Published online Mar 6, 2023. doi: 10.12998/wjcc.v11.i7.1498
Peer-review started: September 20, 2022
First decision: December 26, 2022
Revised: January 7, 2023
Accepted: February 10, 2023
Article in press: February 10, 2023
Published online: March 6, 2023
Processing time: 162 Days and 21.8 Hours
Liver metastasis is the most common form of distant metastasis in colorectal cancer, and the only possible curative treatment for patients with colorectal liver metastases (CRLM) is hepatectomy. However, approximately 25% of patients with CRLM have indications for liver resection at the initial diagnosis. Strategies aimed at downstaging large or multifocal tumors to enable curative resection are appealing.
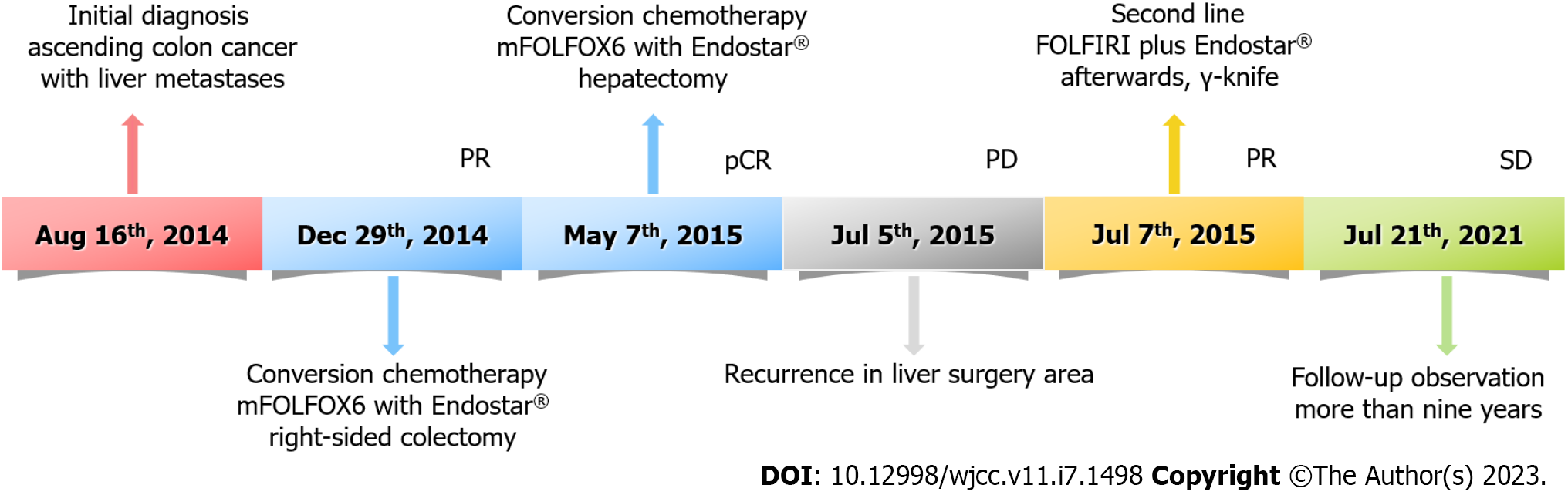
A 42-year-old man was diagnosed with ascending colon cancer and liver metastases. Due to the huge lesion size and compression of the right portal vein, the liver metastases were initially diagnosed as unresectable lesions. The patient was treated with preoperative transcatheter arterial chemoembolization (TACE) consisting of 5-fluorouracil/Leucovorin/oxaliplatin/Endostar®. After four cour
Multidisciplinary treatment can promote the conversion of initially unresectable colorectal liver metastasis and facilitate complete pathological remission of liver lesions.
Core Tip: We report a multidisciplinary strategy, including 5-fluorouracil/Leucovorin/oxaliplatin/Endostar® (mFOLFOX6 plus Endostar®) and transcatheter arterial chemoembolization, that may help improve resectability of initially unresectable colorectal liver metastasis (CRLM) and achieve pathologically complete response (pCR). After the recurrence of liver metastasis, the patient received TACE comprising irinotecan/Leucovorin/fluorouracil therapy plus Endostar® and was treated with γ-knife. The patient's overall survival time exceeded 9 years. To date, this is the first case that mFOLFOX6 combined with Endostar® in conversion therapy of initially unresectable CRLM and liver metastases that achieved pCR. Our study implies that Endostar® has a potential value in conversion therapy and combination therapy of initially unresectable CRLM.
- Citation: Tan XR, Li J, Chen HW, Luo W, Jiang N, Wang ZB, Wang S. Successful multidisciplinary therapy for a patient with liver metastasis from ascending colon adenocarcinoma: A case report and review of literature. World J Clin Cases 2023; 11(7): 1498-1505
- URL: https://www.wjgnet.com/2307-8960/full/v11/i7/1498.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i7.1498
Colorectal cancer (CRC) is one of the most common cancers worldwide, ranking third in terms of incidence (9.7% of all cancer cases worldwide) and second in mortality (9.4% of all cancer mortality) globally[1]. Liver metastasis is the most common cause of death in CRC patients, and liver metastasis prevalence is approximately 15%-42% in this population[2,3]. The only possible curative treatment for patients with colorectal liver metastases (CRLM) is hepatectomy[4]. However, only about 25% of CRLM patients have indications for liver resection at the initial diagnosis[5]. For patients with irresectable CRLM, the standard care remains first-line systemic chemotherapy combined with antiangiogenic or targeted therapy to shrink tumors to allow patients to receive resection[6]. Multidisciplinary treatments, including regional hepatic intra-arterial chemotherapy[7], chemoembolization[8], stereotactic radiation therapy[9], targeted therapy[10], anti-angiogenic therapy[11], immunotherapy[12], and ablation procedures (radiofrequency ablation and microwave ablation)[13,14], improve the survival rate and prognosis of patients with CRLM[15,16]. Here, we report a case of conversion chemotherapy, including 5-fluorouracil/Leucovorin/oxaliplatin/Endostar® (mFOLFOX6 plus Endostar®) and transcatheter arterial chemoembolization (TACE), which promoted the successful conversion of initially unresectable CRLM into resectable CRLM with surgical indications and resulted in pathologically complete response (pCR).
A 42-year-old man presented to the hospital with pain in his right upper abdomen and anorexia in July 2013.
To date, there has been no evidence of disease progression, and the patient's overall survival (OS) time was > 9 years.
He had no previous history of hepatitis B or C, serious diseases, operations, or hospitalizations.
The patient had no significant personal or family history.
Physical examination showed a temperature of 36.3 °C, a heart rate of 87 bpm, and a blood pressure of 15.7/9.2 kPa. There was no tenderness, rebound pain, or muscle tension in the abdomen. The upper boundary of the liver was located within the fourth intercostal space of the right midclavicular line, and the lower boundary of the liver was located approximately 2 cm below the costal margin. There were no other obvious abnormalities.
Serum indicators were as follows: Carcinoembryonic antigen, 23.22 ng/mL (0-5 ng/mL), and aspartate aminotransferase, 115.6 U/L (15-40 U/L).
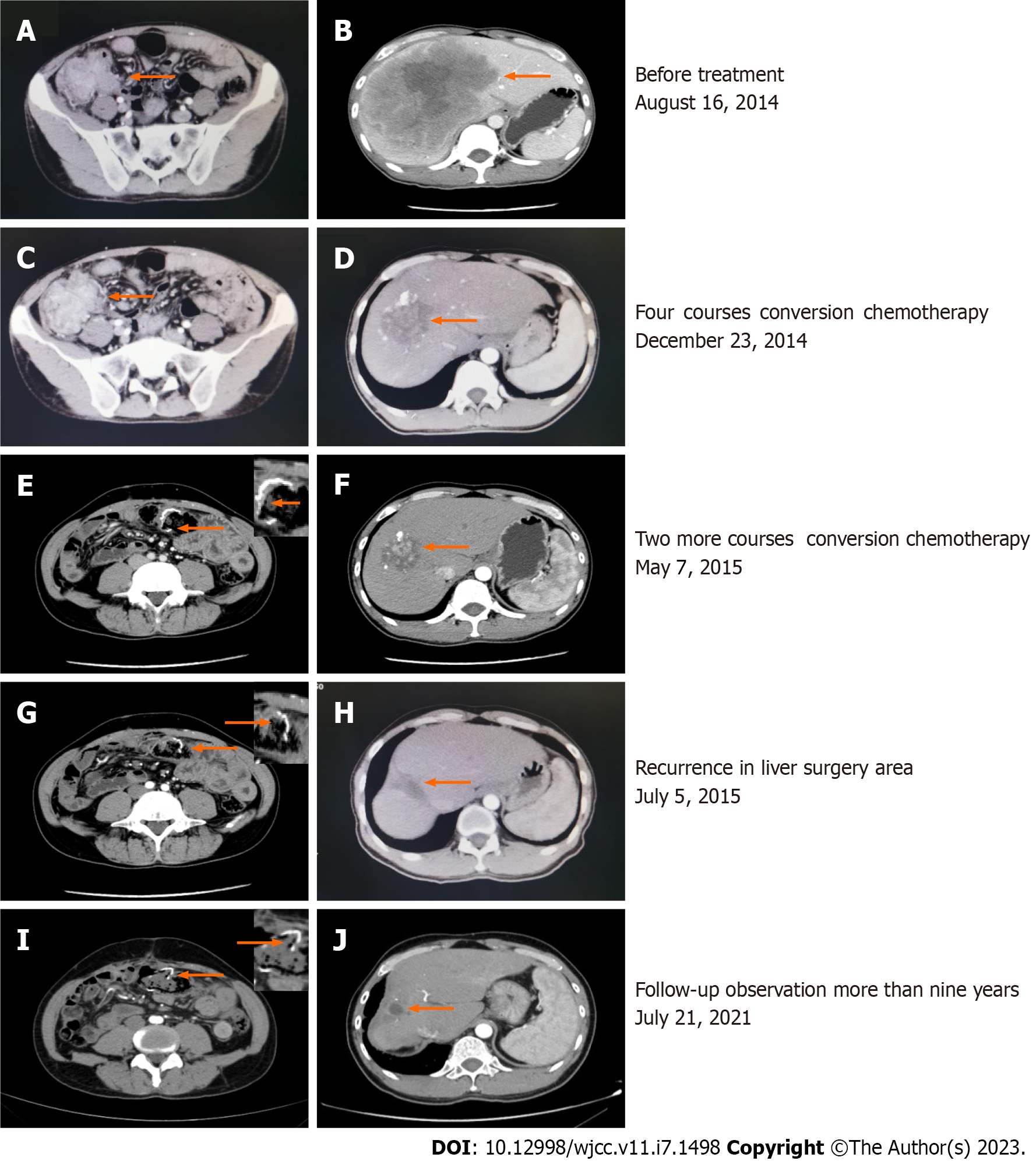
A colonoscopy revealed a moderately differentiated adenocarcinoma. The expression of BRAF-V600E and RAS was not determined. Enhanced computer tomography (CT) of the whole abdomen revealed ascending colon cancer (Figure 1A) with a single large low-density lesion in the liver (14.1 cm in length, Figure 1B). A CT scan of the chest, brain, and bone revealed no other abnormalities.
The patient was diagnosed with colon cancer and liver metastases. The clinical stage was T3N0M1a stage IVa (American Joint Committee on Cancer's Cancer Staging Manual 2010). Due to the huge liver metastatic lesions and compression of the right portal vein, the CRLM were initially diagnosed as unresectable lesions.
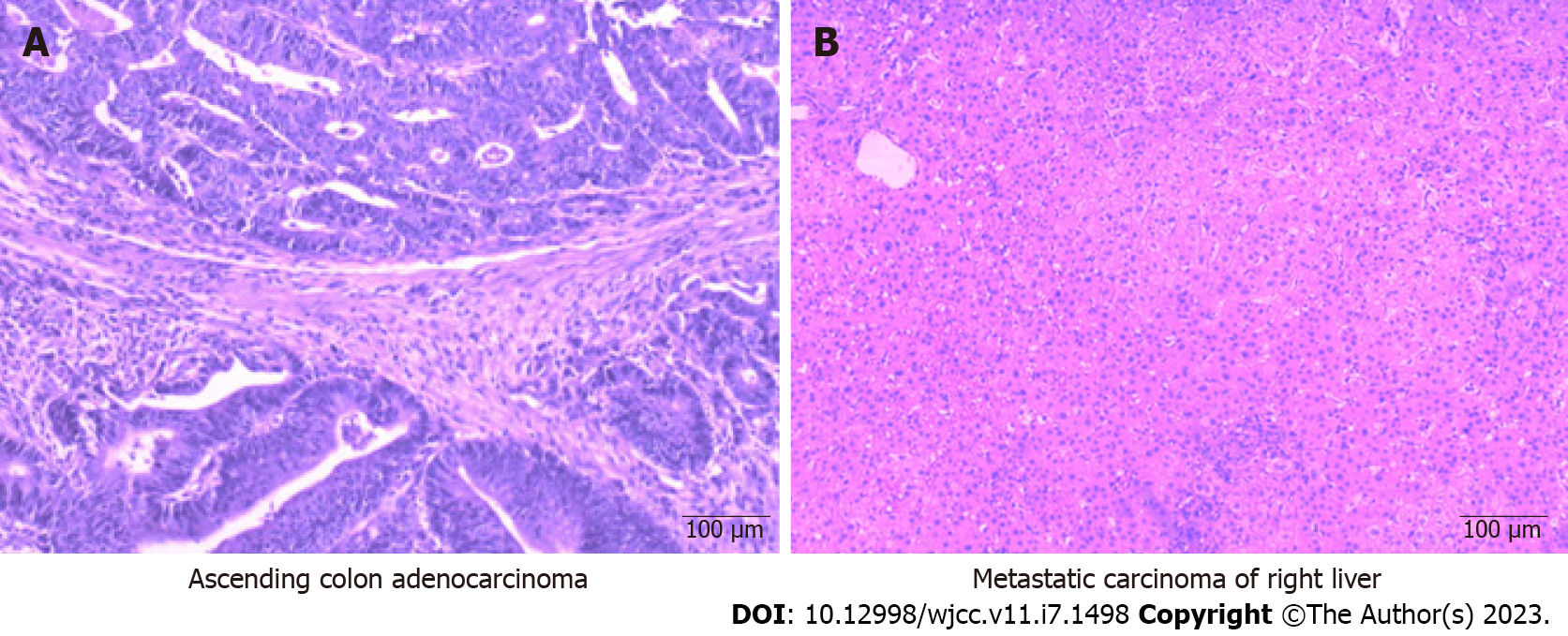
The first multidisciplinary team discussion recommended the conversion therapy model of systemic chemotherapy combined with anti-angiogenesis therapy to strive for the opportunity of surgical resection. mFOLFOX6 has been used as a cornerstone in the combination chemotherapy treatment of CRC[17] and has been considered the first-line standard chemotherapy regimen for advanced CRC. Neutropenia is the most common adverse event of grade 3 or 4 after combination treatment with mFOLFOX6 and bevacizumab[17], and the high cost of bevacizumab continues to be a huge obstacle to its clinical use in China. Moreover, accumulating evidence suggests that the use of Endostar® does not significantly increase the level of chemotherapy toxicity[18] and tends to be accepted by many patients because of the relatively low economic burden. Moreover, studies have shown that Endostar® combined with chemotherapy can prolong progression-free survival and OS rates in patients with advanced CRC[11,19-22]. Hence, combined therapy including mFOLFOX6 plus Endostar® [day 1:5-fluorouracil (5-FU) 400 mg/m2 (perfusion via arterial catheter); leucovorin (LV) 200 mg/(m2·2 h) with oxaliplatin 85 mg/m2 (perfusion via arterial catheter); Endostar® 3 mL (perfusion via arterial catheter), 5-FU 2400 mg/(m2·44 h), and Endostar® 18 mL/(44 h) continuous infusion every 2 wk] was chosen as the conversion chemotherapy. After four courses, enhanced CT of the whole abdomen revealed that the low-density metastatic lesion in the liver had shrunk from 14.1 to 5.9 cm, although the tumor size in the ascending colon was not significantly reduced (6.8 to 5.4 cm) (Figure 1C and D). Therefore, radical right-sided colectomy and ileum transverse colon anastomosis were performed. Histological examination demonstrated moderately differentiated mutant kirsten rat sarcoma viral oncogene homolog adenocarcinoma with necrosis and negative margins. The cancer tissue invaded the whole layer of the intestinal wall and involved the nerve, and the formation of an intravascular tumor thrombus was observed. No lymph node metastasis was observed after surgery (0/13). The pathological response grade of the tumor after chemotherapy was grade 2 (Figure 2A).
Subsequently, the patient received another two cycles of TACE consisting of mFOLFOX6 plus Endostar® after radical resection of colon cancer. A whole abdominal CT scan revealed that the colon cancer surgery area was stable (Figure 1E), but the low-density metastatic lesion in the liver had shrunk from 5.9 to 5.2 cm (Figure 1F). As imaging examinations suggested that the liver lesions were further reduced, a second multidisciplinary team discussion was immediately performed. Resection of the hepatic metastasis was performed one month after the Endostar® was stopped. A pathological biopsy of the resected specimen revealed no cancerous cells in the liver metastases; necrosis was observed in most areas of the specimen. The pathological response grade of the tumor after chemotherapy was grade 0 (Figure 2B). The patient recovered promptly after both surgical procedures.
Unfortunately, more than 2 mo after liver metastasis resection, the patient's serum carcinoembryonic antigen (CEA) level rose to 38 ng/mL, and a CT scan revealed a single low-density lesion in the liver surgery area (3.0 cm in length, Figure 1G). This was considered a postoperative recurrence. At the same time, the colon cancer surgery area remained stable (Figure 1H). Therefore, TACE consisting of 5-FU/LV/irinotecan and Endostar® (FOLFIRI plus Endostar®) was commenced. Studies have suggested that the γ-knife, a specific form of stereotactic radiotherapy, can avoid damaging the surrounding critical tissue for liver oligo metastases. Owing to the good local control effect and survival rates, γ-knife has become an effective option for patients with advanced CRC[23,24]. Therefore, the γ-knife was utilized to treat the recurrent liver lesions with a total dose of 35 Gray after two courses of TACE consisting of FOLFIRI plus Endostar®. The patient attended regular follow-up appointments for the analysis of serum CEA levels and an abdominal CT scan.
By the time of submission of this paper, there was no evidence of disease progression (Figure 1I and J), and the survival time had been more than 9 years. The timeline of the patient's treatment is shown in Figure 3.
Over 1.9 million new cases of CRC (including cancer of the anus) and 935000 CRC-related deaths were estimated to have occurred in 2020; approximately one in 10 cancer cases or deaths was related to CRC[1]. Currently, hepatic resection is the only possible radical treatment for patients with CRLM[4]. However, only about 25% of patients with CRLM have indications for liver resection at first diagnosis[5]. The most common metastatic site is the liver. For patients with unresectable CRLM, the standard care remains first-line systemic chemotherapy combined with antiangiogenic or targeted therapy to shrink tumors enough to allow for resection. mFOLFOX6 is commonly used as a cornerstone of combination therapy[17] and is considered the first-line standard treatment for advanced CRC. However, chemotherapy response rates are low, and severe dose-limiting toxicities can occur.
In 1971, researchers[25] proposed that the formation of new tumor blood vessels led to the growth and metastasis of cancer; thus, angiogenesis inhibitors, such as bevacizumab and Endostar®, have been developed for cancer treatment[26,27]. Endostar® and bevacizumab showed competitive anti-tumor efficacy. Bevacizumab is a recombinant human monoclonal antibody that blocks angiogenesis by inhibiting vascular endothelial growth factor A (VEGF-A)[28]. Endostar®, developed in China, is a recombinant human vascular endothelial inhibitor and multi-targeted tumor cell inhibitor. Endostar® directly inhibits the proliferation of vascular endothelial cells and exerts its anti-angiogenic effects through several targets, including VEGF, VEGF receptor-2 (VEGFR-2), and the platelet-derived growth factor receptor[29]. It can also normalize tumor blood vessels and exert anti-tumor effects[30]. The combination treatment of mFOLFOX6 with bevacizumab may lead to the most common grade 3 or 4 adverse events of neutropenia[17] and the high treatment cost of bevacizumab is still a huge obstacle to its clinical use in China. Endostatin is the strongest endogenous angiogenesis inhibitor of vascular endothelial growth factor expression and tumor angiogenesis[31]. Recombinant human endostatin, Endostar®, is a new recombinant human endostatin developed in China and has achieved good results in treating various advanced malignant tumors[21,22]. Notably, Endostar® has shown promise for the treatment of CRC[20,32]. Studies have demonstrated that combination therapy of FOLFOX with Endostar® can improve clinical efficacy and objective response rate and prolong PFS and OS rates[11,19], and chemotherapy combined with Endostar® does not significantly increase chemotherapy toxicity[18] and is easier to be accepted given its relatively lower price. Therefore, combined therapy with mFOLFOX6 and Endostar® was chosen as neoadjuvant chemotherapy in this case.
Liver metastasis is the most common cause of death in CRC patients, and the prevalence of liver metastasis is approximately 15%-42% in this population[2,3]. With the continuous improvement of surgical methods, such as two-stage hepatectomy and TACE[14], more CRLM patients have the opportunity to undergo surgical treatment. Strategies aimed at downstaging large or multifocal tumors to enable curative resection are appealing. The decision of surgery for CRLM patients must consider many factors, including the order of liver surgery. TSH for CRLM is widely used and has satisfactory survival outcomes because it can reduce the huge surgical trauma caused by simultaneous operations[33]. While the 5-year survival rate after resection of CRLM is approximately 47% to 60%[34,35], 50% to 70% of patients still relapse after hepatectomy, and about one-third of them have isolated recurrence in the liver[36].
Stereotactic radiation strategies have become an important treatment for unresectable CRLM. Therefore, stereotactic radiotherapy can be considered for the local control of liver metastases[23,37,38] and postoperative recurrence in the liver. The γ-knife, a specific form of stereotactic radiotherapy, can highly concentrate the target dose into a maximum focal spot and avoid damaging the surrounding critical tissue for liver oligometastases[24]. Thus, the γ-knife for liver metastasis is a safe and effective treatment that achieves high local control rates and enhanced survival rates among CRLM.
The groundbreaking progress in cancer immunotherapy in recent years has revolutionized the field of oncology with unprecedented survival rates in multiple cancer types[39]. Tumor escape and immune coordination are related to the recurrence of CRC, and major discoveries about the immune response in the recurrence of CRC have been made[40]. In the future, the combination therapy of mFOLFOX6 with immunotherapy will be chosen as the conversion treatment for CRLM. Unfortunately, this patient had not undergone next-generation sequencing to determine the status of immunotherapy markers in the past. Therefore, we could not determine the possibility of immunotherapy.
We report a case of initially unresectable advanced colon cancer with liver metastases that were successfully converted into resectable CRLM using multidisciplinary strategies, including mFOLFOX6+Endostar® and TACE. Surprisingly, a pCR to liver metastases was achieved. By the time of submission of this paper, the patient's OS time had exceeded 9 years. This strategy may help improve the resectability of initially unresectable CRLM and prolong OS.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68834] [Article Influence: 13766.8] [Reference Citation Analysis (202)] |
| 2. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2268] [Cited by in RCA: 3367] [Article Influence: 561.2] [Reference Citation Analysis (3)] |
| 3. | Pinson H, Cosyns S, Ceelen WP. The impact of surgical resection of the primary tumor on the development of synchronous colorectal liver metastasis: a systematic review. Acta Chir Belg. 2018;118:203-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Bellier J, De Wolf J, Hebbar M, Amrani ME, Desauw C, Leteurtre E, Pruvot FR, Porte H, Truant S. Repeated Resections of Hepatic and Pulmonary Metastases from Colorectal Cancer Provide Long-Term Survival. World J Surg. 2018;42:1171-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Dörr NM, Bartels M, Morgul MH. Current Treatment of Colorectal Liver Metastasis as a Chronic Disease. Anticancer Res. 2020;40:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Miller CL, Taylor MS, Qadan M, Deshpande V, Worthington S, Smalley R, Collura C, Ryan DP, Allen JN, Blaszkowsky LS, Clark JW, Murphy JE, Parikh AR, Berger D, Tanabe KK, Lillemoe KD, Ferrone CR. Prognostic Significance of Surgical Margin Size After Neoadjuvant FOLFOX and/or FOLFIRI for Colorectal Liver Metastases. J Gastrointest Surg. 2017;21:1831-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Allard MA, Malka D. Place of hepatic intra-arterial chemotherapy in the treatment of colorectal liver metastases. J Visc Surg. 2014;151 Suppl 1:S21-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Fiorentini G, Aliberti C, Mulazzani L, Coschiera P, Catalano V, Rossi D, Giordani P, Ricci S. Chemoembolization in colorectal liver metastases: the rebirth. Anticancer Res. 2014;34:575-584. [PubMed] |
| 9. | Joo JH, Park JH, Kim JC, Yu CS, Lim SB, Park IJ, Kim TW, Hong YS, Kim KP, Yoon SM, Park J, Kim JH. Local Control Outcomes Using Stereotactic Body Radiation Therapy for Liver Metastases From Colorectal Cancer. Int J Radiat Oncol Biol Phys. 2017;99:876-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Baba K, Oshita A, Kohyama M, Inoue S, Kuroo Y, Yamaguchi T, Nakamura H, Sugiyama Y, Tazaki T, Sasaki M, Imamura Y, Daimaru Y, Ohdan H, Nakamitsu A. Successful treatment of conversion chemotherapy for initially unresectable synchronous colorectal liver metastasis. World J Gastroenterol. 2015;21:1982-1988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Zhao X, Pan J, Song Y, Ding H, Qin L, Pan Y. Recombined human endostatin (Endostar) enhances cisplatin delivery and potentiates chemotherapy by decompressing colorectal cancer vessels. Int J Clin Exp Pathol. 2017;10:10792-10801. [PubMed] |
| 12. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6096] [Cited by in RCA: 7507] [Article Influence: 682.5] [Reference Citation Analysis (2)] |
| 13. | Meijerink MR, Puijk RS, van Tilborg AAJM, Henningsen KH, Fernandez LG, Neyt M, Heymans J, Frankema JS, de Jong KP, Richel DJ, Prevoo W, Vlayen J. Radiofrequency and Microwave Ablation Compared to Systemic Chemotherapy and to Partial Hepatectomy in the Treatment of Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cardiovasc Intervent Radiol. 2018;41:1189-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 14. | Yuan P, Zhang Z, Kuai J. Analysis on efficacy and safety of TACE in combination with RFA and MWA in the treatment of middle and large primary hepatic carcinoma. J BUON. 2019;24:163-170. [PubMed] |
| 15. | Arnold D, Seufferlein T. Targeted treatments in colorectal cancer: state of the art and future perspectives. Gut. 2010;59:838-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | de Haas RJ, Wicherts DA, Andreani P, Pascal G, Saliba F, Ichai P, Adam R, Castaing D, Azoulay D. Impact of expanding criteria for resectability of colorectal metastases on short- and long-term outcomes after hepatic resection. Ann Surg. 2011;253:1069-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Nishina T, Takano Y, Denda T, Yasui H, Takeda K, Ura T, Esaki T, Okuyama Y, Kondo K, Takahashi Y, Sugiyama Y, Muro K. A phase II clinical study of mFOLFOX6 plus bevacizumab as first-line therapy for Japanese advanced/recurrent colorectal cancer patients. Jpn J Clin Oncol. 2013;43:1080-1086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Chen Z, Guo W, Cao J, Lv F, Zhang W, Qiu L, Li W, Ji D, Zhang S, Xia Z, Wang J, Li J. Endostar in combination with modified FOLFOX6 as an initial therapy in advanced colorectal cancer patients: a phase I clinical trial. Cancer Chemother Pharmacol. 2015;75:547-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Jia Y, Liu M, Huang W, Wang Z, He Y, Wu J, Ren S, Ju Y, Geng R, Li Z. Recombinant human endostatin endostar inhibits tumor growth and metastasis in a mouse xenograft model of colon cancer. Pathol Oncol Res. 2012;18:315-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Li BL, Hu XL, Zhao XH, Sun HG, Zhou CY, Zhang Y. Endostar combined with irinotecan/calcium folinate/5-fluorouracil (FOLFIRI) for treating advanced colorectal cancer: A clinical study. J Chemother. 2015;27:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Han B, Xiu Q, Wang H, Shen J, Gu A, Luo Y, Bai C, Guo S, Liu W, Zhuang Z, Zhang Y, Zhao Y, Jiang L, Zhou J, Jin X. A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy of paclitaxel-carboplatin alone or with endostar for advanced non-small cell lung cancer. J Thorac Oncol. 2011;6:1104-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Sun L, Ye HY, Zhang YH, Guan YS, Wu H. Epidermal growth factor receptor antibody plus recombinant human endostatin in treatment of hepatic metastases after remnant gastric cancer resection. World J Gastroenterol. 2007;13:6115-6118. [PubMed] [DOI] [Full Text] |
| 23. | Schefter TE, Kavanagh BD, Timmerman RD, Cardenes HR, Baron A, Gaspar LE. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys. 2005;62:1371-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 304] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 24. | Hoffmann K, Glimm H, Radeleff B, Richter G, Heining C, Schenkel I, Zahlten-Hinguranage A, Schirrmacher P, Schmidt J, Büchler MW, Jaeger D, von Kalle C, Schemmer P. Prospective, randomized, double-blind, multi-center, Phase III clinical study on transarterial chemoembolization (TACE) combined with Sorafenib versus TACE plus placebo in patients with hepatocellular cancer before liver transplantation - HeiLivCa [ISRCTN24081794]. BMC Cancer. 2008;8:349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5115] [Cited by in RCA: 5989] [Article Influence: 108.9] [Reference Citation Analysis (1)] |
| 26. | Liao WJ, Shen P, Wu Wy, Shi M, Luo RC. [Short-term therapeutic effect and safety of endostar combined with XELIRI regimen in the treatment of advanced colorectal cancer]. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30:813-814. [PubMed] |
| 27. | Emmanouilides C, Sfakiotaki G, Androulakis N, Kalbakis K, Christophylakis C, Kalykaki A, Vamvakas L, Kotsakis A, Agelaki S, Diamandidou E, Touroutoglou N, Chatzidakis A, Georgoulias V, Mavroudis D, Souglakos J. Front-line bevacizumab in combination with oxaliplatin, leucovorin and 5-fluorouracil (FOLFOX) in patients with metastatic colorectal cancer: a multicenter phase II study. BMC Cancer. 2007;7:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Jin Y, Wei L, Jiang Q, Song X, Teng C, Fan C, Lv Y, Liu Y, Shen W, Li L, Huang D, Xin T. Comparison of efficacy and toxicity of bevacizumab, endostar and apatinib in transgenic and human lung cancer xenograftzebrafish model. Sci Rep. 2018;8:15837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Ling Y, Yang Y, Lu N, You QD, Wang S, Gao Y, Chen Y, Guo QL. Endostar, a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of endothelial cells. Biochem Biophys Res Commun. 2007;361:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 199] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 30. | Guan Y, Li A, Xiao W, Liu S, Chen B, Lu T, Zhao C, Han F. The efficacy and safety of Endostar combined with chemoradiotherapy for patients with advanced, locally recurrent nasopharyngeal carcinoma. Oncotarget. 2015;6:33926-33934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Folkman J. Antiangiogenesis in cancer therapy--endostatin and its mechanisms of action. Exp Cell Res. 2006;312:594-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 472] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 32. | Xu HX, Huang XE, Qian ZY, Xu X, Li Y, Li CG. Clinical observation of Endostar® combined with chemotherapy in advanced colorectal cancer patients. Asian Pac J Cancer Prev. 2011;12:3087-3090. [PubMed] |
| 33. | Quénet F, Pissas MH, Gil H, Roca L, Carrère S, Sgarbura O, Rouanet P, de Forges H, Khellaf L, Deshayes E, Ychou M, Bibeau F. Two-stage hepatectomy for colorectal liver metastases: Pathologic response to preoperative chemotherapy is associated with second-stage completion and longer survival. Surgery. 2019;165:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Leal JN, Bressan AK, Vachharajani N, Gonen M, Kingham TP, D'Angelica MI, Allen PJ, DeMatteo RP, Doyle MB, Bathe OF, Greig PD, Wei A, Chapman WC, Dixon E, Jarnagin WR. Time-to-Surgery and Survival Outcomes in Resectable Colorectal Liver Metastases: A Multi-Institutional Evaluation. J Am Coll Surg. 2016;222:766-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Lok HT, Fung AKY, Chong CCN, Lee KF, Wong J, Cheung SYS, Lai PBS, Ng KKC. Comparison of long-term survival outcome after curative hepatectomy between selected patients with non-colorectal and colorectal liver metastasis: A propensity score matching analysis. Asian J Surg. 2021;44:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Yan TD, Sim J, Black D, Niu R, Morris DL. Systematic review on safety and efficacy of repeat hepatectomy for recurrent liver metastases from colorectal carcinoma. Ann Surg Oncol. 2007;14:2069-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Ushigome M, Funahashi K, Yoshida K, Koda T, Miura Y, Kagami S, Kaneko T, Nagashima Y, Terahara A. [Five-Year Local Control by Stereotactic Body Radiation Therapy (SBRT) for Liver Metastasis from Colorectal Cancer-A Case Report]. Gan To Kagaku Ryoho. 2019;46:1981-1983. [PubMed] |
| 38. | Flamarique S, Campo M, Asín G, Pellejero S, Viúdez A, Arias F. Stereotactic body radiation therapy for liver metastasis from colorectal cancer: size matters. Clin Transl Oncol. 2020;22:2350-2356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Baimas-George M, Baker E, Kamionek M, Salmon JS, Sastry A, Levi D, Vrochides D. A Complete Pathological Response to Pembrolizumab following ex vivo Liver Resection in a Patient with Colorectal Liver Metastases. Chemotherapy. 2018;63:90-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Camus M, Tosolini M, Mlecnik B, Pagès F, Kirilovsky A, Berger A, Costes A, Bindea G, Charoentong P, Bruneval P, Trajanoski Z, Fridman WH, Galon J. Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res. 2009;69:2685-2693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 229] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Osera S, Japan; Surlin VM, Romania; Teragawa H, Japan S-Editor: Wang DM L-Editor: A P-Editor: Wang DM