Published online Feb 16, 2023. doi: 10.12998/wjcc.v11.i5.1040
Peer-review started: August 30, 2022
First decision: November 25, 2022
Revised: December 7, 2022
Accepted: January 20, 2023
Article in press: January 20, 2023
Published online: February 16, 2023
Processing time: 167 Days and 23.3 Hours
Radiation pneumonitis (RP) is a severe complication of thoracic radiotherapy that may lead to dyspnea and lung fibrosis, and negatively affects patients’ quality of life.
To carry out multiple regression analysis on the influencing factors of radiation pneumonitis.
Records of 234 patients receiving chest radiotherapy in Huzhou Central Hospital (Huzhou, Zhejiang Province, China) from January 2018 to February 2021, and the patients were divided into either a study group or a control group based on the presence of radiation pneumonitis or not. Among them, 93 patients with radiation pneumonitis were included in the study group and 141 without radiation pneumonitis were included in the control group. General characteristics, and radiation and imaging examination data of the two groups were collected and compared. Due to the statistical significance observed, multiple regression analysis was performed on age, tumor type, chemotherapy history, forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), carbon monoxide diffusion volume (DLCO), FEV1/FVC ratio, planned target area (PTV), mean lung dose (MLD), total number of radiation fields, percentage of lung tissue in total lung volume (vdose), probability of normal tissue complications (NTCP), and other factors.
The proportions of patients aged ≥ 60 years and those with the diagnosis of lung cancer and a history of chemotherapy in the study group were higher than those in the control group (P < 0.05); FEV1, DLCO, and FEV1/FVC ratio in the study group were lower than those in the control group (P < 0.05), while PTV, MLD, total field number, vdose, and NTCP were higher than in the control group (P < 0.05). Logistic regression analysis showed that age, lung cancer diagnosis, chemotherapy history, FEV1, FEV1/FVC ratio, PTV, MLD, total number of radiation fields, vdose, and NTCP were risk factors for radiation pneumonitis.
We have identified patient age, type of lung cancer, history of chemotherapy, lung function, and radiotherapy parameters as risk factors for radiation pneumonitis. Comprehensive evaluation and examination should be carried out before radiotherapy to effectively prevent radiation pneumonitis.
Core Tip: Radiotherapy is a common treatment method for malignant tumors. However, it is associated with a high risk of radiation pneumonitis. Therefore, the early prevention of radiation pneumonitis is very important. We retrospectively compared medical records and analyzed the relevant clinical data of 234 patients with and without radiation pneumonitis to demonstrate that the risk factors for radiation pneumonitis include patient age, lung cancer diagnosis, history of chemotherapy, lung function, and radiotherapy parameters. Comprehensive evaluation and examination for each patient should be carried out before radiotherapy to effectively prevent radiation pneumonitis.
- Citation: Shi LL, Yang JH, Yao HF. Multiple regression analysis of risk factors related to radiation pneumonitis. World J Clin Cases 2023; 11(5): 1040-1048
- URL: https://www.wjgnet.com/2307-8960/full/v11/i5/1040.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i5.1040
Radiotherapy is a common treatment method for malignant tumors. About 70.0% of patients with malignant tumors need radiotherapy at a certain stage of the disease[1,2]. However, radiotherapy in patients with breast cancer, lung cancer, esophageal cancer, and other chest malignant tumors is associated with a higher risk of radiation pneumonitis[3,4]. In the process of treatment, radiation can directly damage the vascular endothelial cells and parenchymal cells of the lung, cause inflammatory changes, promote the continuous activation of stromal cells, and lead to irreversible pulmonary fibrosis injury[5]. It may not only affect the smooth implementation of the treatment plan and reduce treatment efficacy, but also increase pain and seriously reduce quality of life of cancer patients[6,7]. Radiation pneumonitis is a common complication of radiotherapy for thoracic tumors, and is mainly caused by radiation-induced damage to the pulmonary vascular endothelial cells and parenchymal cells. It can develop into chronic inflammation and cause pulmonary fibrosis[8]. Radiation pneumonitis symptoms include low fever, irritating dry cough, dyspnea, and even respiratory distress and respiratory failure[9]. While corticosteroid treatment that is often given to patients with radiation pneumonitis can improve the symptoms, it is associated with a risk of pulmonary fibrosis[10]. Therefore, early prevention of radiation pneumonitis, based on the clinical continuous comprehensive analysis of associated risk factors and the formulation of targeted preventative methods, is crucial. The current study retrospectively compared medical records of 234 patients with and without radiation pneumonitis treated at Huzhou Central Hospital, and analyzed the relevant clinical data.
Records of 234 patients (126 males and 108 females) who underwent radiotherapy at Huzhou Central Hospital (Huzhou, Zhejiang Province, China) from January 2018 to February 2021 were collected.
The inclusion criteria were: (1) Receiving intensity modulated conformal radiotherapy for the first time; (2) Complete radiotherapy process; (3) Complete medical records and a follow-up time > 6 mo; (4) Kappa functional status score ≥ 70; and (5) Patients were aware of the study and cooperated with informed consent.
The exclusion criteria were: (1) Radiotherapy for other parts at the same time; (2) Interruption of radiotherapy; (3) Suffering from severe basic lung diseases, such as pulmonary fibrosis; and (4) History of lung surgery.
The Medical Ethics Association of Huzhou Central Hospital approved the study and obtained the informed consent from all enrolled patients (approval number: HZFY-L21035478; date: August 28, 2021).
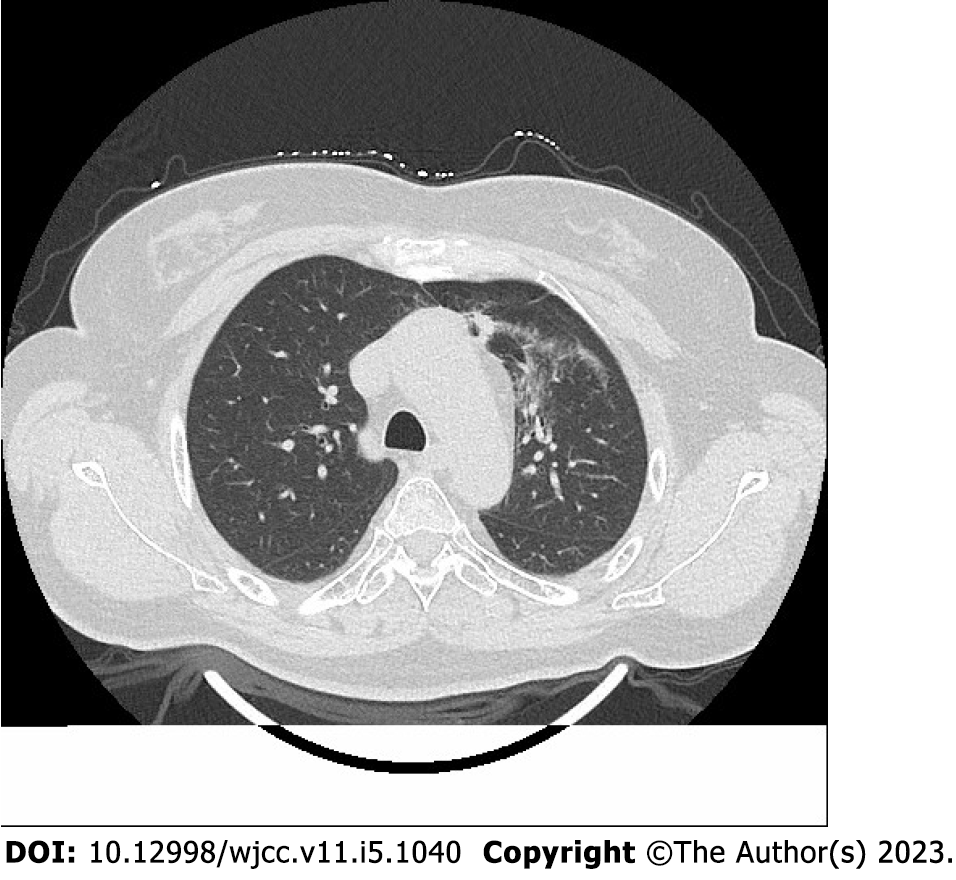
Radiation pneumonitis was diagnosed based on the following criteria[11,12]: (1) Lung irradiation dose > 8 Gy; (2) Within 1-6 mo after irradiation; (3) Chest tightness, low fever, irritating dry cough, dyspnea, and other symptoms; (4) Physical examination showed a decrease in respiratory sound, dry and wet rales could be heard, and the skin at the radiation field had signs of erythema and pigmentation; (5) Laboratory examination showed increased or decreased leukocyte count, and blood gas analysis showed increased carbon dioxide partial pressure and decreased blood oxygen partial pressure; (6) Pulmonary function examination showed that carbon monoxide diffusion volume (DLCO) and ventilation/blood flow ratio decreased, or there was no abnormality; and (7) Chest computed tomography (CT) showed flake ground glass shadow, consolidation shadow, and fiber strip shadow in the irradiated lung field.
Gender, age, smoking history, tumor type, tumour-node-metastasis (TNM) stage, chemotherapy, and operation data of the two groups were collected and recorded. On the end day of radiotherapy, forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), and DLCO of the two groups were measured with a pulmonary function instrument, and the FEV1/FVC ratio was calculated. Concurrently, planned target area (PTV), mean lung dose (MLD), total number of radiation fields, percentage of lung tissue in total lung volume (vdose), and the probability of normal tissue complications (NTCP) were recorded.
General data and laboratory indexes of the two groups were compared. Data were processed with SPSS 22.0 (IBM, Chicago, IL, United States). Categorical data are presented as n (%) and were compared using the χ2 test. Measurement data are presented as the mean ± standard deviation; the t-test was used for measurement data with a normal distribution and the rank sum test was used for those with a non-normal distribution. The risk factors were analyzed by multivariate logistic regression. P < 0.05 was considered statistically significant.
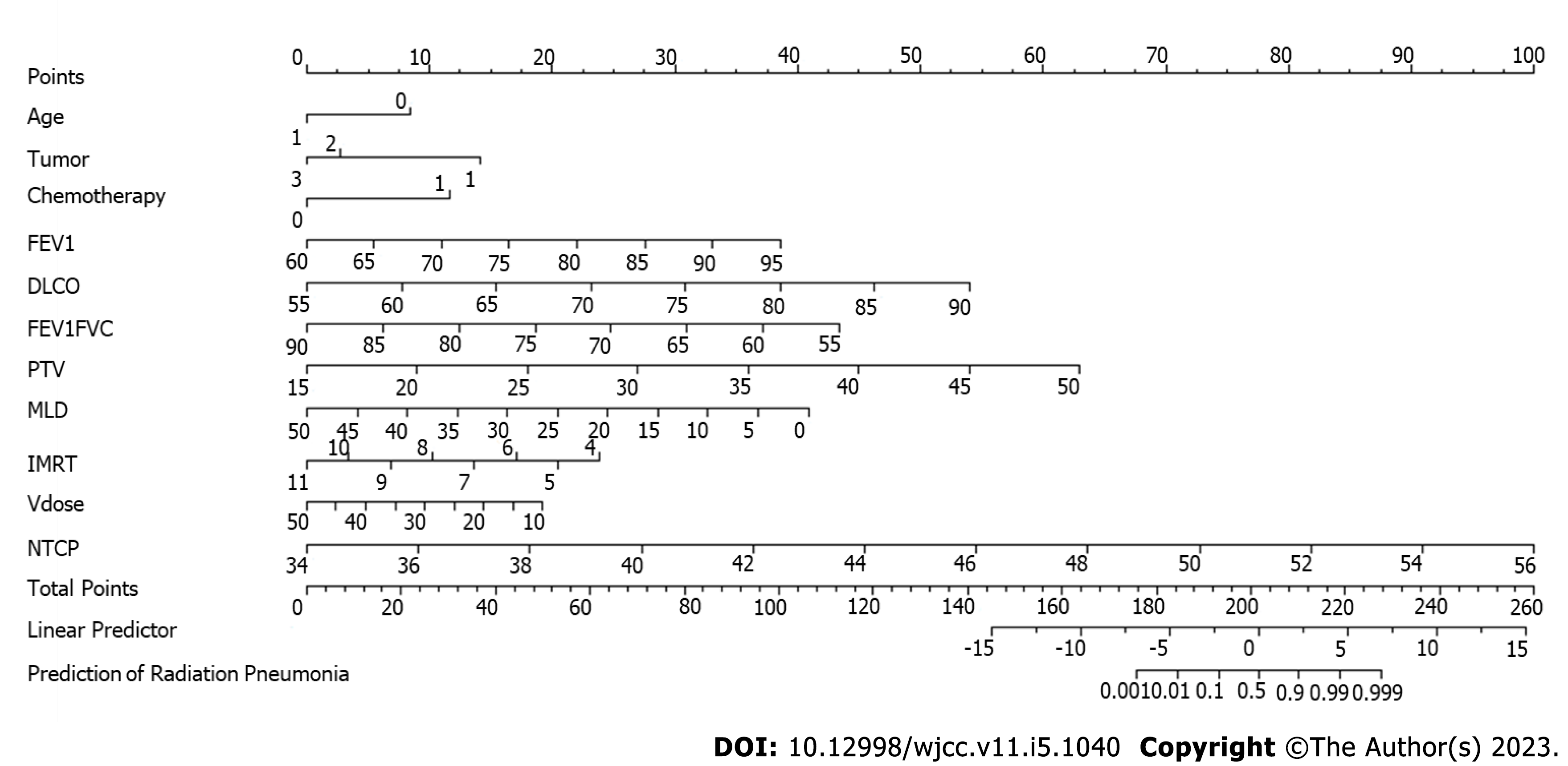
A Kattan-style nomogram was generated as a graphical representation of the logistic regression model used to predict radiation pneumonitis. The position and length of the scale for each independent variable were based on its respective beta-coefficient calibrated against the Points scale at the top[13].
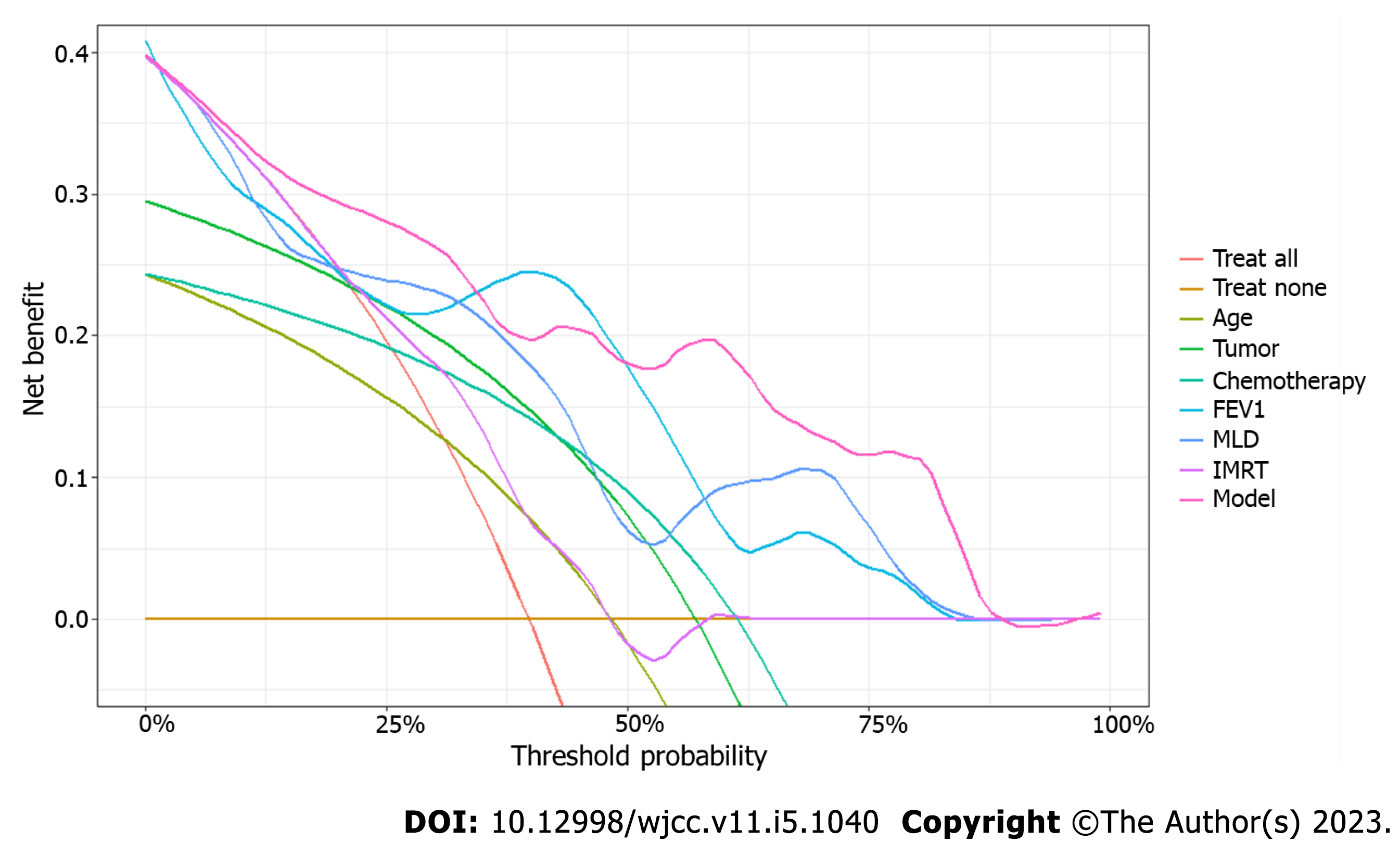
Decision curve analysis was performed to calculate a clinical “net benefit” for the radiation treatment. Net benefit was calculated across a range of threshold probabilities (minimum probability of disease at which treatment would be warranted) as described previously[14]. Prediction by all independent variables in the model was also plotted separately, with continuous variables transformed based on probability. The independent variables in the regression model were assessed for multicollinearity and any variables with a variance inflation factor of 10 or higher were removed. This allowed for a less complex and more applicable decision curve analysis, by limiting the variables that a physician would need to take into consideration when assessing net benefit.
A total of 234 patients (126 males and 108 females) met the inclusion criteria; 93 patients with radiation pneumonitis within 6 mo after radiotherapy were included in the study group. Radiation pneumonitis diagnosis was established as described above and confirmed by chest CT (Figure 1). The remaining 141 patients without radiation pneumonitis within 6 mo were included in the control group. The proportions of patients aged ≥ 60 years and those with a diagnosis of lung cancer, previous chemotherapy history, and operation were higher in the study group than in the control group (P < 0.05). There was no significant difference between the two groups in gender, smoking history, TNM stage, chemotherapy history, and operation (P > 0.05) (Table 1).
| General information | Control group (n = 141) | Study group (n = 93) | χ2 | P | |
| Sex | Male | 70 (49.65) | 56 (60.22) | 2.519 | 0.112 |
| Female | 71 (50.35) | 37 (39.78) | |||
| Age (years) | < 60 | 80 (56.74) | 36 (38.71) | 7.286 | 0.007 |
| ≥ 60 | 61 (43.26) | 57 (61.29) | |||
| Smoking | Yes | 48 (34.04) | 39 (41.94) | 1.495 | 0.221 |
| No | 93 (65.96) | 54 (58.06) | |||
| Tumor type | lung cancer | 52 (36.88) | 69 (74.19) | 36.127 | < 0.001 |
| Mammary cancer | 52 (36.88) | 21 (22.58) | |||
| Esophageal cancer | 37 (26.24) | 3 (2.23) | |||
| TNM stage | II | 43 (30.50) | 39 (41.94) | 5.065 | 0.079 |
| III | 54 (38.30) | 36 (38.71) | |||
| IV | 44 (31.20) | 18 (19.35) | |||
| History of chemotherapy | Yes | 36 (25.53) | 57 (61.29) | 29.921 | < 0.001 |
| No | 105 (74.47) | 36 (38.71) | |||
| Operation | Yes | 47 (33.33) | 39 (41.94) | 1.784 | 0.182 |
| No | 94 (66.67) | 54 (58.06) | |||
FEV1, DLCO, and FEV1/FVC ratio in the study group were lower than those in the control group (P < 0.05), while PTV, MLD, total field number, vdose, and NTCP were higher than those in the control group (P < 0.05) (Table 2).
| Group (n) | FEV1 (%) | DLCO (%) | FEV1/FVC (%) | PTV (mL) | MLD (Gy) | Total number of radiation fields | Vdose (%) | NTCP (%) |
| Control group (n = 141) | 76.89 ± 6.25 | 71.97 ± 10.03 | 72.19 ± 7.44 | 28.26 ± 8.31 | 35.29 ± 6.57 | 7.07 ± 2.07 | 24.54 ± 10.33 | 42.45 ± 3.60 |
| Study group (n = 93) | 70.26 ± 7.50 | 64.19 ± 9.71 | 64.45 ± 7.20 | 38.48 ± 8.27 | 40.71 ± 5.42 | 8.00 ± 1.71 | 36.06 ± 9.95 | 49.29 ± 4.72 |
| t | 7.339 | 5.918 | 7.940 | 9.235 | 6.599 | 3.731 | 8.589 | 12.540 |
| P | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
Taking the above statistically significant factors (age, tumor type, chemotherapy history, FEV1, DLCO, FEV1/FVC ratio, PTV, MLD, total number of radiation fields, vdose, and NTCP) as independent variables and the diagnosis of radiation pneumonitis as a dependent variable, logistic regression analysis was carried out. It was found that age ≥ 60 years old, lung cancer diagnosis, chemotherapy history, FEV1, DLCO, FEV1/FVC ratio, PTV, MLD, total number of radiation fields, and NTCP were risk factors for radiation pneumonitis (Table 3).
| Factor | B | S.E. | Wald | P | OR | 95%CI |
| Age | -2.232 | 0.842 | 7.028 | 0.008 | 0.11 | 0.02-0.48 |
| Tumor type (Lung) | ||||||
| Mammary | -3.038 | 0.938 | 8.313 | 0.001 | 0.05 | 0.01-0.26 |
| Esophageal | -3.753 | 1.075 | 8.313 | < 0.001 | 0.02 | 0.00-0.16 |
| History of chemotherapy | 3.108 | 0.854 | 13.259 | < 0.001 | 22.37 | 4.85-143.77 |
| FEV1 | 0.292 | 0.096 | 9.199 | 0.002 | 1.34 | 1.13-1.65 |
| DLCO | 0.410 | 0.093 | 19.226 | < 0.001 | 1.51 | 1.28-1.85 |
| FEV1/FVC ratio | -0.329 | 0.119 | 7.656 | 0.006 | 0.72 | 0.59-0.90 |
| PTV | 0.477 | 0.149 | 10.206 | 0.001 | 1.61 | 1.23-2.22 |
| MLD | -0.217 | 0.118 | 3.377 | 0.066 | 0.80 | 0.65-1.03 |
| Total number of radiation fields | -0.905 | 0.244 | 13.812 | < 0.001 | 0.40 | 0.23-0.62 |
| Vdose | -0.127 | 0.086 | 2.189 | 0.139 | 0.88 | 0.74-1.04 |
| NTCP | 1.206 | 0.211 | 32.636 | < 0.001 | 3.34 | 2.33-5.41 |
Based on the logistic regression analysis, a nomogram was proposed to predict an individual patient’s probability of developing radiation pneumonitis (Figure 2). When the position of the totaled points on the total points scale lines up with the linear and probability-based predictor scales, 0 (linear) and 0.5 (probability), respectively, indicate the risk of radiation pneumonitis. As shown in Figure 2, age, lung cancer diagnosis, history of chemotherapy, FEV1, DLCO, FEV1/FVC ratio, PTV, MLD, and total number of radiation fields have a greater capacity for providing points in the nomogram and can serve as indicators of the risk of radiation pneumonitis.
Decision curve analysis was then created (Figure 3), with two standard plots, showing the benefit of assuming that the patient will not develop radiation pneumonitis (“Treat None”) and the benefit of assuming that the patient will develop radiation pneumonitis (“Treat All”), respectively. Differences in the predictive power of variables were plotted within these standard plots to predict the risk of radiation pneumonitis.
This study analyzed the risk factors for radiation pneumonitis. We found that these factors include age, diagnosis of lung cancer, history of chemotherapy, lung function, and radiotherapy parameters.
With increased age, tolerance of radiotherapy in patients decreases and the risk of radiation pneumonitis increases. Vogelius and Bentzen[15] conducted a meta-analysis to assess the impact of various clinical risk factors on the incidence of symptomatic radiation pneumonitis. Age was one of the significant risk factors for radiation pneumonitis (odds ratio = 1.7, P < 0.0001). With the increase in age, all body functions of radiotherapy patients were reduced. Additionally, there may be underlying pulmonary diseases, making elderly patients more sensitive to radiation damage, increasing the damage to lung tissue caused by radiotherapy, and increasing the risk of radiation pneumonitis[16]. The onset of lung cancer is obscure, as there are generally no specific symptoms in the early stage. Therefore, most patients are diagnosed at the middle and late stages when the lung tissue has already suffered a certain degree of damage. This often requires a clinical increase of the radiation dose to efficiently kill tumor cells, resulting in further lung tissue damage and subsequently, radiation pneumonitis. Consistent with our findings, Zhang et al[17] also showed that lung disease and tumor that is located in the middle or lower lobe, are risk factors for radiation pneumonitis.
Chemotherapy is a commonly used method to treat cancer. However, increased toxicity and side effects of chemotherapy, coupled with the impact of increased tumor consumption, can lead to malnutrition. Subsequently, this can lead to a decline in epidemic immunity function, the dysfunction of respiratory muscles that may affect effective cough, and eventually, an increased risk of radiation pneumonitis[18]. Palma et al[19] retrospectively analyzed data of 836 patients with non-small cell lung cancer who received radiotherapy and chemotherapy in Europe, North America, and Asia. In recursive partition analysis, it was found that patients over 65 years old after carboplatin/paclitaxel chemotherapy had the highest risk of radiation pneumonitis (> 50%).
Relevant studies have shown that poor pulmonary function reduces the tolerance of lung tissue to radiation, and leads to the reduction of the safety threshold of radiotherapy parameters, and subsequently, radiation pneumonitis[20-22].
An increase in radiotherapy parameters such as PTV, MLD, total number of radiation fields, vdose, and NTCP is indicative of the increase in radiation dose, the radiation range, and the degree of damage to the lung tissue that are caused by radiotherapy. Accordingly, the possibility of radiation pneumonitis increases. Lu et al[23] showed significant differences in PTV, MLD, total MLD, and V5, V10, V20, and V40 (percentage of lung volumes exceeding 5, 10, 20, and 40 Gy) in patients with radiation and non- radiation pneumonitis. In addition, PTV > 145 cm3, total MLD ≥ 4.7 Gy, V5 ≥ 26.8%, V10 > 12%, and V20 ≥ 5.8 were associated with radiation pneumonitis risk. These published findings are consistent with results of our study showing that radiotherapy parameters are independent risk factors for radiation pneumonitis. Although our logistic regression analysis showed that vdose was not a statistically significant risk factor (P = 0.133), subsequent decision curve analysis demonstrated a very high net benefit across the threshold probability, approximating that of the full model.
Based on the results of our logistic regression analysis, we were able to generate a nomogram, incorporating factors such as age, lung cancer diagnosis, history of chemotherapy, lung function, and radiotherapy parameters. The range of data for each independent variable corresponded with the length of its scale and the extent that they span to right on the Points scale indicated their relative importance in the prediction model. The total points summed for each variable for a given patient indicated the probability of radiation pneumonitis on the prediction scale at the bottom. Our results also demonstrated that independent variables that were significant predictors in the regression models had a greater capacity for providing points in the nomogram. Together with the decision curve analysis performed in our study, these results may help physicians to decide on whether the benefit of the radiation treatment outweighs the potential risk of developing radiation pneumonitis. These tools, therefore, can be a useful guide in clinical practice, to evaluate the risk of radiation pneumonitis for a given patient based on their individual variables.
This study had a small sample size of 98 patients who had received radiotherapy at Huzhou Central Hospital within the past year, and there were few observation indexes, which may affect the accuracy of the results. Therefore, further multi-center and large-scale studies are needed, with increased sample size and a number of observation indexes to analyze the risk factors for radiation pneumonitis more comprehensively.
The occurrence of radiation pneumonitis is affected by many factors. Before radiotherapy, clinicians should fully consider all the influencing factors for each patient individually and formulate a scientific and reasonable radiotherapy plan to avoid the occurrence of radiation pneumonitis as much as possible while ensuring the treatment effect. The results of this study show that the risk factors for radiation pneumonitis include patient age, lung cancer diagnosis, chemotherapy, lung function, radiotherapy parameters. We have created a nomogram and performed decision curve analysis to provide clinicians with tools to evaluate the risk of radiation pneumonitis for a given patient based on the identified risk factors.
Radiotherapy is a common treatment method for malignant thoracic tumors. However, it is associated with complications, such as radiation pneumonitis that is mainly caused by the radiation-induced damage to the pulmonary vascular endothelial cells and parenchymal cells. It can develop into chronic inflammation and pulmonary fibrosis and may reduce the effectiveness of the treatment and impact the quality of life of cancer patients.
Early prevention of radiation pneumonitis is very important. Clinical continuous comprehensive analysis of associated risk factors and the formulation of targeted preventative methods may reduce the incidence of this complication.
To carry out multiple regression analysis on the influencing factors of radiation pneumonitis.
Records of patients receiving chest radiotherapy between January 2018 to February 2021 were collected and divided into two groups according to whether radiation pneumonitis was diagnosed. Multiple regression analysis was performed on age, tumor type, chemotherapy history, forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), DLCO, FEV1/FVC ratio, planned target area (PTV), mean lung dose (MLD), total number of radiation fields, percentage of lung tissue in total lung volume (vdose), probability of normal tissue complications (NTCP), and other factors.
The proportions of patients with an age ≥ 60 years, lung cancer diagnosis, and a history of chemotherapy in the study group were higher than those in the control group (P < 0.05); FEV1, DLCO, and FEV1/FVC ratio in the study group were lower than those in the control group (P < 0.05), while PTV, MLD, total field number, vdose, and NTCP were higher than those in the control group (P < 0.05). Logistic regression analysis showed that age, lung cancer diagnosis, chemotherapy history, FEV1, FEV1/FVC ratio, PTV, MLD, total number of radiation fields, vdose, and NTCP were risk factors for radiation pneumonitis.
The risk factors for radiation pneumonitis include patient age, lung cancer diagnosis, history of chemotherapy, lung function, and radiotherapy parameters.
A comprehensive evaluation and examination should be carried out before beginning the radiotherapy treatment. Clinicians should fully consider all the influencing factors based on the individual situation of each patient, to design a scientific and reasonable radiotherapy plan.
| 1. | Jiao Y, Shen Y, Yan H, Liu Y, Tan H, Li J. Short-term clinical effect of conformal radiotherapy combined with tegafur gimeracil oteracil potassium in treating recurrent esophagus cancer. Pak J Med Sci. 2016;32:1141-1145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Bakkal BH, Ugur MB, Bahadir B. Bilateral synchronous squamous cell tonsil carcinoma treated with chemoradiotherapy. J Pak Med Assoc. 2014;64:468-470. [PubMed] |
| 3. | Nisaa Zia NU, Khokhar MA, Qamar S, Hanif A, Goraya AW, Awan NU. Concurrent radiotherapy and chemotherapy with erlotinib followed by maintenance erlotinib in patients with Epidermal Growth Factor Receptor mutation- positive adenocarcinoma lung. J Pak Med Assoc. 2019;69:1605-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Hanania AN, Mainwaring W, Ghebre YT, Hanania NA, Ludwig M. Radiation-Induced Lung Injury: Assessment and Management. Chest. 2019;156:150-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 454] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 5. | Chen Z, Wu Z, Ning W. Advances in Molecular Mechanisms and Treatment of Radiation-Induced Pulmonary Fibrosis. Transl Oncol. 2019;12:162-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | Simsek FS, Koroglu R, Elmali F, Comak A, Ulutas H, Balci TA, Asik M, Akatli A, Kekilli E, Oner AO. Is it possible to achieve more accurate mediastinal nodal radiotherapy planning for NSCLC with PET/CT? J Pak Med Assoc. 2020;70:29-34. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Kim W, Lee S, Seo D, Kim D, Kim K, Kim E, Kang J, Seong KM, Youn H, Youn B. Cellular Stress Responses in Radiotherapy. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 231] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 8. | Giridhar P, Mallick S, Rath GK, Julka PK. Radiation induced lung injury: prediction, assessment and management. Asian Pac J Cancer Prev. 2015;16:2613-2617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Koulenti D, Zhang Y, Fragkou PC. Nosocomial pneumonia diagnosis revisited. Curr Opin Crit Care. 2020;26:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Ullah T, Patel H, Pena GM, Shah R, Fein AM. A contemporary review of radiation pneumonitis. Curr Opin Pulm Med. 2020;26:321-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Bledsoe TJ, Nath SK, Decker RH. Radiation Pneumonitis. Clin Chest Med. 2017;38:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 155] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 12. | Giuranno L, Ient J, De Ruysscher D, Vooijs MA. Radiation-Induced Lung Injury (RILI). Front Oncol. 2019;9:877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 276] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 13. | Ing EB, Ing R. The Use of a Nomogram to Visually Interpret a Logistic Regression Prediction Model for Giant Cell Arteritis. Neuroophthalmology. 2018;42:284-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Vickers AJ, Van Calster B, Steyerberg EW. Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. BMJ. 2016;352:i6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 718] [Article Influence: 71.8] [Reference Citation Analysis (1)] |
| 15. | Vogelius IR, Bentzen SM. A literature-based meta-analysis of clinical risk factors for development of radiation induced pneumonitis. Acta Oncol. 2012;51:975-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 181] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 16. | Zhao J, Yorke ED, Li L, Kavanagh BD, Li XA, Das S, Miften M, Rimner A, Campbell J, Xue J, Jackson A, Grimm J, Milano MT, Spring Kong FM. Simple Factors Associated With Radiation-Induced Lung Toxicity After Stereotactic Body Radiation Therapy of the Thorax: A Pooled Analysis of 88 Studies. Int J Radiat Oncol Biol Phys. 2016;95:1357-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 17. | Zhang XJ, Sun JG, Sun J, Ming H, Wang XX, Wu L, Chen ZT. Prediction of radiation pneumonitis in lung cancer patients: a systematic review. J Cancer Res Clin Oncol. 2012;138:2103-2116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 18. | Scobioala S, Eich HT. Risk stratification of pulmonary toxicities in the combination of whole lung irradiation and high-dose chemotherapy for Ewing sarcoma patients with lung metastases: a review. Strahlenther Onkol. 2020;196:495-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Palma DA, Senan S, Tsujino K, Barriger RB, Rengan R, Moreno M, Bradley JD, Kim TH, Ramella S, Marks LB, De Petris L, Stitt L, Rodrigues G. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85:444-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 510] [Cited by in RCA: 515] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 20. | Harris AA, Stang K, Small C, Hutten R, Alite F, Emami B, Harkenrider M. Pretreatment Factors Influencing Radiation Pneumonitis after Stereotactic Body Radiation Therapy in the Treatment of Lung Cancer. Cureus. 2020;12:e7462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Chen X, Sheikh K, Nakajima E, Lin CT, Lee J, Hu C, Hales RK, Forde PM, Naidoo J, Voong KR. Radiation Versus Immune Checkpoint Inhibitor Associated Pneumonitis: Distinct Radiologic Morphologies. Oncologist. 2021;26:e1822-e1832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Zhang A, Yang F, Gao L, Shi X, Yang J. Research Progress on Radiotherapy Combined with Immunotherapy for Associated Pneumonitis During Treatment of Non-Small Cell Lung Cancer. Cancer Manag Res. 2022;14:2469-2483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 23. | Lu C, Lei Z, Wu H, Lu H. Evaluating risk factors of radiation pneumonitis after stereotactic body radiation therapy in lung tumor: Meta-analysis of 9 observational studies. PLoS One. 2018;13:e0208637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Respiratory system
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ait Addi R, Morocco; Saha L, India; Shariati MBH, Iran; Taooka Y, Japan S-Editor: Chang KL L-Editor: Wang TQ P-Editor: Chang KL