Published online Mar 6, 2023. doi: 10.12998/wjcc.v11.i7.1458
Peer-review started: November 23, 2022
First decision: January 5, 2023
Revised: January 23, 2023
Accepted: February 13, 2023
Article in press: February 13, 2023
Published online: March 6, 2023
Processing time: 99 Days and 2.6 Hours
Lymphoma, which is highly malignant, stems from lymph nodes and lymphoid tissue. Lymphoma cells express programmed death-ligand 1/2 (PD-L1/PD-L2), which binds with programmed cell death 1 protein (PD-1) to establish inhibitory signaling that impedes the normal function of T cells and allows tumor cells to escape immune system surveillance. Recently, immune checkpoint inhibitor immunotherapies such as PD-1 inhibitors (nivolumab and pembrolizumab) have been introduced into the lymphoma treatment algorithm and have shown remarkable clinical efficacy and greatly improve prognosis in lymphoma patients. Accordingly, the number of lymphoma patients who are seeking treatment with PD-1 inhibitors is growing annually, which results in an increasing number of patients developing immune-related adverse events (irAEs). The occurrence of irAEs inevitably affects the benefits provided by immunotherapy, particularly when PD-1 inhibitors are applied. However, the mechanisms and characteristics of irAEs induced by PD-1 inhibitors in lymphoma need further investigation. This review article summarizes the latest research advances in irAEs during treatment of lymphoma with PD-1 inhibitors. A comprehensive understanding of irAEs incurred in immunotherapy can help to achieve better efficacy with PD-1 inhibitors in lymphoma.
Core Tip: Much work on immune checkpoint immunotherapy as cancer therapy has been made in recent years. In lymphoma, the immune checkpoint pathway is used to evade the host immune system and suppress immune cell function. Use of programmed cell death 1 protein (PD-1) inhibitors in lymphoma is supported by their unprecedented clinical efficacy in a range of tumors. Lymphoma patients treated with PD-1 inhibitors inevitably experience immune-related adverse events (irAEs), ranging from mild to life-threatening. Although irAEs can be managed with therapy, severe irAEs may necessitate suspension or even interruption of patient-beneficial PD-1 antibody therapy. It is essential that clinicians take adequate care when irAEs occur.
- Citation: Hou YZ, Zhang Q, Bai H, Wu T, Chen YJ. Immune-related adverse events induced by programmed death protein-1 inhibitors from the perspective of lymphoma immunotherapy. World J Clin Cases 2023; 11(7): 1458-1466
- URL: https://www.wjgnet.com/2307-8960/full/v11/i7/1458.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i7.1458
Lymphoma, a malignant tumor of the lymphohematopoietic system, comprises a variety of subtypes, some of which have a complicated pathogenesis, and progression of the disease is associated with immune dysfunction.
Immune checkpoint inhibitor (ICI) immunotherapy is an effective therapeutic strategy for lymphoma patients because it can suppress tumor immune escape signaling pathways such as programmed cell death 1 protein (PD-1)[1]. Programmed death-ligand 1 (PD-L1) and programmed death-ligand 2 (PD-L2) are expressed on lymphoma cells, and binding with PD-1 on T cells invokes inhibitory signal transduction, reducing cytotoxicity and inducing T-cell exhaustion, which eventually results in immunological escape by lymphoma cells[2]. One of the representative ICI medications is a PD-1 inhibitor. PD-1 inhibitors bind to PD-1 on T lymphocytes, suppressing the PD-1/PD-L1 signaling pathway, promoting T lymphocyte activation and proliferation, and restoring the antitumor effect of T cells[3].
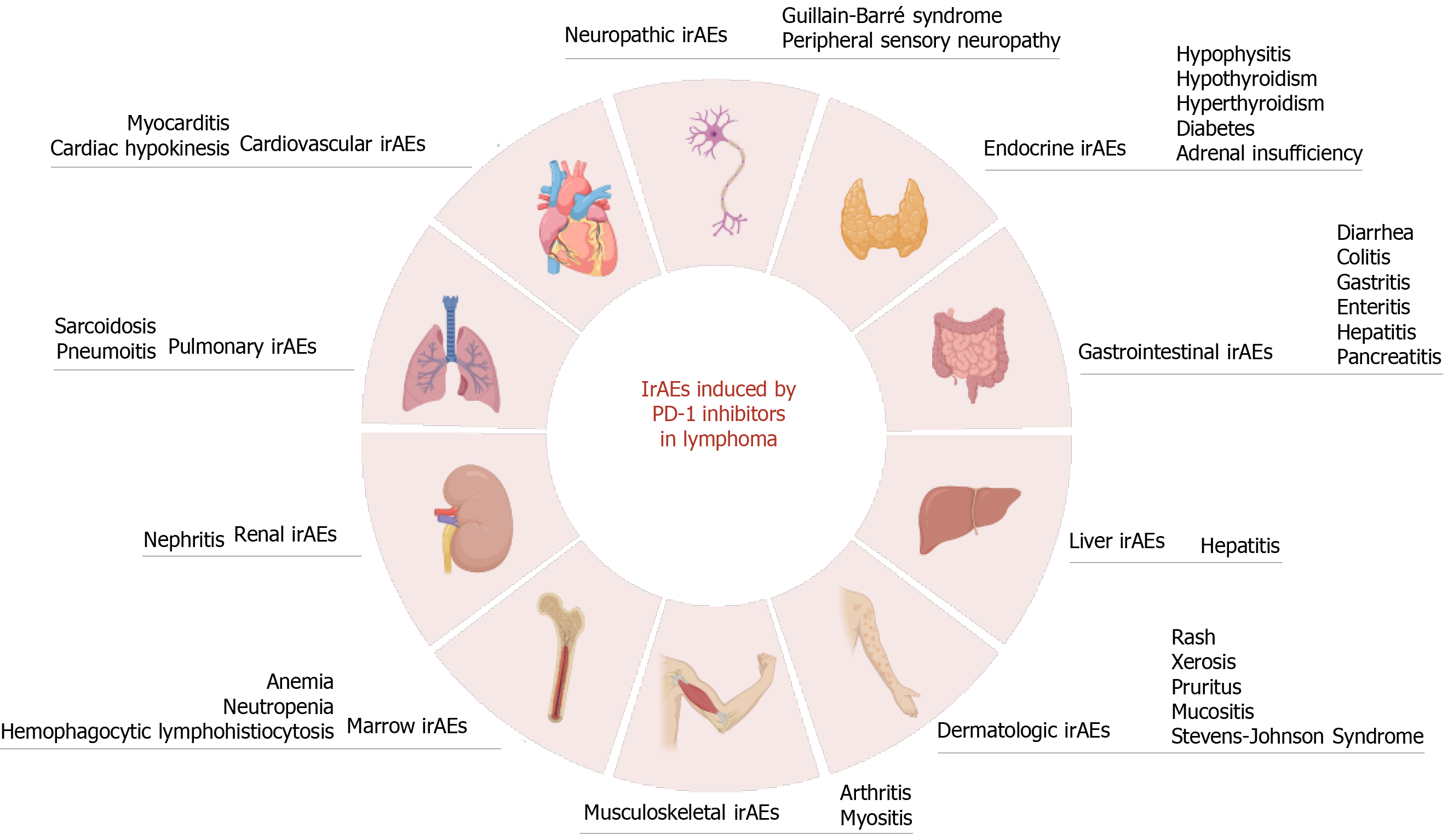
The United States Food and Drug Administration (FDA) approved nivolumab and pembrolizumab in 2016 and 2017, respectively, for treatment of classic Hodgkin lymphoma (cHL), showing outstanding clinical efficacy and substantially improving prognosis in patients. Although lymphoma patients do benefit from PD-1 inhibitor therapy, immune-related adverse events (irAEs) should not be neglected. Theoretically, PD-1 inhibitors block the inhibitory signaling pathway of T cells and induce T-cell activation, which might overactivate the immune system, resulting in an imbalance of immune tolerance and thus producing a unique series of toxic effects in multiple systems from T-cell tissue infiltration (Figure 1). As immunotherapy for treatment of hematologic malignancies develops quickly, the rate of irAEs related to various organ systemic toxicity brought on by PD-1 inhibitors for lymphoma is increasing. As a consequence, patients will be compelled to discontinue PD-1 inhibitor therapy early, which would compromise the benefits they receive from ICI therapy. Therefore, clinicians, especially those in hematology, need to be aware of the occurrence of irAEs during treatment of lymphoma patients with PD-1 inhibitors.
Tumor cells shape and regulate the tumor microenvironment (TME), and the prevailing view is that alterations in all immune cell lineages in the TME affect the efficacy of cancer immunotherapy[4]. A better understanding of the tumor TME will provide the necessary assistance for immunotherapy. Tumor cells in the TME aberrantly express PD-L1 and PD-L2. PD-L1 is a transmembrane protein that is abnormally expressed in both solid and hematologic tumors[5]. PD-L1 on tumor cells and the PD-1 receptor form a protein complex that acts as a pair of negative coinhibitory signals and constitutes the PD-1/PD-L1 signaling pathway, which inhibits the proliferation and activation of T cells and allows for evasion of the body's antitumor response[6]. This complex also enhances the resistance of tumor cells to proapoptotic signals, activates oncogenic signaling pathways (e.g., ERK and mTOR), and promotes tumor cell proliferation and drug resistance[7,8].
ICIs, monoclonal antibodies targeting certain immune checkpoints, for example, anti-cytotoxic T lymphocyte antigen-4 (CTLA-4) antibodies and anti-PD-1 antibodies, have essentially expanded our toolkit against cancer, including lymphoma. PD-1 inhibitors bind to PD-1 on T lymphocytes, prevent PD-1 from binding to its ligand, enhance tumor-specific central memory T lymphocyte metastasis, increase the number of CD8+ T lymphocytes in tumors, restore the cytotoxicity of T cells and enhance the T-cell-killing effect against lymphoma[9]. In addition, factors such as the TME, expression of PD-L1 and PD-L1 gene deletion need to be considered before PD-1 inhibitors are administered. cHL results in increased expression of PD-L1 Ligands on tumor cells in the TME through amplification and translocation of the 9p24.1 Locus and a high response rate to PD-1 inhibitors; in diffuse large B-cell lymphoma (DLBCL), the 9p24.1 alteration and PD-L1 expression frequency are usually low, and thus PD-1 inhibitors are not recommended[10].
Current monoclonal antibody studies mainly focus on the PD-1/PD-L1 signaling pathway. Anti-PD-1 antibodies have been proven to prevent the binding of PD-1 to PD-L1 and PD-L2. This inhibition has shown promise in the treatment of a wide variety of cancers and other diseases and is an important step forward in the development of effective immunotherapies. The FDA has authorized PD-1 inhibitors (nivolumab, pembrolizumab, etc.) for treatment of cHL, non-small cell lung cancer, melanoma, and other malignancies in the past decade[11,12]. In addition, China's National Medical Products Administration approved camrelizumab in 2019 for treatment of relapsed/refractory cHL (R/R cHL)[13]. Together, these PD-1 inhibitors, which significantly boost the T-cell immune response, lead to a strong objective response rate (ORR) in the treatment of R/R HL patients, along with substantial clinical efficacy and a superior prognosis[14].
Here, we primarily focus on the research status of immunotherapy with nivolumab, pembrolizumab, and camrelizumab monoclonal antibodies in patients with lymphoma. In a multicenter phase 1/2 trial on nivolumab that enrolled 64 patients with R/R HL, Diefenbach et al[15] evaluated the safety and effectiveness of nivolumab in these patients. The ORR was 89% (95%CI 65-99) in the nivolumab group, and the complete response (CR) was 61% (95%CI 36-83) with a median follow-up of 2.4 years[15]. Kuruvilla et al[16] conducted a study among 151 patients with R/R cHL who were ineligible for autologous hematopoietic stem cell transplantation or who experienced relapse after transplantation, and the median progression-free survival with pembrolizumab was 13.2 mo (95%CI 10.9-19.4), with significant clinical improvement and safety. Decitabine with camrelizumab provided considerable antitumor efficacy and safety for patients with R/R HL according to a study by Nie et al[17] published in 2019. The median time to CR was 2.85 mo (95%CI 2.81-2.89), and the ORR and CR in the most patients were 95% and 71%, respectively. Currently, PD-1 inhibitors are mainly used to treat R/R HL patients. Despite significant clinical efficacy, sustained remission rates are unsatisfactory. Moreover, PD-1 inhibitors block the inhibitory signaling pathway of T cells, which can overactivate the immune system, unbalance immune tolerance, and lead to the occurrence of irAEs.
From a pathophysiological point of view, PD-1 inhibitors produce specific toxicity profiles in lymphoma. The specific pathophysiological mechanisms by which PD-1 inhibitors trigger irAEs are not fully understood but should be associated with the role played by the PD-1/PD-L1 signaling pathway in the maintenance of immune balance. The current hypothesis is that diminished autoimmune tolerance, proliferation of activated T-cell-, T-cell- and antigen-presenting cell-mediated responses, and release of other proinflammatory cytokines (e.g., IL-6 and IL-17) cause and provoke the occurrence of auto irAEs[18].
Interestingly, PD-1 inhibitors and CTLA-4 inhibitors probably produce irAEs through different mechanisms. In general, CTLA-4 antibodies enhance T-cell action at the beginning of the immune response, whereas PD-1 inhibitors act at a later step by reactivating T-cell response[19]. Notably, the incidence and characteristics of irAEs associated with PD-1 inhibitors depend on the patient's profile, baseline overall tumor burden, and high PD-L1 expression[20].
In addition, the irAEs induced by PD-1 inhibitors show a unique signature. The occurrence of irAEs predict better overall survival for patients using anti-PD-1 therapy in a manner unrelated to anti-CTLA4 therapy[21]. However, this must be clearly confirmed in prospective studies. Clinical manifestations of irAEs can vary widely, from mild to life-threatening, and occur at any time during treatment. The most common irAEs include rash, diarrhea, colitis, hepatitis, and endocrinopathies. Although most irAEs are manageable with treatment, they can be severe and even life-threatening. Notably, fatal adverse effects (especially cardiovascular and pulmonary toxicity) require urgent investigation, and early recognition and intervention are critical for severe irAEs[22]. Most irAEs are treatable with steroids, and most irAEs are reversible[23]. Depending on the type of irAE, adverse reaction remission times vary.
As irAEs occur prior to regular response assessment and can be very heterogeneous in clinical presentation, it is important for clinicians to identify irAEs early and formulate effective treatment regimens, which are vital to optimize the efficacy of PD-1 inhibitors in lymphoma.
Dermatologic irAEs: Skin toxicity is the most frequent irAE induced by anti-PD-1 antibodies. Pruritus and rash are the most common skin-related adverse effects of PD-1 inhibitors. Compared to anti-CTLA-4 inhibitors, skin damage due to anti-PD-1 inhibitors for lymphoma seems less prevalent and of lower severity, but combining the two medications increases the incidence of dermatologic toxicity[24].
Armand et al[25]'s 2016 study on pembrolizumab for patients with R/R cHL found xerosis in 2% of patients and skin-related toxicity in a few patients. Recently, Armand et al[26] reported that the most common irAE in lymphoma patients treated with nivolumab was rash (12%). Moreover, in patients receiving nivolumab for lymphoma, there were 9 cases (21%) of pruritus and 14 (34%) of skin rash. All of these cases were grade 1-3, and one patient even experienced grade 4 Stevens-Johnson syndrome[15]. Among patients with cHL treated with decitabine-plus-camrelizumab, rash occurred in 5 (12%)[27].
Topical corticosteroids and antihistamines should be administered symptomatically for grade 1 and 2 cutaneous adverse effects. Systemic glucocorticoid treatment is used for grade 3 and grade 4 cutaneous adverse effects, and PD-1 inhibitors are interrupted until ≤ grade 1[28]. Skin complications are often mild and manageable. However, early recognition and management of dermatologic irAEs are critical to ensure the best possible outcome for patients.
Pulmonary irAEs: One of the common side effects of anti-PD-1 antibodies is pulmonary toxicity, which can manifest as reactive airway disease, pneumonitis, or pulmonary fibrosis. Pneumonitis manifestations entail coughing, dyspnea, chest discomfort, and shortness of breath, and the diagnostic method is a high-resolution CT scan[29]. Only in the absence of clinically substantial new lung infection or tumor progression should immune-associated pneumonitis be considered. Interestingly, the incidence of pneumonitis is higher in patients receiving anti-PD-1 antibodies (3%-5%) than in those receiving anti-CTLA-4 antibodies (< 1%)[30]. Pneumonitis can occur at any point during oncologic immunotherapy.
Bonhomme-Faivre et al[31] described a patient with refractory HL who developed interstitial pneumonitis following nivolumab therapy and experienced remission of pneumonia after discontinuing nivolumab for eight months. Four percent of cHL patients who receive nivolumab monotherapy experience grade 1-2 pneumonia[26]. Liu et al[27] found pneumonitis in 10% of patients treated with camrelizumab for the R/R cHL Phase II Study, among whom 5% had grade ≥ 3. Moreover, Maruyama et al[32] first reported a case of emergence of late-onset pulmonary toxicities in lymphoma patients treated with PD-1 inhibitors (nivolumab).
Usually, close monitoring is adequate for grade 1 pneumonitis, glucocorticoid treatment for grades 2 and 3, and steroid pulse therapy for grade 4 pneumonitis. For steroid-refractory pneumonitis, medications such as infliximab and mycophenolate mofetil are available[29]. In the presence of grade 3-4 pneumonitis, withdrawing PD-1 inhibitors is suggested, but clinicians should consider reintroducing PD-1 inhibitors depending on the patient's state.
Digestive system irAEs: Gastrointestinal (GI) irAEs are a common side effect of anti-PD-1 antibody treatment. The most common symptoms include abdominal pain, diarrhea, and colitis. Colitis is more likely to occur with anti-CTLA-4 antibodies and combination therapy than with anti-PD-1 antibodies, with incidences of 10%-25%, 20%, and 1%-5%, respectively[33]. Most GI irAEs occur within the first few weeks of treatment, but they can occasionally occur later.
Phase 2 found that 6% of patients treated with nivolumab experience any grade of diarrhea[32]. Among patients with cHL treated with decitabine-plus-camrelizumab, diarrhea occurred in 4 (12%). Furthermore, typhlitis, colitis, and enteritis can occur with nivolumab, and pembrolizumab can cause colitis, duodenitis, and pancreatitis[34]. No grade 5 GI irAEs have been reported thus far.
Prompt treatment of GI irAEs is essential to minimize the risk of serious complications. Therapy for diarrhea, such as loperamide and electrolyte replacement, is sufficient. Steroid treatment is possible for other GI irAEs, such as prednisolone or budesonide. In steroid-refractory cases, infliximab and vedolizumab are recommended[34].
Another digestive system irAE induced by PD-1 inhibitors is liver-related dysfunction. Immune-related hepatitis is uncommon, but when hepatitis occurs, patients experience high alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin levels, along with fever and fatigue.
Diefenbach et al[15] found a 47%/32% ALT/AST elevation rate in the nivolumab+brentuximab vedotin (BV) group in a phase 1/2 clinical study for R/R cHL patients, with all treatment-related hepatotoxicity of grade 1-2 severity. Seventeen cHL patients were enrolled in Japanese phase II research on nivolumab; by the end of the trial, all had adverse drug responses, with two having grade 1-2 abnormal hepatic function and one having grade 3-4 Liver dysfunction and temporary medication withdrawal[32]. In the study by Armand et al[26] of nivolumab for R/R cHL, the incidence of hepatitis was approximately 5%. A phase II study of pembrolizumab for R/R mature T-cell lymphoma reported grade 1-2 abnormal hepatic function in 6% of patients[35].
Hepatitis induced by PD-1 inhibitors is treated routinely with steroids. Mycophenolate mofetil is an excellent choice if the patient's symptoms have not improved after three days. Weekly AST, ALT, and bilirubin tests are also needed[36].
Endocrine irAEs: PD-1 inhibitors can cause endocrine toxicity, which is a type of side effect that can occur when the body's hormones are disrupted. The most common side effects of PD-1 inhibitors are hypothyroidism and thyroiditis. Other endocrine side effects include adrenal insufficiency, hypophysitis, and diabetes.
Pembrolizumab was used to treat 31 individuals with R/R cHL in a phase 2 trial, and the most frequent adverse event was hypothyroidism (16%)[25]. Nivolumab induces 29% of grade 1-2 hypothyroidism in R/R cHL, according to Maruyama et al[32]. Furthermore, nivolumab, pembrolizumab, and camrelizumab for lymphoma are associated with less hyperthyroidism, adrenal insufficiency, and diabetic endocrine irAEs[34].
Endocrine disruption during PD-1 inhibitor treatment is usually mild to moderate (grade 1 and 2). Thyroid hormone replacement therapy or beta-blockers are typically effective treatments for thyroid toxicity[37]. In rare cases, patients may require surgery to remove the thyroid gland. However, treatment of endocrine toxicity usually involves managing symptoms and monitoring the patient's hormone levels.
Rare irAEs: Rare adverse reactions can be severe. Some of the rare toxicities of PD-1 inhibitors include cardiotoxicity, nephrotoxicity, and neurotoxicity[38].
Following treatment with nivolumab and an allogeneic stem cell transplant, Charles et al[39] described a case of a cHL patient who had severe and eventually fatal multisystem organ failure, including acute pneumonia, widespread rash, myositis, colitis, hepatic cytolysis, acute renal failure, pancreatitis, and complete heart block. In a case study, Bonhomme-Faivre et al[31] described a refractory HL patient who, upon therapy with nivolumab, experienced global cardiac hypokinesis. However, the patient's cardiopathy recovered after interruption of nivolumab for eight months[31]. Ansell et al[40] found that 4% of DLBCL patients develop grade 3 to 4 nephritis and renal dysfunction after treatment with nivolumab. Rare irAEs induced by PD-1 inhibitors include rheumatic irAEs, peripheral sensory neuropathy, hemophagocytic lymphohistiocytosis, and Guillain-Barré syndrome[34].
These side effects can be serious, and patients should be monitored closely for them. Additionally, there are no guidelines or sizable clinical trials to direct how patients should utilize PD-1 inhibitors to restart therapy after recovering from severe irAEs. There is still much to learn about irAEs, and more research is needed in this area.
A current study suggests that cancer patients treated with PD-1 inhibitors may have an increased incidence of cutaneous toxic irAEs after coronavirus disease 2019 (COVID-19) vaccination[41]. Qi et al[42] indicated that cancer patients treated with PD-1 inhibitors had an increased incidence of grade 1 and 2 irAEs after COVID-19 vaccination.
Until now, there are few data regarding the implications of COVID-19 vaccination on the safety and efficacy of PD-1 inhibitor treatment against lymphoma. However, many patients with hematologic malignancies risk not producing antibodies after vaccination with mRNA SARS-CoV-2 vaccines, and considerable populations of vaccinated blood cancer patients (including lymphoma) are at high risk of COVID-19[43]. Additionally, in the context of the ongoing COVID-19 pandemic, immunocompromised lymphoma patients are more vulnerable to infection. It is unclear whether COVID-19 impacts irAEs, though risk of death is higher in tumor patients with COVID-19.
Therefore, further studies are needed to corroborate whether COVID-19 vaccination or infection with COVID-19 in lymphoma patients treated with PD-1 inhibitors impacts irAEs and establish immunotherapy regimens for protecting this vulnerable population of patients with hematologic malignancies.
In recent years, our understanding of recognizing and treating irAEs has increased along with use of immunotherapeutic drugs such as PD-1 inhibitors in hematologic malignancies. However, irAEs induced by PD-1 inhibitors are different in hematologic and solid tumors. PD-1 inhibitors may increase risk of concurrent irAEs in hematologic malignancies. Therefore, it is imperative for patients and their caregivers to be aware of the signs and symptoms of irAEs and to know when to seek medical help. There is no one-size-fits-all approach to managing irAEs, and treatment will vary depending on the individual patient's situation. Nevertheless, common management concepts include early detection and treatment of irAEs, frequent patient monitoring, and personalized treatment strategies[44].
First, corticosteroid therapy and immunosuppressive drugs such as tacrolimus, infliximab, and mycophenolate mofetil should be administered. Additionally, the doctor should decide whether to stop using PD-1 inhibitors based on the severity of the disease. Second, although severe cases of irAEs are rare, they do occur and are usually treated with high-dose corticosteroids or other immunosuppressive agents while PD-1 inhibitors are permanently discontinued. Finally, as PD-1 inhibitor monotherapy and PD-1 inhibitor combination therapy are applied more frequently in hematologic cancers, irAEs will increase in frequency. Early detection is critical for better treating irAE patients and increasing quality of life.
There is still much to learn about why and how irAEs develop. Identifying biomarkers that can predict who is most likely to experience irAEs and how severe they may be is an important area of future research. In addition, developing immunosuppressive treatments that address toxicity without interfering with the therapeutic benefits of anti-PD-1 antibody immunotherapy is a key goal.
Altogether, PD-1 inhibitors exhibit good overall efficacy with few adverse effects for lymphoma treatment. However, given the nonspecific activation of the PD-1/PD-L1 signaling pathway in vivo by PD-1 inhibitors, PD-1 inhibitors for lymphoma can lead to irAEs. A deeper understanding of the pathophysiology of PD-1 inhibitors and identifying reliable biomarkers to predict the efficacy and toxicity of treatment are crucial goals. Such knowledge would enable clinicians to select patients who may respond to treatment more accurately and to tailor treatment plans to individual patients. There is still room for more investigation and discussion on the safe and efficient use of PD-1 inhibitors in lymphoma and ways to avoid and mitigate irAEs.
We sincerely thank the first clinical college of Gansu University of Chinese medicine for providing account number to allow us access to global database support.
| 1. | Takiar R, Karimi Y. Novel Salvage Therapy Options for Initial Treatment of Relapsed/Refractory Classical Hodgkin's Lymphoma: So Many Options, How to Choose? Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Saber MM. Diagnostic Performance of PD-L1 versus PD-1 Expression in Circulating CD20 Cells in Diffuse Large B-Cell Lymphoma. Antibodies (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Chen X, Kong H, Luo L, Han S, Lei T, Yu H, Guo N, Li C, Peng S, Dong X, Yang H, Wu M. High efficacy of PD-1 inhibitor after initial failure of PD-L1 inhibitor in Relapsed/Refractory classical Hodgkin Lymphoma. BMC Cancer. 2022;22:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. 2021;21:345-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 974] [Article Influence: 194.8] [Reference Citation Analysis (0)] |
| 5. | Mansorunov D, Apanovich N, Apanovich P, Kipkeeva F, Muzaffarova T, Kuzevanova A, Nikulin M, Malikhova O, Karpukhin A. Expression of Immune Checkpoints in Malignant Tumors: Therapy Targets and Biomarkers for the Gastric Cancer Prognosis. Diagnostics (Basel). 2021;11:2370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 6. | Bolandi N, Derakhshani A, Hemmat N, Baghbanzadeh A, Asadzadeh Z, Afrashteh Nour M, Brunetti O, Bernardini R, Silvestris N, Baradaran B. The Positive and Negative Immunoregulatory Role of B7 Family: Promising Novel Targets in Gastric Cancer Treatment. Int J Mol Sci. 2021;22:10719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 7. | Cao Y, Liang W, Fang L, Liu MK, Zuo J, Peng YL, Shan JJ, Sun RX, Zhao J, Wang J. PD-L1/PD-L1 signalling promotes colorectal cancer cell migration ability through RAS/MEK/ERK. Clin Exp Pharmacol Physiol. 2022;49:1281-1293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 8. | Zhao Q, Guo J, Zhao Y, Shen J, Kaboli PJ, Xiang S, Du F, Wu X, Li M, Wan L, Li X, Wen Q, Li J, Zou C, Xiao Z. Comprehensive assessment of PD-L1 and PD-L2 dysregulation in gastrointestinal cancers. Epigenomics. 2020;12:2155-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | Xia W, Zhang S, Duan H, Wang C, Qian S, Shen H. The combination therapy of Everolimus and anti-PD-1 improves the antitumor effect by regulating CD8(+) T cells in bladder cancer. Med Oncol. 2022;39:37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 10. | Xie W, Medeiros LJ, Li S, Yin CC, Khoury JD, Xu J. PD-1/PD-L1 Pathway and Its Blockade in Patients with Classic Hodgkin Lymphoma and Non-Hodgkin Large-Cell Lymphomas. Curr Hematol Malig Rep. 2020;15:372-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Halahleh K, Al Sawajneh S, Saleh Y, Shahin O, Abufara A, Ma'koseh M, Abdel-Razeq R, Barakat F, Abdelkhaleq H, Al-Hassan N, Atiyyat R, Al-Faker N, Omari Z, Ghatasheh H, Jaradat I, Muradi I, Iyad S, Bazarbachi A. Pembrolizumab for the Treatment of Relapsed and Refractory Classical Hodgkin Lymphoma After Autologous Transplant and in Transplant-Naïve Patients. Clin Lymphoma Myeloma Leuk. 2022;22:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Goto Y, Yoh K, Kato T, Hosomi Y, Usui K, Fukui T, Hirano K, Tanaka H, Taguri M, Kunitoh H. Observational study to predict the efficacy and optimal duration of nivolumab treatment in patients with previously treated advanced or recurrent non-small cell lung cancer. Jpn J Clin Oncol. 2023;53:153-160. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Markham A, Keam SJ. Camrelizumab: First Global Approval. Drugs. 2019;79:1355-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 185] [Article Influence: 26.4] [Reference Citation Analysis (1)] |
| 14. | Yohannan B, Rios A, Buja M. Durable Remission in Hodgkin Lymphoma Treated With One Cycle of Bleomycin, Vinblastine, Dacarbazine and Two Doses of Nivolumab and Brentuximab Vedotin. J Hematol. 2022;11:154-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Diefenbach CS, Hong F, Ambinder RF, Cohen JB, Robertson MJ, David KA, Advani RH, Fenske TS, Barta SK, Palmisiano ND, Svoboda J, Morgan DS, Karmali R, Sharon E, Streicher H, Kahl BS, Ansell SM. Ipilimumab, nivolumab, and brentuximab vedotin combination therapies in patients with relapsed or refractory Hodgkin lymphoma: phase 1 results of an open-label, multicentre, phase 1/2 trial. Lancet Haematol. 2020;7:e660-e670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 16. | Kuruvilla J, Ramchandren R, Santoro A, Paszkiewicz-Kozik E, Gasiorowski R, Johnson NA, Fogliatto LM, Goncalves I, de Oliveira JSR, Buccheri V, Perini GF, Goldschmidt N, Kriachok I, Dickinson M, Komarnicki M, McDonald A, Ozcan M, Sekiguchi N, Zhu Y, Nahar A, Marinello P, Zinzani PL; KEYNOTE-204 investigators. Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): an interim analysis of a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2021;22:512-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 189] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 17. | Nie J, Wang C, Liu Y, Yang Q, Mei Q, Dong L, Li X, Liu J, Ku W, Zhang Y, Chen M, An X, Shi L, Brock MV, Bai J, Han W. Addition of Low-Dose Decitabine to Anti-PD-1 Antibody Camrelizumab in Relapsed/Refractory Classical Hodgkin Lymphoma. J Clin Oncol. 2019;37:1479-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 162] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 18. | Fan Y, Geng Y, Shen L, Zhang Z. Advances on immune-related adverse events associated with immune checkpoint inhibitors. Front Med. 2021;15:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Wei SC, Anang NAS, Sharma R, Andrews MC, Reuben A, Levine JH, Cogdill AP, Mancuso JJ, Wargo JA, Pe'er D, Allison JP. Combination anti-CTLA-4 plus anti-PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc Natl Acad Sci U S A. 2019;116:22699-22709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 264] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 20. | Sumi T, Koshshino Y, Sekikawa M, Nagahisa Y, Matsuura K, Shijubou N, Kamada K, Watanabe H, Michimata H, Nagayama D, Tanaka Y, Yamada Y, Chiba H. Risk factors for severe immune-related adverse events after first-line pembrolizumab monotherapy or combination chemotherapy for non-small-cell lung cancer. Invest New Drugs. 2022;40:1298-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 21. | Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? BMC Med. 2020;18:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 277] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 22. | Murakata Y, Tajiri K. A Diverse Range of Cardiac Adverse Events Associated with Immune Checkpoint Inhibitor Therapy. Intern Med. 2022;61:2099-2100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 23. | Zhang L, Zlotoff DA, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, Thuny F, Zubiri L, Chen CL, Sullivan RJ, Alvi RM, Rokicki A, Murphy SP, Jones-O'Connor M, Heinzerling LM, Barac A, Forrestal BJ, Yang EH, Gupta D, Kirchberger MC, Shah SP, Rizvi MA, Sahni G, Mandawat A, Mahmoudi M, Ganatra S, Ederhy S, Zatarain-Nicolas E, Groarke JD, Tocchetti CG, Lyon AR, Thavendiranathan P, Cohen JV, Reynolds KL, Fradley MG, Neilan TG. Major Adverse Cardiovascular Events and the Timing and Dose of Corticosteroids in Immune Checkpoint Inhibitor-Associated Myocarditis. Circulation. 2020;141:2031-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 204] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 24. | Darnell EP, Mooradian MJ, Baruch EN, Yilmaz M, Reynolds KL. Immune-Related Adverse Events (irAEs): Diagnosis, Management, and Clinical Pearls. Curr Oncol Rep. 2020;22:39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 288] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 25. | Armand P, Shipp MA, Ribrag V, Michot JM, Zinzani PL, Kuruvilla J, Snyder ES, Ricart AD, Balakumaran A, Rose S, Moskowitz CH. Programmed Death-1 Blockade With Pembrolizumab in Patients With Classical Hodgkin Lymphoma After Brentuximab Vedotin Failure. J Clin Oncol. 2016;34:3733-3739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 541] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 26. | Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, Timmerman JM, Collins GP, Ramchandren R, Cohen JB, De Boer JP, Kuruvilla J, Savage KJ, Trneny M, Shipp MA, Kato K, Sumbul A, Farsaci B, Ansell SM. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J Clin Oncol. 2018;36:1428-1439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 394] [Cited by in RCA: 546] [Article Influence: 68.3] [Reference Citation Analysis (1)] |
| 27. | Liu Y, Wang C, Li X, Dong L, Yang Q, Chen M, Shi F, Brock M, Liu M, Mei Q, Liu J, Nie J, Han W. Improved clinical outcome in a randomized phase II study of anti-PD-1 camrelizumab plus decitabine in relapsed/refractory Hodgkin lymphoma. J Immunother Cancer. 2021;9:e002347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 28. | Collins LK, Chapman MS, Carter JB, Samie FH. Cutaneous adverse effects of the immune checkpoint inhibitors. Curr Probl Cancer. 2017;41:125-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 29. | Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, Gerber DE, Hamad L, Hansen E, Johnson DB, Lacouture ME, Masters GA, Naidoo J, Nanni M, Perales MA, Puzanov I, Santomasso BD, Shanbhag SP, Sharma R, Skondra D, Sosman JA, Turner M, Ernstoff MS. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9:e002435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 571] [Article Influence: 114.2] [Reference Citation Analysis (0)] |
| 30. | Shibata Y, Murakami S, Kato T. Overview of checkpoint inhibitor pneumonitis: incidence and associated risk factors. Expert Opin Drug Saf. 2021;20:537-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Bonhomme-Faivre L, Guarino V, Misra SC. Nivolumab-induced pneumonitis and cardiopathy in a patient with relapsed Hodgkin's lymphoma. J Oncol Pharm Pract. 2023;29:479-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 32. | Maruyama D, Terui Y, Yamamoto K, Fukuhara N, Choi I, Kuroda J, Ando K, Hattori A, Tobinai K. Final results of a phase II study of nivolumab in Japanese patients with relapsed or refractory classical Hodgkin lymphoma. Jpn J Clin Oncol. 2020;50:1265-1273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Som A, Mandaliya R, Alsaadi D, Farshidpour M, Charabaty A, Malhotra N, Mattar MC. Immune checkpoint inhibitor-induced colitis: A comprehensive review. World J Clin Cases. 2019;7:405-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 201] [Cited by in RCA: 204] [Article Influence: 29.1] [Reference Citation Analysis (15)] |
| 34. | Hradska K, Hajek R, Jelinek T. Toxicity of Immune-Checkpoint Inhibitors in Hematological Malignancies. Front Pharmacol. 2021;12:733890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Barta SK, Zain J, MacFarlane AW 4th, Smith SM, Ruan J, Fung HC, Tan CR, Yang Y, Alpaugh RK, Dulaimi E, Ross EA, Campbell KS, Khan N, Siddharta R, Fowler NH, Fisher RI, Oki Y. Phase II Study of the PD-1 Inhibitor Pembrolizumab for the Treatment of Relapsed or Refractory Mature T-cell Lymphoma. Clin Lymphoma Myeloma Leuk. 2019;19:356-364.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 36. | Shah P, Sundaram V, Björnsson E. Biologic and Checkpoint Inhibitor-Induced Liver Injury: A Systematic Literature Review. Hepatol Commun. 2020;4:172-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 37. | Yoon JH, Hong AR, Kim HK, Kang HC. Characteristics of Immune-Related Thyroid Adverse Events in Patients Treated with PD-1/PD-L1 Inhibitors. Endocrinol Metab (Seoul). 2021;36:413-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 38. | Iranzo P, Callejo A, Assaf JD, Molina G, Lopez DE, Garcia-Illescas D, Pardo N, Navarro A, Martinez-Marti A, Cedres S, Carbonell C, Frigola J, Amat R, Felip E. Overview of Checkpoint Inhibitors Mechanism of Action: Role of Immune-Related Adverse Events and Their Treatment on Progression of Underlying Cancer. Front Med (Lausanne). 2022;9:875974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 39. | Charles J, Giovannini D, Terzi N, Schwebel C, Sturm N, Masson D, Leccia MT, Cahn JY, Manches O, Bulabois CE, Chaperot L. Multi-organ failure induced by Nivolumab in the context of allo-stem cell transplantation. Exp Hematol Oncol. 2019;8:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Ansell SM, Minnema MC, Johnson P, Timmerman JM, Armand P, Shipp MA, Rodig SJ, Ligon AH, Roemer MGM, Reddy N, Cohen JB, Assouline S, Poon M, Sharma M, Kato K, Samakoglu S, Sumbul A, Grigg A. Nivolumab for Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Patients Ineligible for or Having Failed Autologous Transplantation: A Single-Arm, Phase II Study. J Clin Oncol. 2019;37:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 298] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 41. | Luo B, Li J, Hou X, Yang Q, Zhou Y, Ye J, Wu X, Feng Y, Hu T, Xu Z, He Y, Sun J. Indications for and contraindications of immune checkpoint inhibitors in cancer patients with COVID-19 vaccination. Future Oncol. 2021;17:3477-3484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 42. | Mei Q, Hu G, Yang Y, Liu B, Yin J, Li M, Huang Q, Tang X, Böhner A, Bryant A, Kurts C, Yuan X, Li J. Impact of COVID-19 vaccination on the use of PD-1 inhibitor in treating patients with cancer: a real-world study. J Immunother Cancer. 2022;10:e004157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 43. | Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39:1031-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 44. | Chhabra N, Kennedy J. A Review of Cancer Immunotherapy Toxicity: Immune Checkpoint Inhibitors. J Med Toxicol. 2021;17:411-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Shahriari M, Iran; Watanabe T, Japan S-Editor: Wang LL L-Editor: A P-Editor: Wang LL