia, loss of appetite | Fever, asthenia | Fever, asthenia | Fever, cough | Fever, asthenia, cough, dyspnea |
| Duration of illness before admission (days) | 4 | 7 | 7 | 12 | 7 |
| SpO2 at admission (%) | 88 | 80 | 75 | 70 | 90 |
| Time from admission to bleeding (days) | 10 | 19 | 12 | 10 | 11 |
| Type of bleed | RSH, extraperitoneal | SRH | SRH | SRH | SRH and RSH |
| Signs and symptoms of bleed | Abdominal, back pain | Abdominal pain, low hemoglobin | Abdominal, back pain | Abdominal pain | Low hemoglobin, abdominal pain, asthenia, swelling (right) |
| Hemodynamic instability | No | Yes | No | Yes | No |
| Maximum O2 therapy prior to bleed | 7 L, mask | CPAP 85% | 15 L, mask | 15 L, mask | 12 L, mask |
| Pulmonary changes | Moderate | Severe | Severe | Moderate | Severe |
| Length of hospitalization (days) | 17 | 31 | 20 | 10 | 18 |
| Anticoagulation (prophylactic) | No | No | No | No | No |
| Anticoagulation therapeutic | LMWH 6000 IU, BID; Acenocumarol | ivHEP, LMWH 6000 IU, BID | LMWH 6000 IU, BID | ivHEP | ivHEP |
| Antiplatelet therapy | No | ASA, clopidogrel | ASA | ASA | ASA |
| Steroids | Methyl-prednisolone | Methyl-prednisolone | Methyl-prednisolone | Methyl-prednisolone | Methyl-prednisolone |
| Bioproduct units (pRBC/FFP) | None | 5/6 | 0/2 | No | 4/2 |
| Surgery | Yes | Yes (x 2) | No | No | Yes |
| Outcome | Discharged | Died | Discharged | Died | Discharged |
All 11 patients had fever, myalgia, and asthenia as main complaints. However, their severity progressed during attempted home treatment, prompting presentation to the hospital. Additionally, 3 patients had dyspnea with productive cough, 2 had a loss of appetite, and another had diarrhea at admission.
The patients differ in terms of the length of time from admission to onset of bleeding, ranging from 2 d to 19 d (mean 9.45 d). The major symptoms were abdominal and back pain, with abdominal pain being predominant in RSH and back and lateral abdominal pain in SRH. Besides the pain, one patient with RSH and extraperitoneal hematoma had leg swelling accompanied by profound asthenia due to blood loss and low hemoglobin levels. Another patient with bilateral RSH was intubated, mechanically ventilated, and sedated when the bleeding occurred. The only symptom in this patient was swelling of the bilateral abdominal rectus muscles.
The period from the onset of symptoms to the time of admission to our hospital ranged between 5 and 20 d (mean 8.4 d). Two of the patients were admitted first to other hospitals, treated there for 7-10 d, then transferred to our institution for additional treatment. All patients had undertaken home treatment, including nonspecific antivirals, vitamins, antipyretics, and antibiotics. In addition, 2 were taking oral steroids, 5 were taking antiplatelet drugs (e.g., acetylsalicylic acid, dipyridamole, nattokinase), 4 were on chronic anticoagulation for either history of atrial fibrillation or pulmonary embolism (2 on acenocoumarol and 2 on rivaroxaban).
On the day of hospitalization, all patients underwent plain chest CT and were diagnosed with bilateral pneumonia, except for 1 patient with unilateral lung involvement. The typical morphological changes (ground-glass opacities, "crazy-paving" pattern, interlobar septal thickening, dilated pulmonary vessels, etc) were present in all patients. Two had more chronic changes on CT (e.g., coarse consolidation of the parenchyma, fibrotic changes), as a more extended period had passed from the onset of symptoms.
Regarding severity, 2 patients had mild involvement of the lung parenchyma, 5 had moderate involvement, and 4 had more severe changes. During the clinical course of the viral disease, oxygen (O2) needs varied. The maximum O2 therapy prior to bleeding ranged from 5-7 L/min by mask or nasal cannula in 4 patients, 9-15 L/min reservoir mask in 5 patients, 1 patient requiring a continuous positive airway pressure mask with 85% O2, and 1 patient requiring mechanical ventilation.
All 11 patients had ongoing arterial hypertension. In addition, 4 patients had ischemic heart disease, 3 had atrial fibrillation and chronic kidney disease, 2 had asthma, and 1 had chronic obstructive pulmonary disease and diabetes.
One of the patients had a history of renal transplant recipient (14 years ago) and had undergone abdominal surgery for a mesenteric tumor. Another patient had acute pulmonary embolism 2 wk before hospitalization, which was managed with local fibrinolysis. This same patient also had implantation of a pacemaker type dual chamber system for trifascicular block (3 years ago), left ventricular dysfunction, and pulmonary arterial hypertension. One patient had undergone surgery for thyroid cancer (1 year ago). Another patient had a recent ischemic stroke (8 mo ago) and recent radiation for treatment of squamous cell carcinoma of the left tonsil. One had a history of tuberculosis, and another had a history of 2 ischemic strokes. One of the patients with ischemic heart disease underwent stent implantation of the right coronary artery after a myocardial infarction (8 years ago). None of the patients had chronic liver disease or a history of major bleeding. This was a particularly important factor to be excluded to prevent confounding by other liver disease or other coagulopathy unrelated to COVID-19.
No patient had a family history of liver disease, bleeding disorder, or other coagulopathy.
On physical examination at admission, varying signs of viral infection and bilateral pneumonia were observed in all patients, except for 1 with only unilateral (left) lung involvement. The measured initial oxygen saturation (SpO2) on room air ranged between 70% and 97%.
At the onset of symptoms that led to bleeding complications being considered, the most common finding was abdominal pain, the location of which depended on the site of bleeding. Four patients with RSH had visible swelling in the region of the affected rectus muscle, which was painful upon palpation. One had abdominal rigidity with guarding. Three of the patients had hemodynamic instability with low blood pressure and tachycardia accompanied by impaired respiratory function.
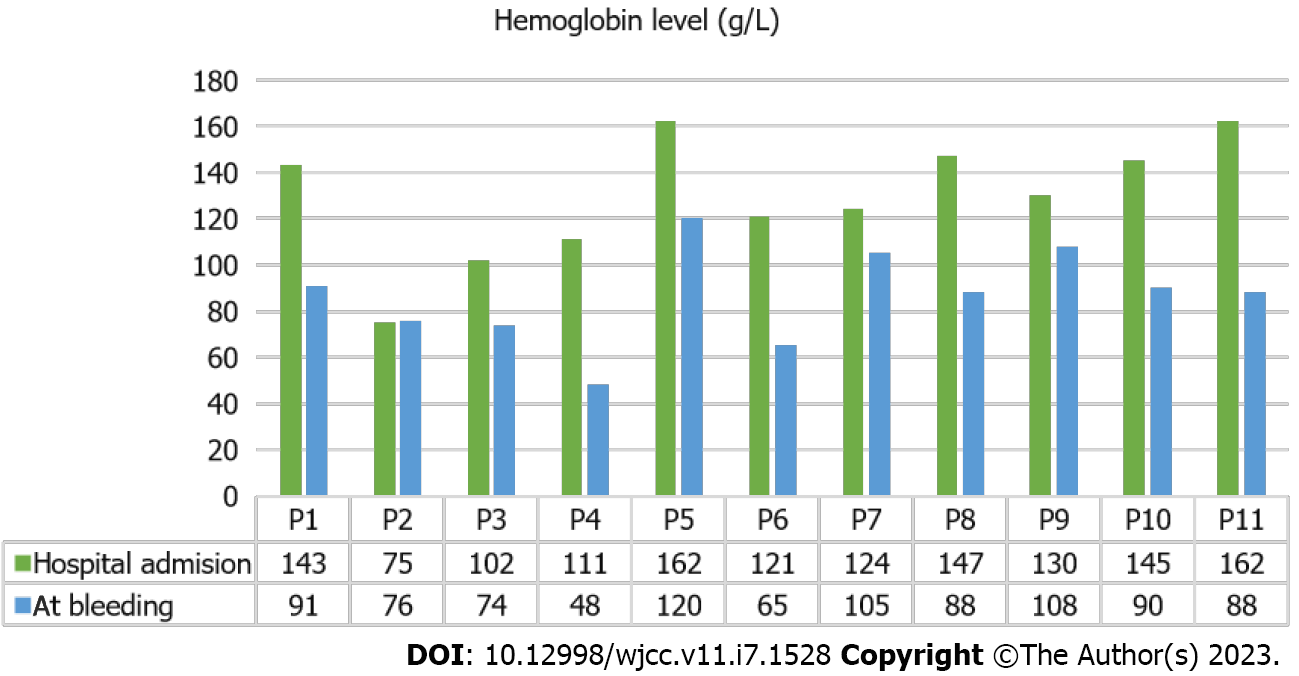
All patients underwent routine laboratory testing. Serum inflammatory and hematological markers found at admission are shown in Table 3. Of note, 10 patients had low hemoglobin (between 11 g/L and 52 g/L) (Figure 1).
| Marker | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | Patient 11 |
| At admission | |||||||||||
| Hgb in g/L | 143 | 75 | 102 | 111 | 162 | 121 | 124 | 147 | 130 | 145 | 162 |
| PLT as 109/L | 197 | 262 | 179 | 254 | 325 | 198 | 354 | 80 | 178 | 131 | 103 |
| Ly as 109/L | 0.74 | 0.83 | 0.58 | 0.49 | 1.1 | 0.66 | 0.75 | 0.47 | 0.64 | 0.21 | 0.57 |
| Cr in μmol/L | 124 | 224 | 53 | 72 | 48 | 55 | 71 | 60 | 72 | 57 | 140 |
| LDH in μ/L | 330 | 451 | 154 | 230 | 502 | 405 | 241 | 491 | 638 | 360 | 760 |
| Ferritin in ng/mL | 238.8 | 535 | 1027 | 198.9 | > 1675.5 | 939 | 1635 | > 1675.5 | > 1675.5 | 632 | > 1675.5 |
| D-dimer in μg/mL | 0.68 | 1.1 | 0.30 | 0.98 | 1.57 | 1.07 | 1.55 | 1.95 | 2.03 | 0.61 | 0.77 |
| CRP in mg/L | 65.6 | 108 | 3.1 | 13 | 20.4 | 57.7 | 7.5 | 6.4 | 113.9 | 1.0 | 72.5 |
| INR | 1.0 | 1.1 | 1.07 | 1.07 | 1.02 | 1.0 | 1.1 | 1.09 | 1.01 | 1.1 | 1.33 |
| At bleeding | |||||||||||
| Hgb in g/L | 91 | 76 | 74 | 48 | 120 | 65 | 105 | 88 | 108 | 90 | 88 |
| PLT as 109/L | 271 | 151 | 206 | 93 | 200 | 279 | 245 | 57 | 222 | 129 | 149 |
| Ly as 109/L | 1.2 | 0.55 | 0.59 | 1.7 | 1.4 | 0.94 | 0.89 | 0.37 | 0.55 | 0.37 | 0.28 |
| Cr in μmol/L | 71 | 251 | 46 | 92 | 48 | 56 | 130 | 43 | 55 | 80 | 96 |
| LDH in μ/L | 335 | 437 | 155 | 335 | 441 | 717 | 311 | 503 | 521 | 719 | 405 |
| Ferritin in ng/mL | 597 | 243 | 889 | 166.3 | 720 | 689 | 971 | > 1675.5 | 906 | 437 | 1292 |
| D-dimer in μg/mL | 5.46 | 1.7 | 0.27 | 2.7 | 2.2 | 1.22 | 2.36 | 1.63 | 2.5 | 0.61 | 0.99 |
| CRP in mg/L | 8.6 | 31 | 2.8 | 179.5 | 80.5 | 160 | 76.3 | 87 | 22.1 | 5.8 | 14.7 |
| INR | 1.0 | 1.0 | 1.05 | 3.5 | 1.04 | 1.0 | 2.5 | 1.1 | 1.06 | 3.6 | 1.51 |
For all patients, multidetector computed tomography (MDCT) images were reviewed for the presence of extravasated contrast material, a CT finding indicative of active hemorrhage. Imaging studies were performed on a Philips Brilliance CT 256-slice scanner, and images and reconstructions were analyzed on an ISP workstation.
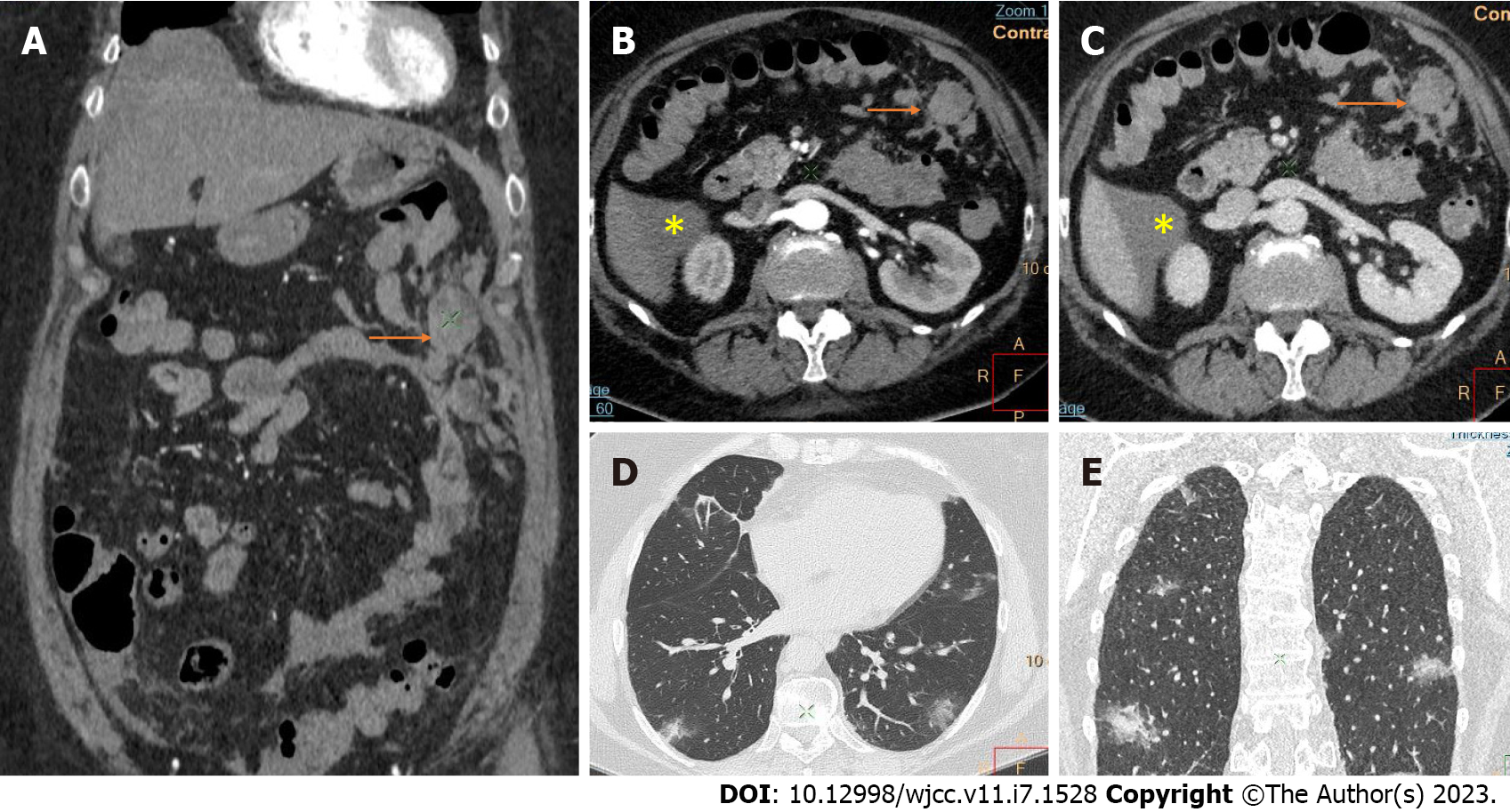
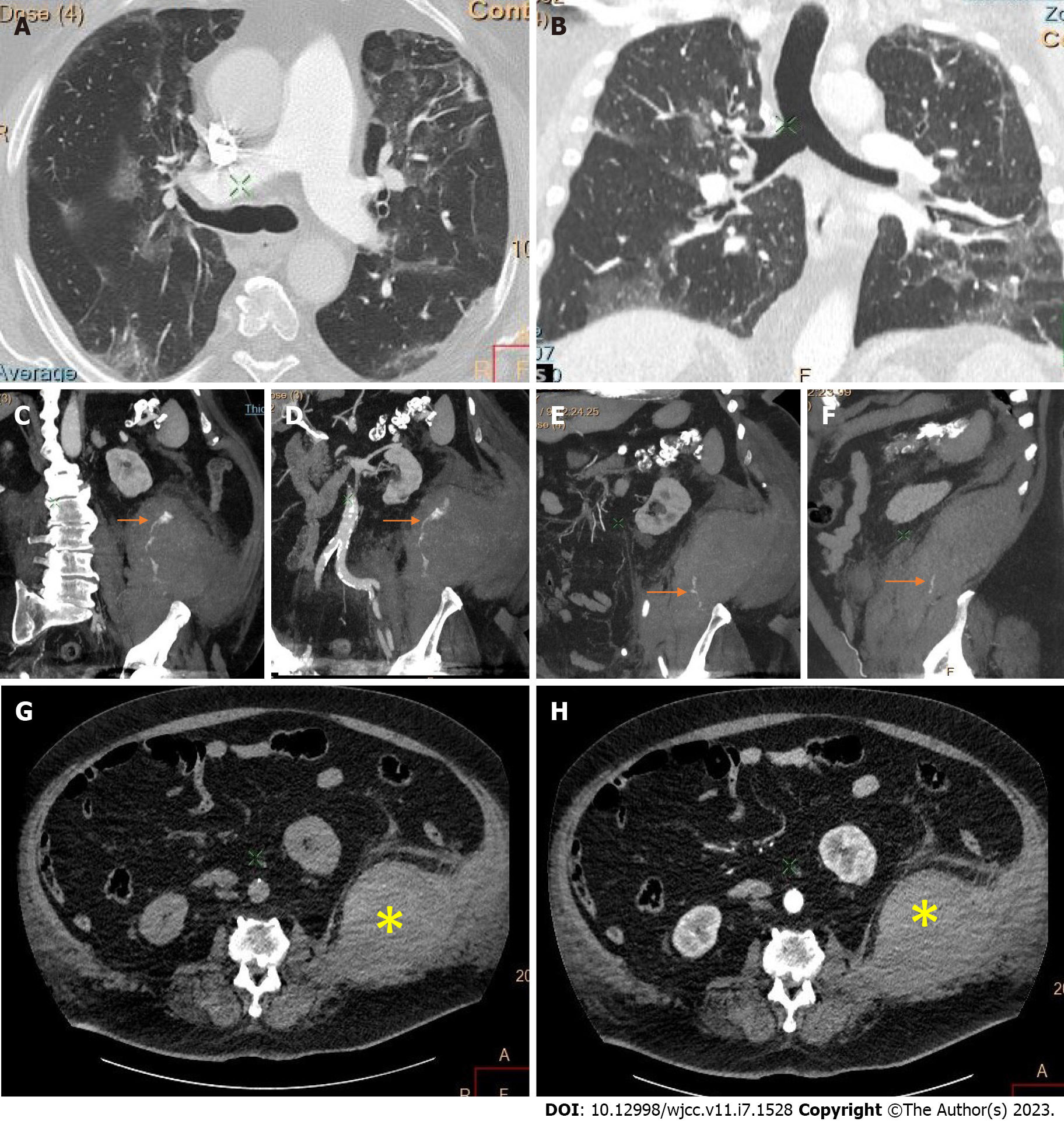
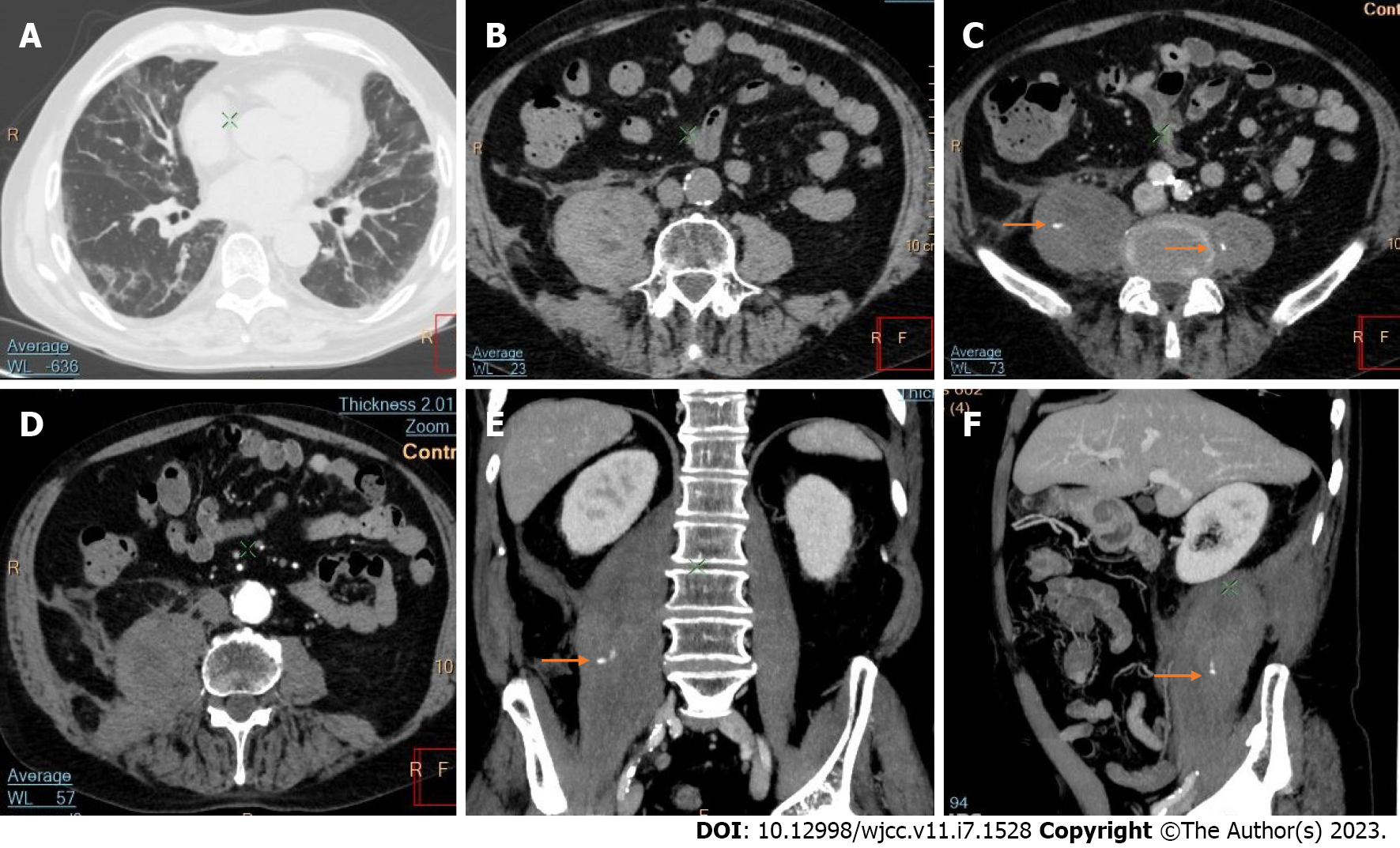
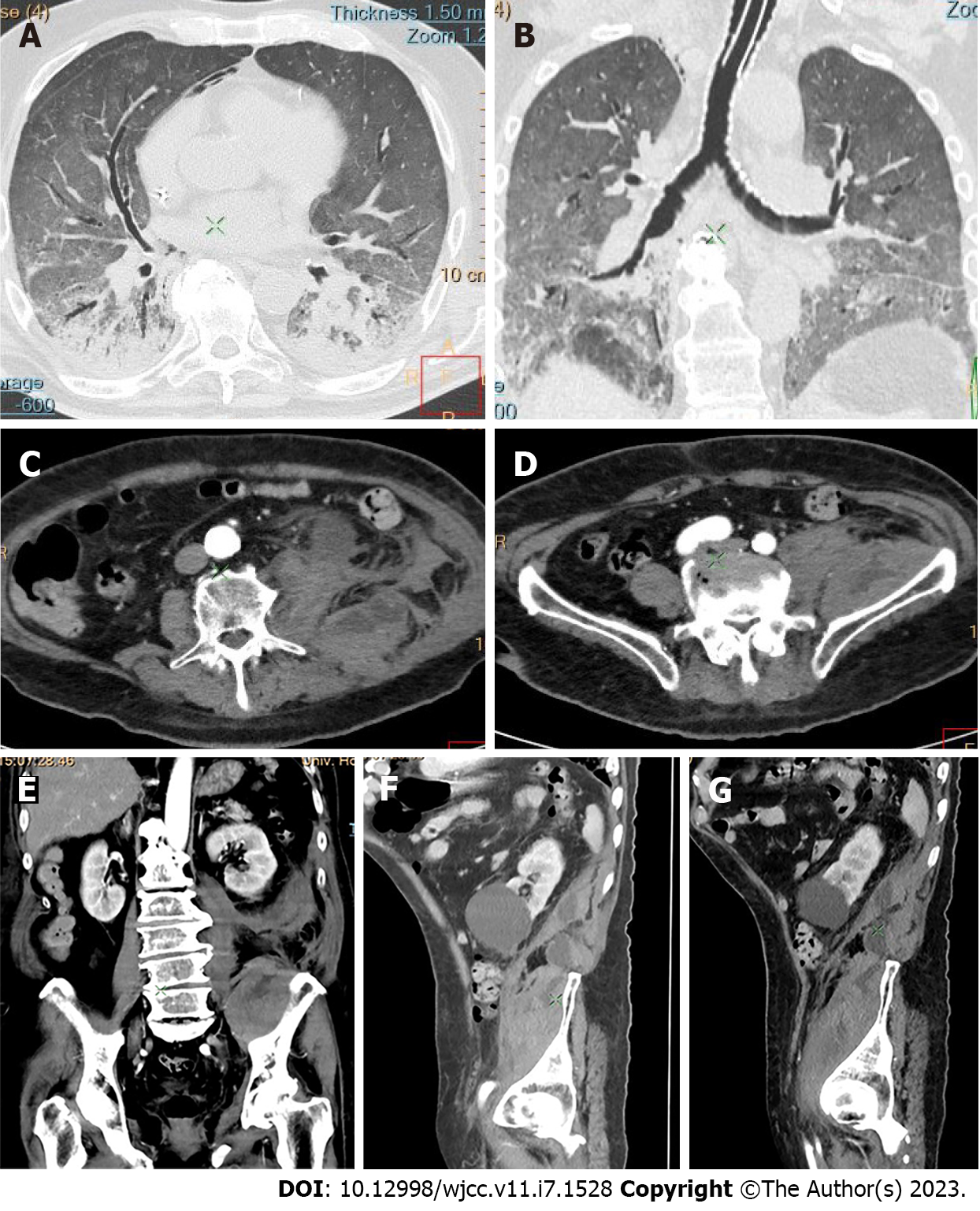
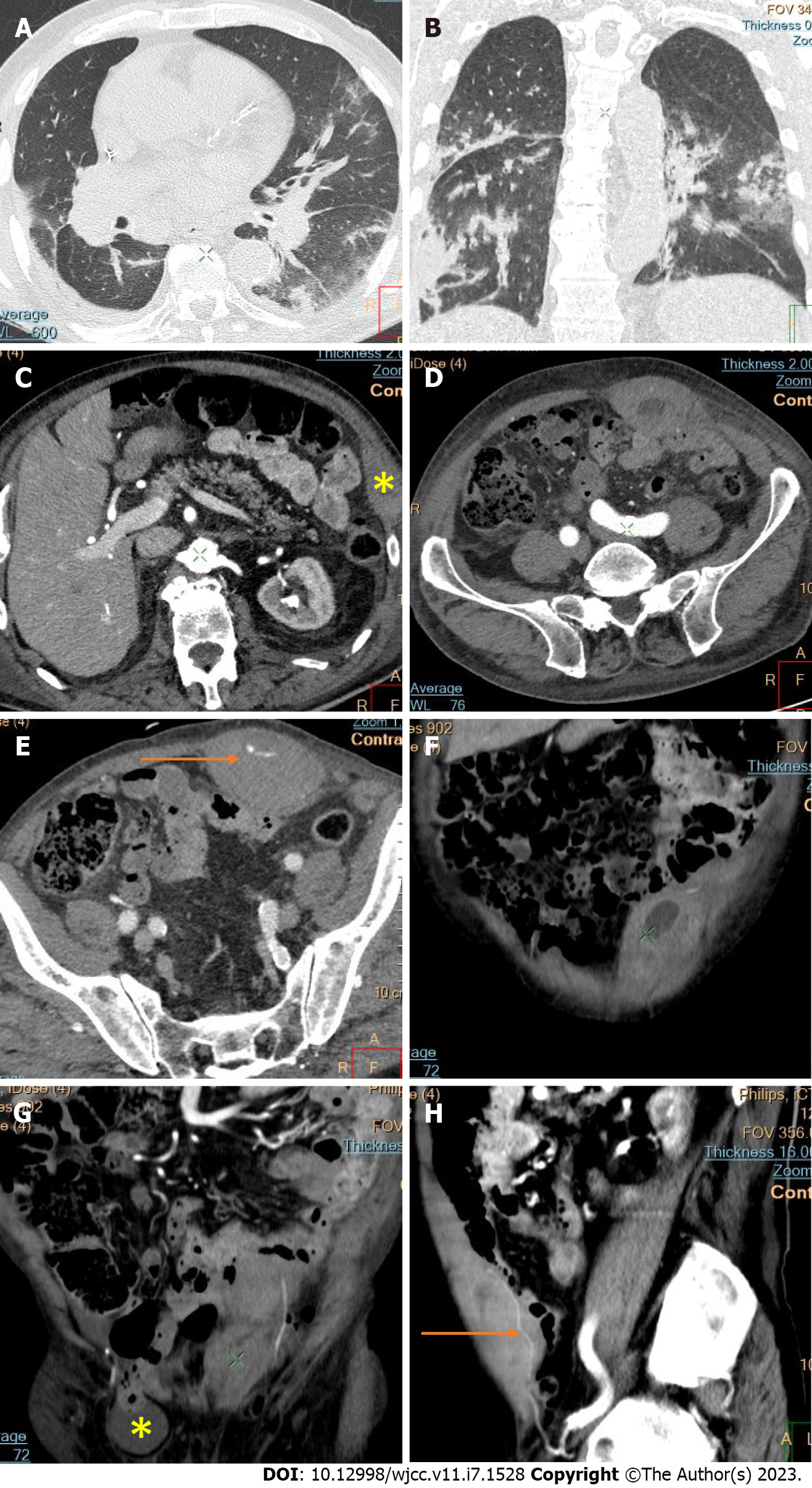
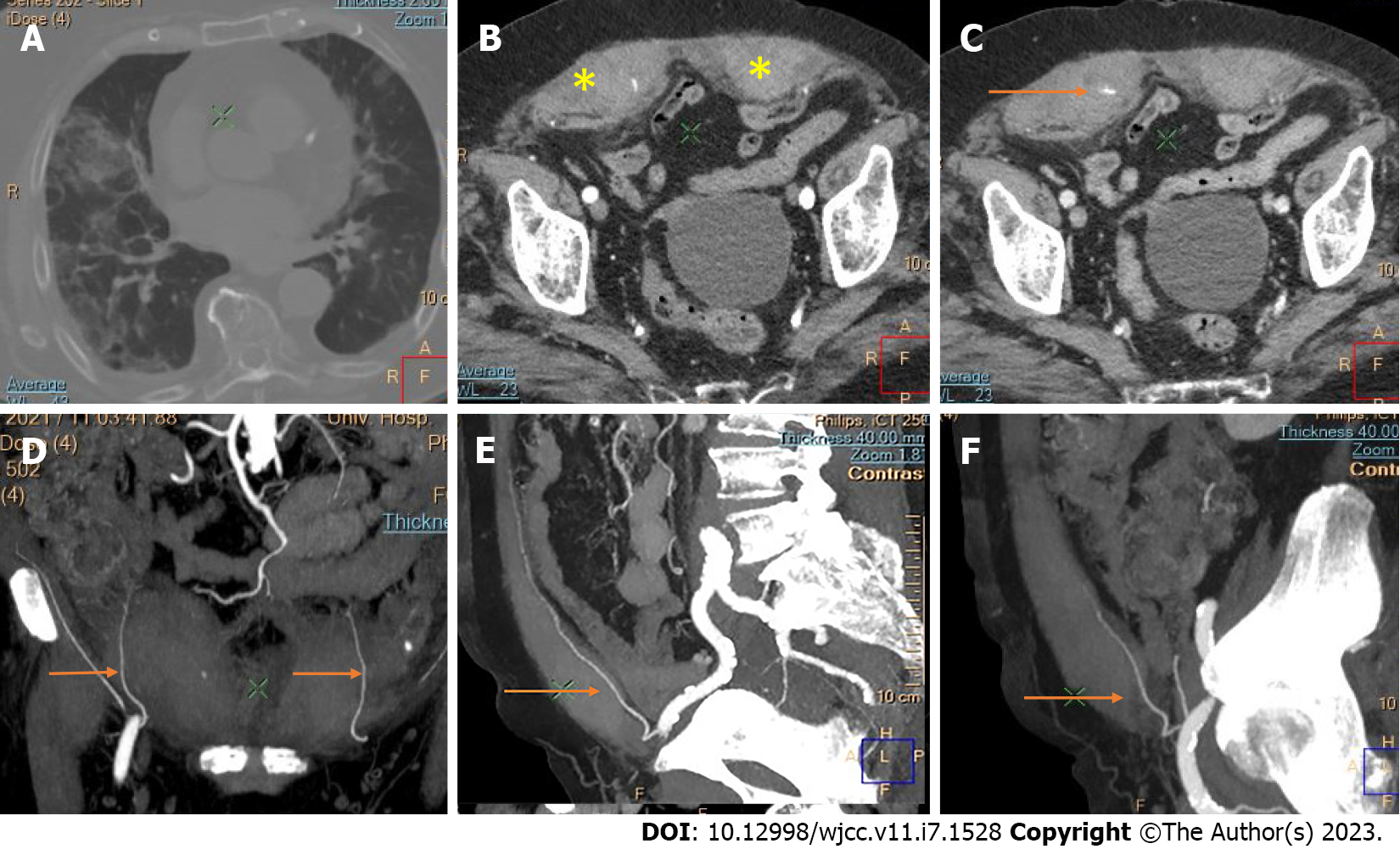
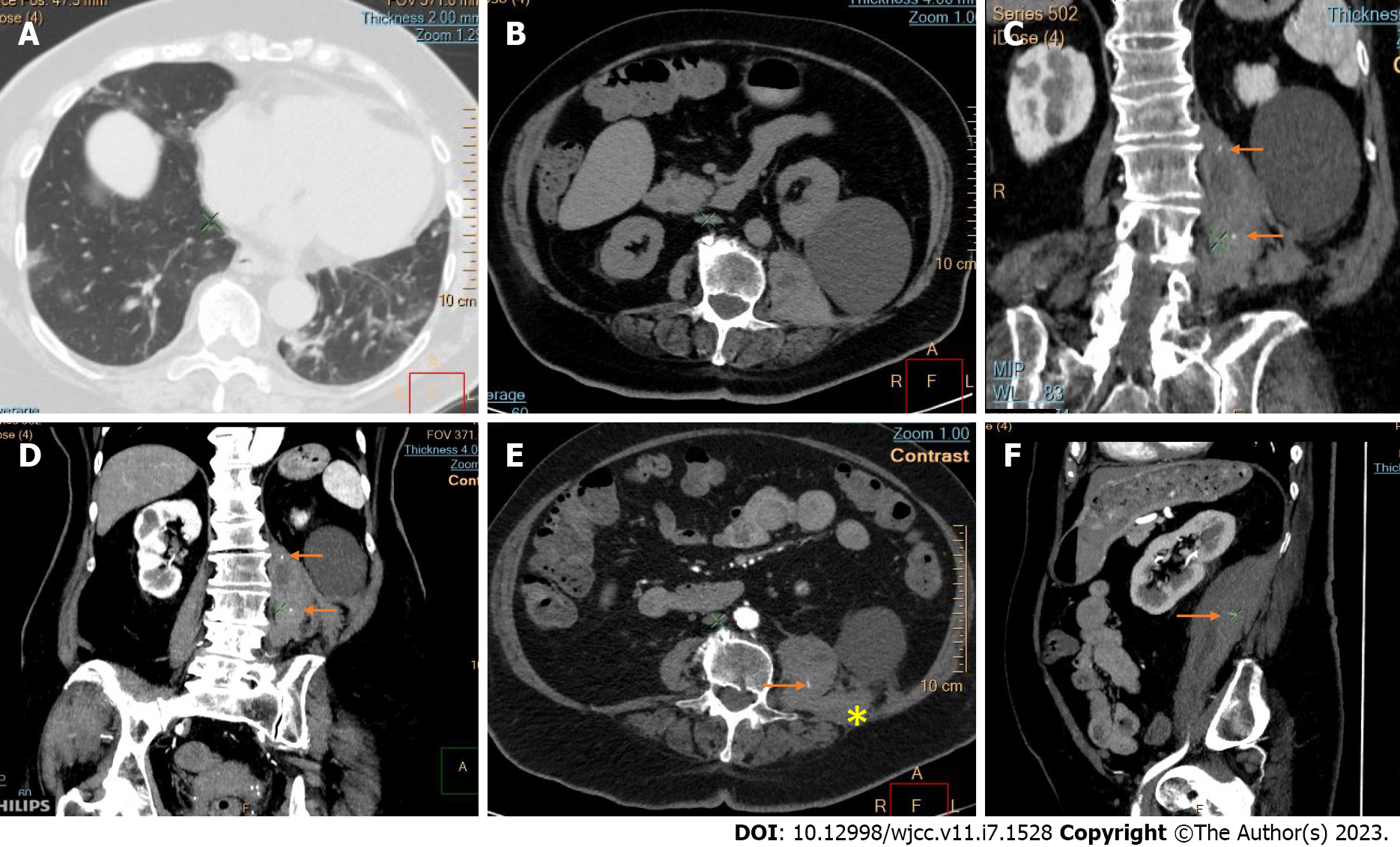
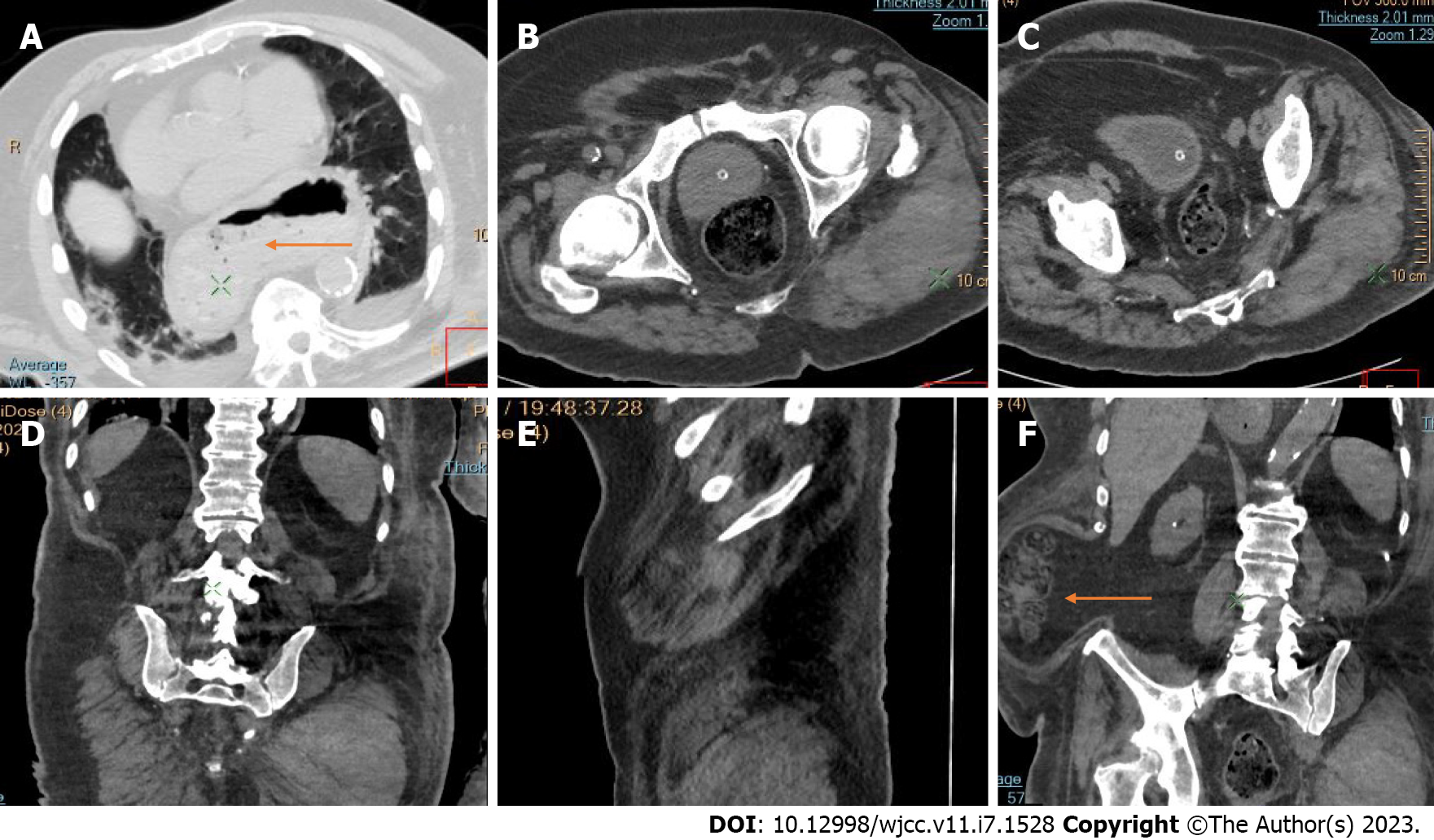
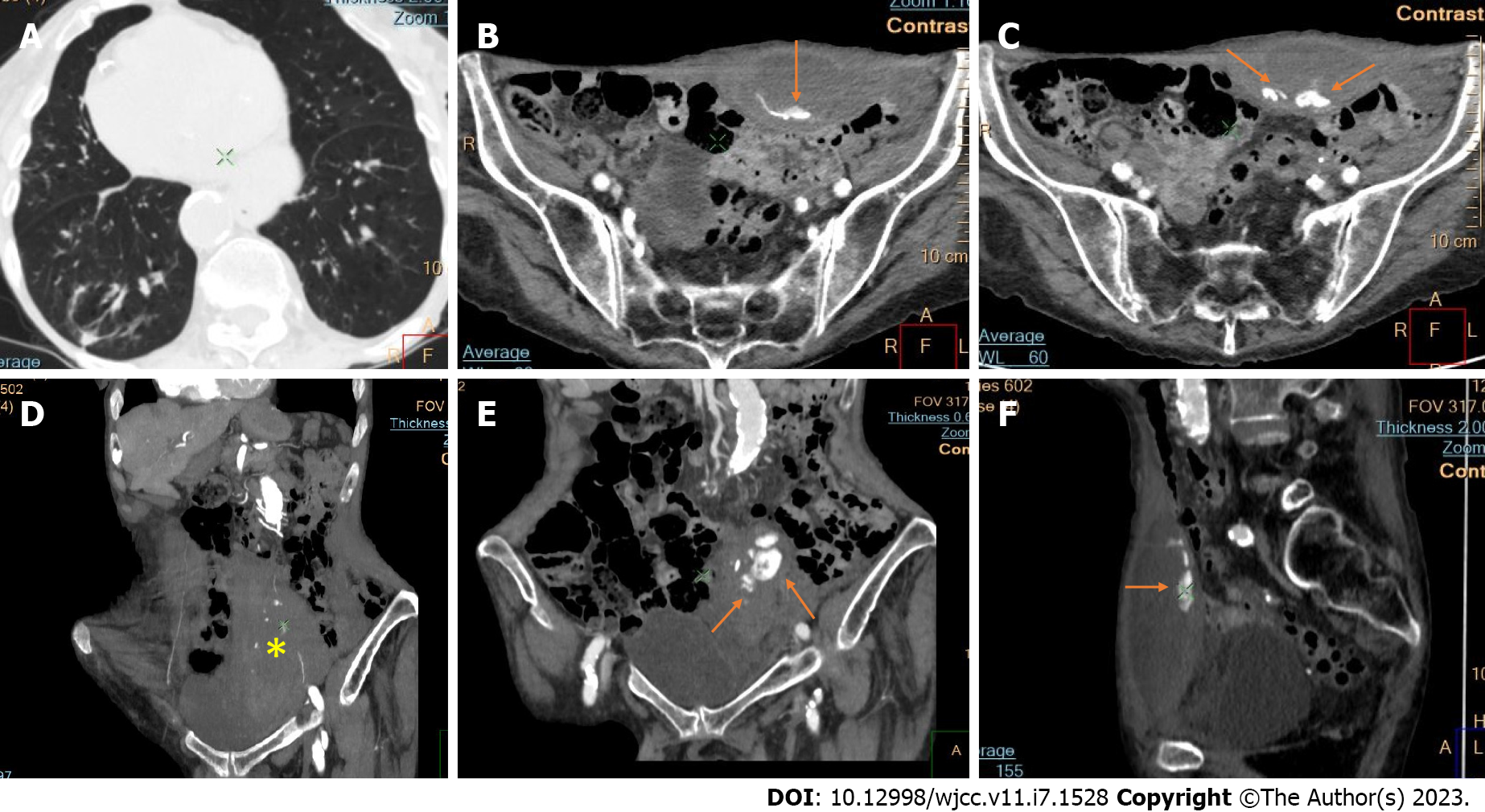
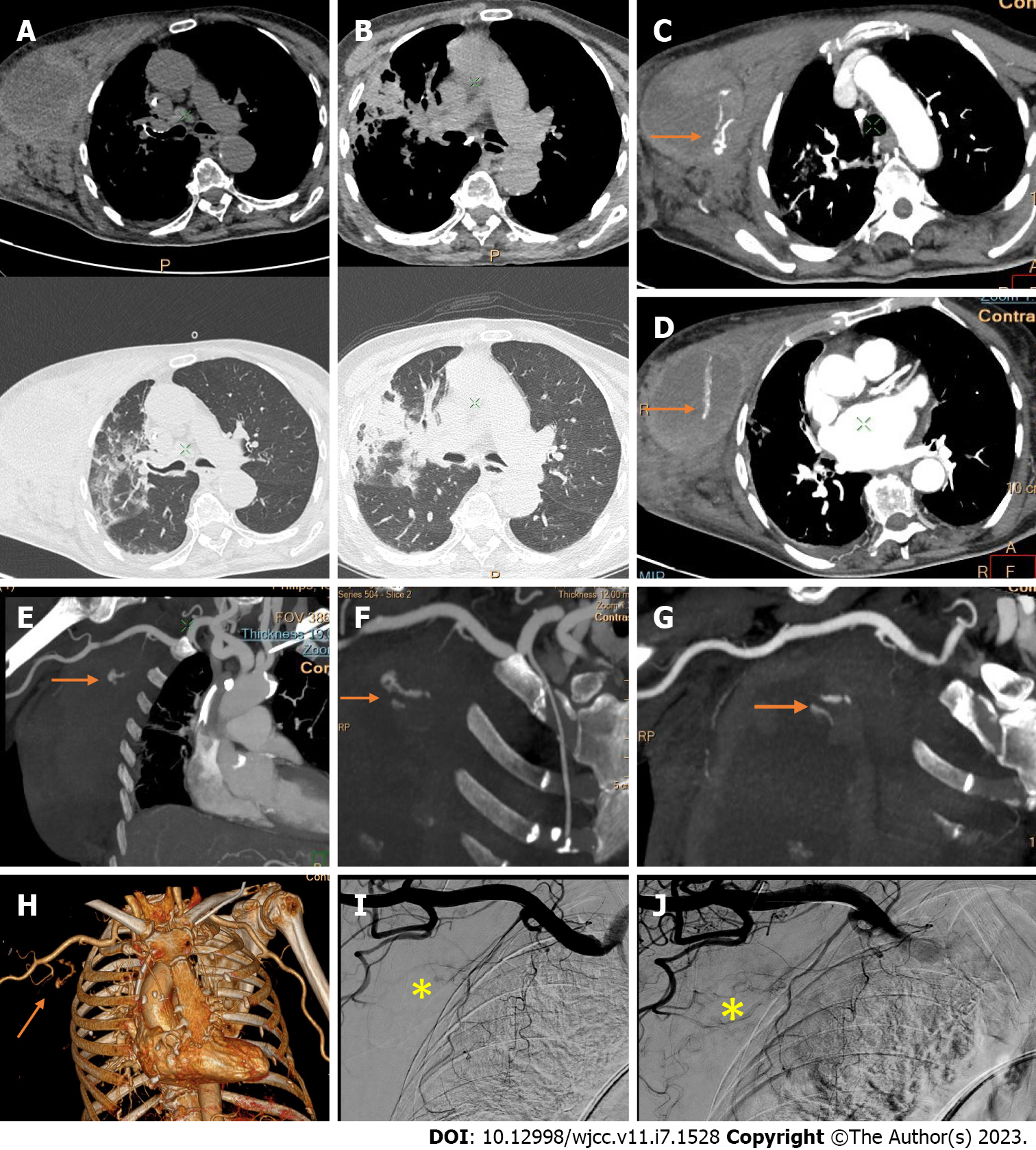
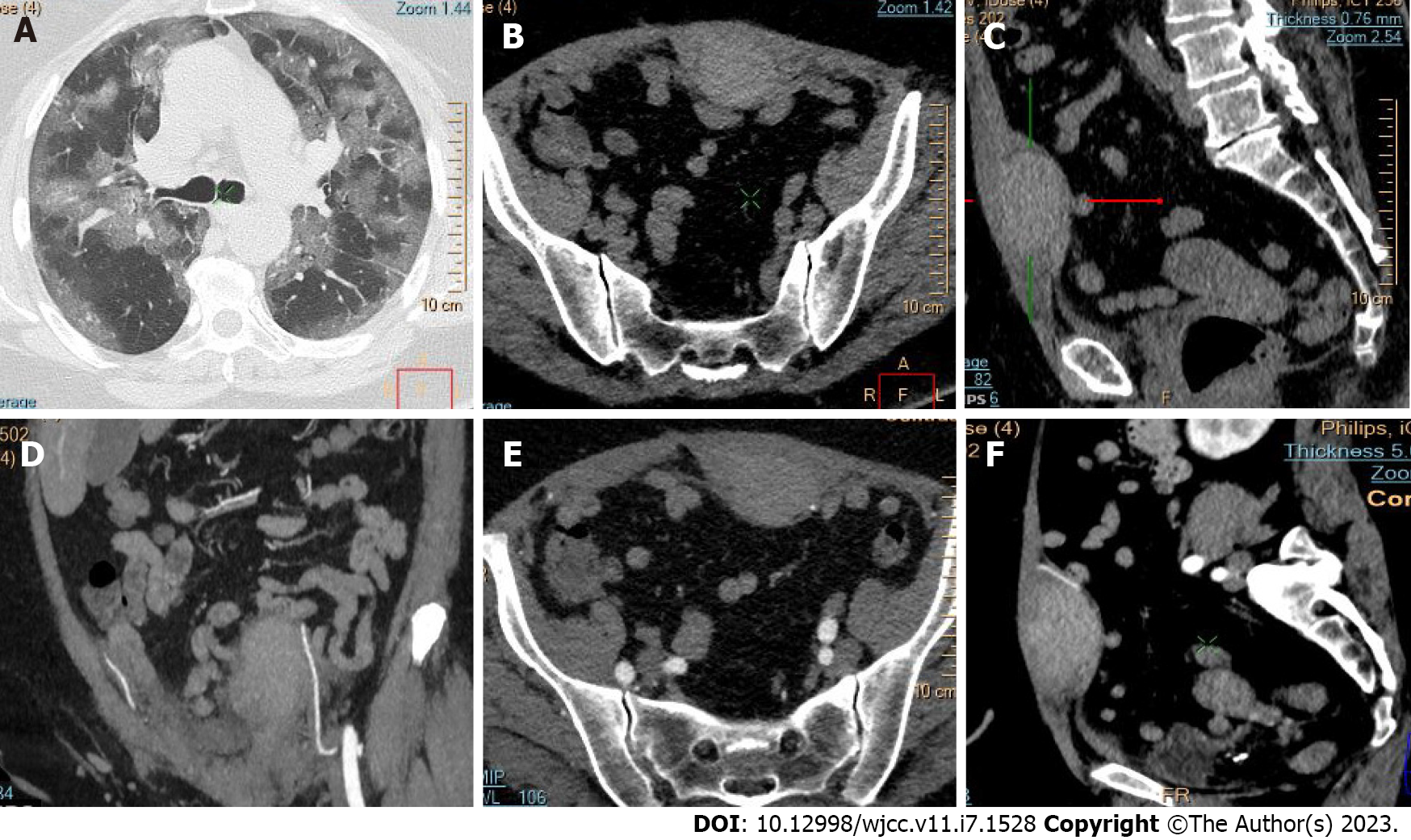
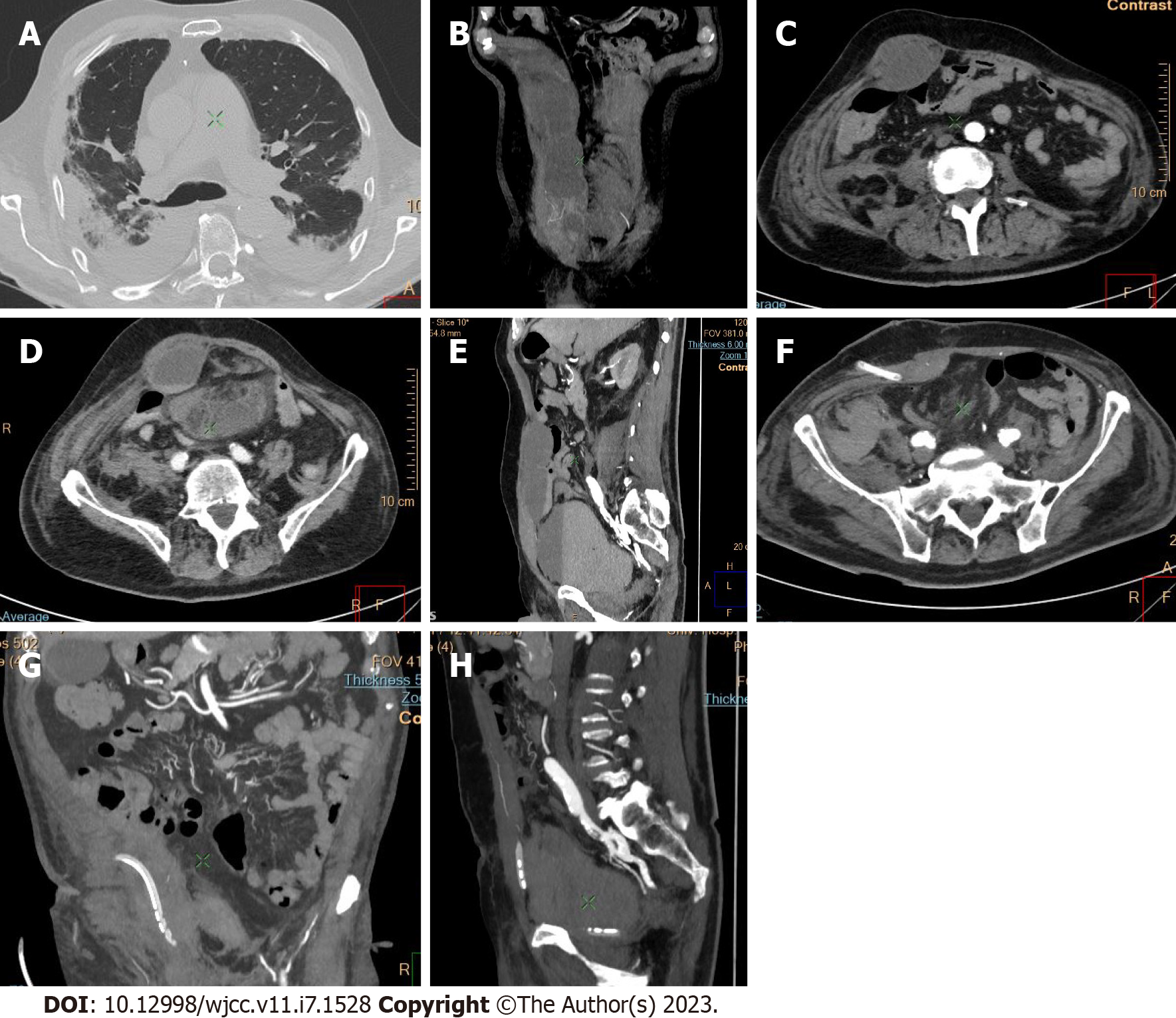
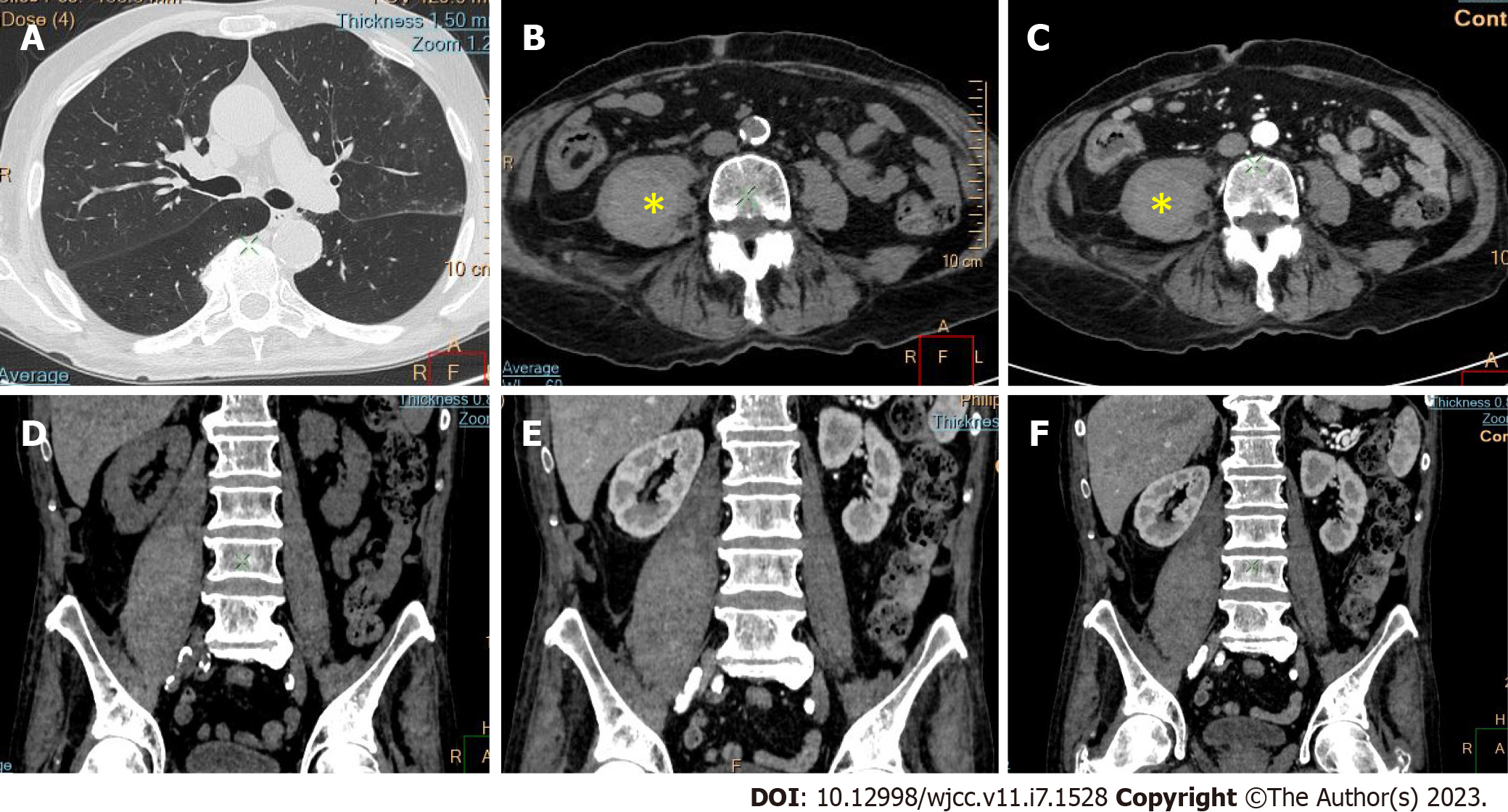
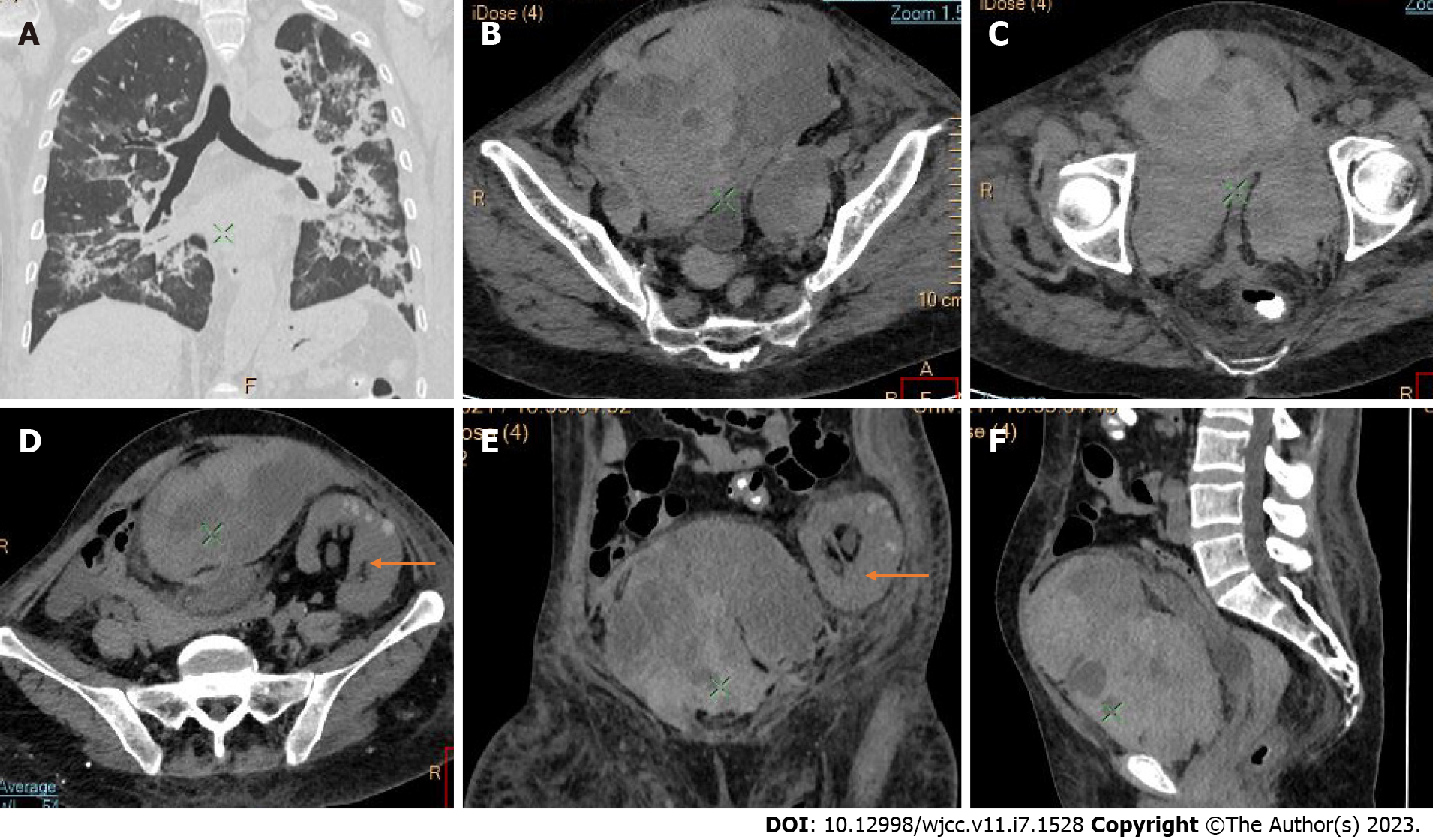
Active bleeding was identified in 6 patients out of 11 with a total of 14 identified sites of bleeding (7 intra- or retroperitoneal and 7 extraperitoneal locations). Five of these patients received immediate surgical treatment. One patient with chest wall hematoma underwent angiographic intervention 2 h after MDCT, which confirmed the imaging findings (Figures 2-12).
Despite the typical intraabdominal and retroperitoneal locations of hematomas, we also observed some uncommon sites of anticoagulant-related hematomas, such as the gluteal region and the chest wall (Figures 13-15).
Each patient was discussed on an interdisciplinary council board, which included a pulmonologist, an abdominal surgeon, a radiologist, and an intensive care specialist. The decision for a conservative or surgical approach was made after detailed assessment of the risk factors and outcomes for both treatment strategies.
Based on clinical and laboratory findings together with the imaging studies, all patients were diagnosed with SRH, RSH, or other abdominal bleeding or hematoma.
Per our hospital's guidelines, all patients received standardized COVID-19 treatments. Oxygen therapy, remdesivir, intravenous corticosteroids, proton pump inhibitors, bromhexine, antipyretics, and anticoagulants were all part of the treatment. The standard treatment protocol was applied to all patients. It included O2 therapy with changing amounts depending on the needs and the clinical course; anticoagulation either with subcutaneous low molecular weight heparin (LMWH) or intravenous heparin (ivHEP) (7 patients were treated with therapeutic doses due to concomitant AF, recent PE, or high risk for thrombotic complications). All patients received anticoagulant therapy, either with subcutaneous LMWH or ivHEP. Those with past vascular incidents and in cases following therapeutic procedures, 7 patients were given antiplatelet medication (acetylsalicylic acid) as well. All patients were given intravenous corticosteroids (methylprednisolone in 10 and dexamethasone in 1) in optimal doses depending on the clinical course and oxygen needs. One patient was also treated with remdesivir for 5 d, and another with 2 units of convalescent plasma. According to the antibiogram and clinical data on bacterial infection, antibiotics were used only in patients with positive microbiological tests. All patients received symptomatic medications.
After confirmation of bleeding complications with contrast CT, anticoagulant and antiplatelet therapies were immediately stopped. Five patients underwent surgery for hemostasis and drainage of hematoma, and 6 were treated with conservative methods (e.g., replacement with blood products, blood pressure control, hemostatic medication, etc). One patient needed a second surgery due to continuing bleeding and a drop in hemoglobin despite replacement with packed red blood cells (pRBC) and fresh frozen plasma (FFP). In 7 patients (63.7%), blood products were transfused according to laboratory markers of blood loss and hemostatic impairment. Product replacement ranged from 0 to 6 units for pRBC and 2 to 6 units for FFP. Anticoagulation with LMWH in reduced dosage was restored as soon as no signs of active bleeding were observed. Three patients with higher risk of thrombotic complication due to AF or recent PE received LMWH or ivHEP sooner, with monitoring of activated partial thromboplastin time. Supportive measures and early rehabilitation were applied to all patients.
Nonsurgical treatment included tranexamic acid and transfusion of bioproducts such as FFP and pRBC. Based on laboratory data, almost two-thirds of our patients received transfusions with varying volumes of these blood products (pRBC and FFP). However, due to low hemoglobin level and clinical signs of ongoing bleeding, only one patient required subsequent surgical treatment. After the second intervention, there was no evidence of active bleeding in the first 24-48 hours, and preventative anticoagulant medication was restarted.
Four of 11 patients died, resulting in a 36.3% mortality rate among patients with the complication of abdominal bleeding. Three of the 4 fatal cases did not undergo surgery, and 1 surgery was revised due to continuous bleeding. Half of the death cases were in patients with RSH, and the other half were in the SRH group. Two of the patients died the same day as the complication of bleeding complication was found, and the deaths were attributed to the hemorrhage in the background of persistent and worsening pulmonary infection. The other 2 patients died following the progression of the viral disease and associated respiratory failure. Hospital stays ranged from 10 to 31 d (mean 17.9 d; mean 15.5 d for those who died and mean 19.2 d for discharged patients). Of the patients who were discharged, 1 developed the complication of delayed wound healing with surgical site infection and infection of the hematoma, which led to a second admission and surgical revision of the wound with lavage and continuous drainage.
Since the beginning of the COVID-19 pandemic, many reports have shown that SARS-CoV-2 is linked with various thrombotic events[31]. Therefore, the putative culprit was thought to be COVID-19-induced coagulopathy, in which micro- and macrovascular thrombosis may occur (e.g., deep venous thrombosis, pulmonary intravascular coagulation, pulmonary embolism)[32,33]. As a result, anticoagulant treatments are often administered to these patients, as suggested by worldwide recommendations[34]. The mortality rate of these complications in COVID-19 units is significant, and it has been reported to surpass 50%[35,36].
Our study presents 11 cases of spontaneous retroperitoneal and abdominal bleeding in hospitalized, anticoagulated COVID-19 patients at our institution (Tables 1 and 2). The average age of our patients was 74.27 ± 11.75-years-old, and there was a male predominance (87.5%). The signs of bleeding appeared 9.45 ± 4.34 d after hospital admission. Abdominal pain was the most common clinical complaint among our group (90%), followed by back pain and leg edema. Ten patients had significant hemoglobin level reductions (> 20 g/L), while only 1 showed no changes in blood count analyses (Figure 1). Seven patients also received antiplatelet therapy with ASA due to previous arterial interventions or incidents such as ischemic heart disease with myocardial infarction or ischemic stroke.
Yeoh et al[37] demonstrated that the diagnosis of retroperitoneal hematoma requires a high level of clinical suspicion, since patients often do not develop clinically visible signs and symptoms until significant blood loss has already occurred. These individuals are at substantial risk of bleeding due to strong anticoagulant dosages, commonly combined with low platelet counts. The authors advise suspecting bleeding in patients with considerable groin, flank, stomach, or back discomfort following an interventional procedure and in the setting of anticoagulation[37].
We performed CE-CT on all patients presented. Active bleeding was identified in 6 of 11 patients, with a total of 14 bleeding sites (7 intra- or retroperitoneal and 7 extraperitoneal locations) (Figures 2-12). With respect to anticoagulation therapy, 9 of 11 patients were on LMWH, while only 2 patients received ivHEP before the bleeding occurred. Aspirin was also administered to 63.3% of patients (Tables 1 and 2). We discontinued the anticoagulation therapy as soon as the bleeding was established. Seven of 11 patients described were conservatively managed with fluid resuscitation, while the other 5 were surgically treated.
Following the identification of bleeding by CE-CT, anticoagulant and antiplatelet medications were immediately discontinued. Once bleeding sources were identified, we stratified the patients into two groups: Patients with stable hemodynamic parameters and patients with hemodynamic instability. In patients with stable hemodynamic parameters (54.55%), conservative therapy was applied, whereas the remaining 5 patients underwent surgical drainage, hemostasis, and drain placement. The average hospital stay was 17.90 ± 6.46 d, and the mortality rate was 36.36% (Tables 1 and 2).
This case series aims to raise awareness in clinicians of the complication of bleeding in COVID-19 patients, with particular emphasis on the necessary vigilant observation of anticoagulated COVID-19 patients. However, despite being an uncommon complication, COVID-19- related bleeding still carries a significant mortality rate. Accordingly, clinicians must look for nonspecific signs of bleeding in this group, including abdominal or flank pain, and signs of hypovolemia or anemia to ensure early and efficient treatment[38]. To this end, anticoagulation therapy has been included in treatment guidelines[39,40].
However, taking into consideration their side effects, there has been substantial discussion regarding whether anticoagulant therapy should be applied in particular conditions and at what dosage[39,41]. A plethora of retrospective studies have indicated that patients receiving anticoagulation in therapeutic doses had significantly higher risks of bleeding and inpatient mortality[24,27,42,43], which was also corroborated in our study[44-48]. However, the impact of anticoagulation on bleeding in hospitalized COVID-19 patients is currently being studied. In a study conducted by Musoke et al[49], COVID-19 patients receiving therapeutic anticoagulation had significantly higher rates of major bleeding than those not receiving anticoagulation. In contrast, subtherapeutic and prophylactic doses had no significant differences in primary bleeding outcomes compared to patients not receiving anticoagulation. Despite the danger of bleeding, anticoagulation has been proven to improve survival in individuals with severe COVID-19 infection[49].
It is thought that microvascular susceptibility caused by atherosclerosis, COVID-19 induced microtrauma, and mechanical disturbance (e.g., cough) might contribute to retroperitoneal bleeding. However, more studies must be conducted to elucidate the exact pathophysiological mechanism[50-52].
CT is a proven technique for evaluating and detecting hemorrhagic lesions. Intravenous contrast material administration is needed to visualize the precise location and site of bleeding and identify the affected vessels. Active bleeding can be seen as a focal jet of extravasated contrast material on multidetector CT images. Maximum Intensity Projection (MIP) and Volume Rendering (VR) techniques are valuable tools for the presentation of various findings of CT angiography. These tools are suited to display even small vessels and facilitate the visualization of any bleeding site. Active hemorrhage can be demonstrated best in the arterial phase, and may involve the peritoneal cavity, the mesentery, or the retroperitoneum. Less common locations of bleeding following anticoagulant therapy in patients with mild or severe COVID-19 infection include the chest wall and various muscles (e.g., gluteus muscles).
Furthermore, there is no confirmed pathognomonic sign to suggest retroperitoneal hemorrhage, especially in the scenario of overt bleeding without significant hemoglobin level changes. Thus, a high level of clinical suspicion is required to identify nonspecific symptoms such as groin, abdominal, back, or lower extremity discomfort[38,43].
Regarding diagnostic management, CE-CT is considered the best imaging technique for evaluating and detecting hemorrhagic lesions. Intravenous contrast material administration is necessary to visualize the precise location and site of bleeding and identify the affected vessels. Active bleeding can be seen as a focal jet of extravasated contrast material on multidetector CE-CT images. MIP and VR techniques are valuable tools for the presentation of various findings of CT angiography. These tools are suited to display even small vessels and facilitate visualization of any bleeding site. Active hemorrhage can be demonstrated best in the arterial phase and may involve the peritoneal cavity, the mesentery, or the retroperitoneum[53,54].
Early diagnosis and prompt management of abdominal bleeding are paramount to prevent hematoma expansion and compression of surrounding tissues[54]. According to the available reports, there is no clear consensus on the best therapeutic strategy for retroperitoneal bleeding in COVID-19 patients[54]. However, the prevailing primary treatment consists of discontinuing anticoagulant medication as soon as possible and replacing lost volume with fluids and bioproduct transfusion (pRBC/FFP). Small hematomas and hemodynamically stable patients are managed conservatively[55]. On the other hand, patients with significant bleeding and hemodynamic instability should undergo arterial embolization or surgical treatment with the goal of increasing survival[55-57].
Our case series has some limitations. Firstly, the number of cases is relatively few, however, our manuscript has gathered the most notable cases of retroperitoneal and abdominal bleeding in anticoagulated COVID-19 patients seen in our institution. Secondly, we are unable to provide follow-up data after patients were discharged. We collected examples of both common and rare cases that illustrate that imaging methods such as CE-CT can be potent tools in the diagnosis and management of bleeding complications in COVID-19 patients. What remains unresolved is how to choose the ideal time for specialized image testing and when to suspect retroperitoneal bleeding in anticoagulated COVID-19 patients; these issues represent fertile topics for future research.
It is thought that microvascular susceptibility caused by atherosclerosis, viral-induced microtrauma, and mechanical disturbance (e.g., cough) may contribute to the development of retroperitoneal bleeding in the setting of COVID-19. However, more studies must be conducted to elucidate the exact pathophysiological mechanism of this serious complication. CT is a proven technique for evaluating and detecting hemorrhagic lesions, where intravenous contrast material administration is needed to visualize the precise bleeding site and identify affected vessels. Active bleeding can be seen as a focal jet of extravasated contrast material on multidetector CT images. Native and contrast-enhanced MDCT are essential imaging modalities in the diagnosis of hematoma. In addition, MIP and VR techniques with contrast media can help identify the possible site of bleeding. At the same time, digital subtraction angiography may be used to embolize the affected vessels.
Active hemorrhage can be demonstrated best in the arterial phase and may involve the peritoneal cavity, the mesentery, or the retroperitoneum. Less common locations of bleeding following anticoagulant therapy in patients with mild and severe COVID-19 infection include the chest wall and different muscle groups (e.g., gluteus muscles). However, there is no consensus regarding the best therapeutic strategy for retroperitoneal bleeding in COVID-19 patients. Nevertheless, CE-CT is considered the best imaging technique for evaluating and detecting hemorrhagic lesions in these patients.
| 1. | Coronavirus Disease (COVID-19): Weekly Epidemiological Update (5 October 2022). [Internet] [cited 6 October 2022]. Available from: https://reliefweb.int/report/world/coronavirus-disease-COVID-19-weekly-epidemiological-update-5-october-2022. |
| 2. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18404] [Article Influence: 3067.3] [Reference Citation Analysis (13)] |
| 3. | Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quéré I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:2950-2973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2178] [Cited by in RCA: 2214] [Article Influence: 369.0] [Reference Citation Analysis (0)] |
| 4. | Velikova TV, Kotsev SV, Georgiev DS, Batselova HM. Immunological aspects of COVID-19: What do we know? World J Biol Chem. 2020;11:14-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 5. | Kotsev SV, Miteva D, Krayselska S, Shopova M, Pishmisheva-Peleva M, Stanilova SA, Velikova T. Hypotheses and facts for genetic factors related to severe COVID-19. World J Virol. 2021;10:137-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (2)] |
| 6. | COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. Available from: https://www.COVID19treatmentguidelines.nih.gov/. |
| 7. | Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, Clark C, Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023-1026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1289] [Cited by in RCA: 1328] [Article Influence: 221.3] [Reference Citation Analysis (0)] |
| 8. | Barnes GD, Burnett A, Allen A, Blumenstein M, Clark NP, Cuker A, Dager WE, Deitelzweig SB, Ellsworth S, Garcia D, Kaatz S, Minichiello T. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50:72-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 325] [Article Influence: 54.2] [Reference Citation Analysis (1)] |
| 9. | Spyropoulos AC, Levy JH, Ageno W, Connors JM, Hunt BJ, Iba T, Levi M, Samama CM, Thachil J, Giannis D, Douketis JD; Subcommittee on Perioperative, Critical Care Thrombosis, Haemostasis of the Scientific, Standardization Committee of the International Society on Thrombosis and Haemostasis. Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1859-1865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 492] [Cited by in RCA: 558] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 10. | Hajra A, Mathai SV, Ball S, Bandyopadhyay D, Veyseh M, Chakraborty S, Lavie CJ, Aronow WS. Management of Thrombotic Complications in COVID-19: An Update. Drugs. 2020;80:1553-1562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Velikov T, Keremidchiev G, Velikova T. How to use Safely COVID-19 Vaccines in Patients on Anticoagulants or Antiaggregants. Int J Preven Cardio. 2021;1:32-33. |
| 12. | Velikova T, Snegarova V, Kukov A, Batselova H, Mihova A, Nakov R. Gastrointestinal mucosal immunity and COVID-19. World J Gastroenterol. 2021;27:5047-5059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Sposato B, Croci L, Di Tomassi M, Puttini C, Olivieri C, Alessandri M, Ronchi MC, Donati E, Garcea A, Brazzi A, Migliorini MG, Chigiotti S, Nikiforakis N, Carli T, Canneti E, Strambio F, Cellini C, Nardangeli C, Allegri MP, Bianchi F, Bettini C, Perruzza M, Lanzarone N, Valentini L, Orselli P, Solari M, Cardaci S, Nofri M, Angeli G, Mangani F, Aloia E, Lanari A, Corridi M, Spargi G, Perrella A, Nencioni C. Spontaneous abdominal bleeding associated with SARS-CoV-2 infection: causality or coincidence? Acta Biomed. 2021;92:e2021199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Wang T, Chen R, Liu C, Liang W, Guan W, Tang R, Tang C, Zhang N, Zhong N, Li S. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7:e362-e363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 274] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 15. | Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med. 2020;173:268-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1577] [Cited by in RCA: 1774] [Article Influence: 295.7] [Reference Citation Analysis (0)] |
| 16. | Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Bertuzzi A, Sandri MT, Barco S; Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1298] [Cited by in RCA: 1545] [Article Influence: 257.5] [Reference Citation Analysis (1)] |
| 17. | Hatahet S, Yacoub MS, Farag M, Gasimova U, Elhamamsy S. Internal Bleeding Extending to the Retroperitoneum and Right Psoas With Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Cureus. 2021;13:e18477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 18. | Trindade AJ, Izard S, Coppa K, Hirsch JS, Lee C, Satapathy SK; Northwell COVID-19 Research Consortium. Gastrointestinal bleeding in hospitalized COVID-19 patients: a propensity score matched cohort study. J Intern Med. 2021;289:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Dubovský M, Hajská M, Panyko A, Vician M. Severe Retroperitoneal Hemorrhage in a COVID-19 Patient on a Therapeutic Dose of Low Molecular Weight Heparin: A Case Report. Cureus. 2022;14:e26275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3992] [Cited by in RCA: 4077] [Article Influence: 679.5] [Reference Citation Analysis (0)] |
| 21. | Jiang H, Liu L, Guo T, Wu Y, Ai L, Deng J, Dong J, Mei H, Hu Y. Improving the safety of CAR-T cell therapy by controlling CRS-related coagulopathy. Ann Hematol. 2019;98:1721-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 22. | Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605-2610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1223] [Article Influence: 58.2] [Reference Citation Analysis (2)] |
| 23. | Chan ASW, Ho JMC, Li JSF, Tam HL, Tang PMK. Impacts of COVID-19 Pandemic on Psychological Well-Being of Older Chronic Kidney Disease Patients. Front Med (Lausanne). 2021;8:666973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Ohn MH, Ng JR, Ohn KM, Luen NP. Double-edged sword effect of anticoagulant in COVID-19 infection. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2419] [Cited by in RCA: 2113] [Article Influence: 352.2] [Reference Citation Analysis (7)] |
| 26. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4227] [Cited by in RCA: 4667] [Article Influence: 777.8] [Reference Citation Analysis (2)] |
| 27. | Mahboubi-Fooladi Z, Pourkarim Arabi K, Khazaei M, Nekooghadam S, Shadbakht B, Moharamzad Y, Sanei Taheri M. Parenteral Anticoagulation and Retroperitoneal Hemorrhage in COVID-19: Case Report of Five Patients. SN Compr Clin Med. 2021;3:2005-2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Bonaffini PA, Franco PN, Bonanomi A, Giaccherini C, Valle C, Marra P, Norsa L, Marchetti M, Falanga A, Sironi S. Ischemic and hemorrhagic abdominal complications in COVID-19 patients: experience from the first Italian wave. Eur J Med Res. 2022;27:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 29. | Javid A, Kazemi R, Dehghani M, Bahrami Samani H. Catastrophic retroperitoneal hemorrhage in COVID-19 patients under anticoagulant prophylaxis. Urol Case Rep. 2021;36:101568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Ilieva E, Boyapati A, Chervenkov L, Gulinac M, Borisov J, Genova K, Velikova T. Imaging related to underlying immunological and pathological processes in COVID-19. World J Clin Infect Dis. 2022;12:1-19. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 31. | Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA, Bouman CCS, Beenen LFM, Kootte RS, Heijmans J, Smits LP, Bonta PI, van Es N. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995-2002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1017] [Cited by in RCA: 1106] [Article Influence: 184.3] [Reference Citation Analysis (0)] |
| 32. | Poli D, Antonucci E, Ageno W, Prandoni P, Barillari G, Bitti G, Imbalzano E, Bucherini E, Chistolini A, Fregoni V, Galliazzo S, Gandolfo A, Grifoni E, Mastroianni F, Panarello S, Pesavento R, Pedrini S, Sala G, Pignatelli P, Preti P, Simonetti F, Sivera P, Visonà A, Villalta S, Marcucci R, Palareti G. Thromboembolic Complications in COVID-19 Patients Hospitalized in Italian Ordinary Wards: Data from the Multicenter Observational START-COVID Register. TH Open. 2022;6:e251-e256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 33. | Chan NC, Weitz JI. COVID-19 coagulopathy, thrombosis, and bleeding. Blood. 2020;136:381-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 34. | COVID-19 Treatment Guidelines. Antithrombotic Therapy in Patients With COVID-19. [Internet] [accessed December 2022]. Available from: https://www.COVID19treatmentguidelines.nih.gov/therapies/antithrombotic-therapy/ 2022. |
| 35. | Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3488] [Cited by in RCA: 3447] [Article Influence: 574.5] [Reference Citation Analysis (0)] |
| 36. | Nahum J, Morichau-Beauchant T, Daviaud F, Echegut P, Fichet J, Maillet JM, Thierry S. Venous Thrombosis Among Critically Ill Patients With Coronavirus Disease 2019 (COVID-19). JAMA Netw Open. 2020;3:e2010478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 239] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 37. | Yeoh WC, Lee KT, Zainul NH, Syed Alwi SB, Low LL. Spontaneous retroperitoneal hematoma: a rare bleeding occurrence in COVID-19. Oxf Med Case Reports. 2021;2021:omab081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Shah M, Colombo JP, Chandna S, Rana H. Life-Threatening Retroperitoneal Hematoma in a Patient with COVID-19. Case Rep Hematol. 2021;2021:8774010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Salabei JK, Fishman TJ, Asnake ZT, Ali A, Iyer UG. COVID-19 Coagulopathy: Current knowledge and guidelines on anticoagulation. Heart Lung. 2021;50:357-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 40. | Moores LK, Tritschler T, Brosnahan S, Carrier M, Collen JF, Doerschug K, Holley AB, Jimenez D, Le Gal G, Rali P, Wells P. Prevention, Diagnosis, and Treatment of VTE in Patients With Coronavirus Disease 2019: CHEST Guideline and Expert Panel Report. Chest. 2020;158:1143-1163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 463] [Cited by in RCA: 475] [Article Influence: 79.2] [Reference Citation Analysis (1)] |
| 41. | Gomez K, Laffan M, Bradbury C. Debate: Should the dose or duration of anticoagulants for the prevention of venous thrombosis be increased in patients with COVID-19 while we are awaiting the results of clinical trials? Br J Haematol. 2021;192:459-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Teta M, Drabkin MJ. Fatal retroperitoneal hematoma associated with COVID-19 prophylactic anticoagulation protocol. Radiol Case Rep. 2021;16:1618-1621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Nakamura H, Ouchi G, Miyagi K, Higure Y, Otsuki M, Nishiyama N, Kinjo T, Nakamatsu M, Tateyama M, Kukita I, Fujita J. Case Report: Iliopsoas Hematoma during the Clinical Course of Severe COVID-19 in Two Male Patients. Am J Trop Med Hyg. 2021;104:1018-1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Erdinc B, Raina JS. Spontaneous Retroperitoneal Bleed Coincided With Massive Acute Deep Vein Thrombosis as Initial Presentation of COVID-19. Cureus. 2020;12:e9772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Guo SH, Zhu SM, Yao YX. Giant Retroperitoneal Hematoma During Extracorporeal Membrane Oxygenation in a Patient With Coronavirus Disease-2019 Pneumonia. J Cardiothorac Vasc Anesth. 2020;34:2839-2840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Vergori A, Pianura E, Lorenzini P, D'Abramo A, Di Stefano F, Grisetti S, Vita S, Pinnetti C, Donno DR, Marini MC, Nicastri E, Ianniello S, Antinori A; ReCOVeRI Study Group. Spontaneous ilio-psoas haematomas (IPHs): a warning for COVID-19 inpatients. Ann Med. 2021;53:295-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 47. | Hazenberg P, Lechareas S, Vasquez Rios M, Taegtmeyer M, McWilliams R, Dutt T. Rectus sheath and retroperitoneal haematomas in patients with Coronavirus 2019 infection. Br J Haematol. 2021;194:923-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Lucatelli P, Rocco B, Nardis PG, Cannavale A, Bezzi M, Catalano C, Corona M. Bleeding in COVID Patients: What We Have Understood So Far. Cardiovasc Intervent Radiol. 2021;44:666-668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Musoke N, Lo KB, Albano J, Peterson E, Bhargav R, Gul F, DeJoy R 3rd, Salacup G, Pelayo J, Tipparaju P, Azmaiparashvili Z, Patarroyo-Aponte G, Rangaswami J. Anticoagulation and bleeding risk in patients with COVID-19. Thromb Res. 2020;196:227-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 50. | Singh SP, Pritam M, Pandey B, Yadav TP. Microstructure, pathophysiology, and potential therapeutics of COVID-19: A comprehensive review. J Med Virol. 2021;93:275-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 51. | Kumar M, Al Khodor S. Pathophysiology and treatment strategies for COVID-19. J Transl Med. 2020;18:353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 52. | Parasher A. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad Med J. 2021;97:312-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 421] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 53. | Mondie C, Maguire NJ, Rentea RM. Retroperitoneal Hematoma. 2022 Jul 12. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. [PubMed] |
| 54. | Janák D, Rohn V. Retroperitoneal hematoma: diagnosis and treatment. Rozhl Chir. 2022;100:569-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Chan YC, Morales JP, Reidy JF, Taylor PR. Management of spontaneous and iatrogenic retroperitoneal haemorrhage: conservative management, endovascular intervention or open surgery? Int J Clin Pract. 2008;62:1604-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 56. | Dorosh J, Lin JC. Retroperitoneal Bleeding. 2022 Oct 3. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. [PubMed] |
| 57. | Gulinac M, Novakov IP, Antovic S, Velikova T. Surgical complications in COVID-19 patients in the setting of moderate to severe disease. World J Gastrointest Surg. 2021;13:788-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Bulgaria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chan ASW, China; Nooripour R, Iran S-Editor: Liu GL L-Editor: Filipodia P-Editor: Liu GL