Published online Dec 26, 2023. doi: 10.12998/wjcc.v11.i36.8581
Peer-review started: October 18, 2023
First decision: November 13, 2023
Revised: November 19, 2023
Accepted: December 13, 2023
Article in press: December 13, 2023
Published online: December 26, 2023
Processing time: 64 Days and 11.3 Hours
One of the major perioperative complications for coronary artery bypass graft (CABG) is stroke. The risk of perioperative stroke after CABG is approximately 2%. Carotid stenosis (CS) is considered an independent predictor of perioperative stroke risk in CABG patients. The optimal management of such patients has been a source of controversy. One of the possible surgical options is synchronous carotid endarterectomy (CEA) and CABG. Here, we have presented 4 cases of successful synchronous CEA and CABG.
Our center’s experience with 4 cases of significant carotid artery stenosis, which were successfully managed with combined CEA and CABG, are detailed. The first case was a female who presented for CABG after a ST-elevation myocardial infarction. She had right internal carotid artery (ICA) occlusion and 90% left ICA stenosis. The second case was a male who was electively admitted for CABG. It was discovered that he had left ICA occlusion and 90% right ICA stenosis. The third case was a male with a history of stroke, two months prior to admission. He presented with non-ST-elevation myocardial infarction. Preoperatively, it was discovered that he had > 90% right ICA stenosis. The final case was a male who was electively admitted for CABG. It was discovered that he had bilateral > 90% ICA stenosis. We have also reviewed the current evidence and guidelines for managing CS in patients undergoing CABG.
Our case series demonstrated that synchronous CEA and CABG was safe. A multicenter study with additional patients is needed. It is necessary for clinicians to screen for CS in high-risk patients with features.
Core Tip: The risk of perioperative stroke after coronary artery bypass graft (CABG) is 2%. A hemodynamically significant carotid artery stenosis is found in 7% of patients undergoing CABG. Carotid stenosis is considered an independent predictor for the risk of perioperative stroke in CABG patients. The optimal management of such patients has been a source of controversy, but one of the possible surgical options is combined carotid endarterectomy and CABG. Our case series suggested that this option is safe for the management of this population of patients.
- Citation: AlGhamdi FK, Altoijry A, AlQahtani A, Aldossary MY, AlSheikh SO, Iqbal K, Alayadhi WA. Synchronous carotid endarterectomy and coronary artery bypass graft: Four case reports. World J Clin Cases 2023; 11(36): 8581-8588
- URL: https://www.wjgnet.com/2307-8960/full/v11/i36/8581.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i36.8581
Ischemic heart disease and ischemic stroke are the two leading causes of death worldwide[1]. Due to similar pathophysiologic processes, coronary artery disease and carotid artery disease can coexist. A hemodynamically significant carotid artery stenosis (> 50% stenosis) is found in 7% of patients undergoing coronary artery bypass grafting (CABG)[2]. One of the major perioperative complications for CABG is stroke. The risk of perioperative stroke after CABG is around 2%[2]. Carotid stenosis (CS) is considered an independent predictor for the risk of perioperative stroke in CABG patients[3]. The optimal management of such patients has been a source of controversy. One of the possible surgical options for treatment is synchronous carotid endarterectomy (CEA) and CABG. In this work, we reported the experience of 4 cases of successful combined CEA and CABG at a single center and briefly reviewed the literature.
Case 1: A 64-year-old female presented with typical ischemic chest pain.
Case 2: A 76-year-old male presented with the complaint of chronic chest discomfort that was related to physical exertion.
Case 3: A 63-year-old male presented with severe chest pain that started 6 h prior to presentation to the emergency department.
Case 4: A 71-year-old male presented with shortness of breath and orthopnea that started the morning of his presentation.
Case 1: The patient presented to our emergency department with chest pain that was retrosternal and radiating to the left. She previously had similar pain that was related to physical activity. The previous pain was aggravated by physical activity and relieved by rest. However, the pain at presentation was more severe and occurred during rest.
Case 2: The patient presented to the clinic with a 6-mo history of chest discomfort and tightness that was triggered by physical exertion and relieved by rest. He denied any shortness of breath, palpitations, or loss of consciousness. The patient was electively admitted to our center for diagnostic coronary angiography (CAG).
Case 3: The patient presented to our emergency department with severe left-sided chest pain that was radiating to the back and left shoulder. The chest pain started while the patient was at rest and was associated with shortness of breath and sweating. He was admitted with an initial diagnosis of non-ST elevation myocardial infarction (NSTEMI).
Case 4: The patient presented to our emergency department with shortness of breath that started that morning while at rest. He was also experiencing chest heaviness and sweating. He was admitted with an initial diagnosis of NSTEMI.
Case 1: The patient had diabetes, which was poorly controlled, and hypertension. In addition, she had hypothyroidism. She previously had an infected foot ulcer that was treated by amputation of the right big toe.
Case 2: The patient had end-stage renal disease and hypertension.
Case 3: The patient had hypertension. He also had a history of pulmonary embolism that was treated with oral anticoagulation medication. He suffered a stroke 2 mo prior to presentation and experienced residual left-sided weakness.
Case 4: The patient had hypothyroidism and bilateral carotid artery stenosis.
There were no family history of cardiac disease or stroke of all patients.
Case 1: The patient was alert, conscious, and oriented with stable vital signs. She had normal bilateral vesicular breathing sounds. Heart sounds were normal with no murmurs or added sounds. Peripheral pulses were palpable with normal volume on both upper and lower limbs.
Case 2: The patient was alert, conscious, and oriented with stable vital signs. He had normal bilateral vesicular breathing sounds. Heart sounds were normal with no murmurs or added sounds. Peripheral pulses were palpable with normal volume on both upper and lower limbs. He had an arteriovenous fistula on his left arm that was used for dialysis.
Case 3: The patient was alert, conscious, and oriented with stable vital signs. He had normal bilateral vesicular breathing sounds. Heart sounds were normal with no murmurs or added sounds. Peripheral pulses were palpable with normal volume on both upper and lower limbs. The motor power of the patient’s upper and lower limbs on the left side was reduced. However, sensation and proprioception were preserved.
Case 4: The patient was alert, conscious, and oriented with stable vital signs. He had normal bilateral vesicular breathing sounds. Heart sounds were normal with no murmurs or added sounds. Peripheral pulses were palpable with normal volume on both upper and lower limbs.
Case 1: The patient’s routine blood work was normal with the exception of the hemoglobin A1c level, which was 9.9% (normal range: < 5.7%).
Case 2: The patient’s routine blood work was normal with the exception of the creatinine level, which was 575 mmol/L (normal range: 53-106 mmol/L). The estimated glomerular filtration rate was very low (8.4 mL/min/1.73 m2; normal range: ≥ 90 mL/min/1.73 m2).
Case 3: The patient’s routine blood work was normal with the exception of the high-sensitivity troponin-T level which was 126 ng/L (normal range: 0.0002-58.9 ng/L).
Case 4: The patient’s routine blood work was normal with the exception of the high-sensitivity troponin-T level which was 200 ng/L. The patient’s hemoglobin A1c level was slightly elevated (6.1%).
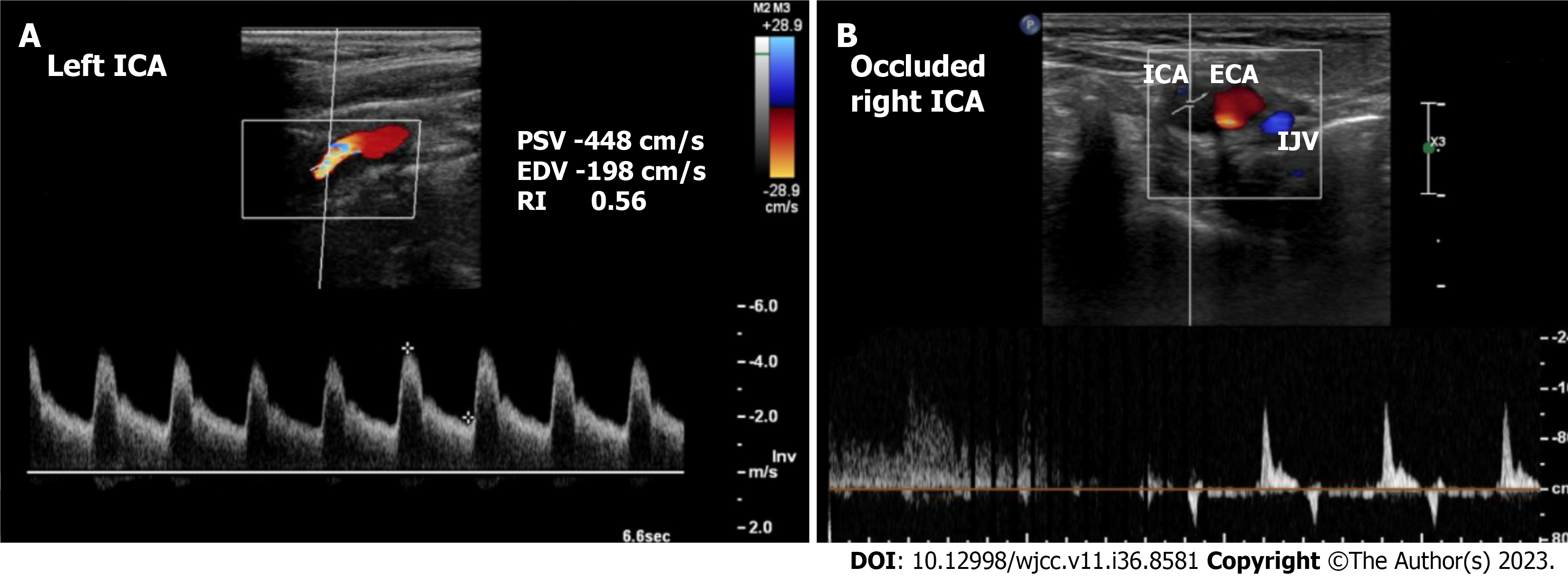
Case 1: Echocardiography showed a 30%-35% left ventricular ejection fraction (LVEF) with no significant valvular abnormalities. CAG showed triple vessel disease. Carotid Doppler showed total occlusion of the right internal carotid artery (ICA) and > 90% stenosis of the left ICA (Figure 1).
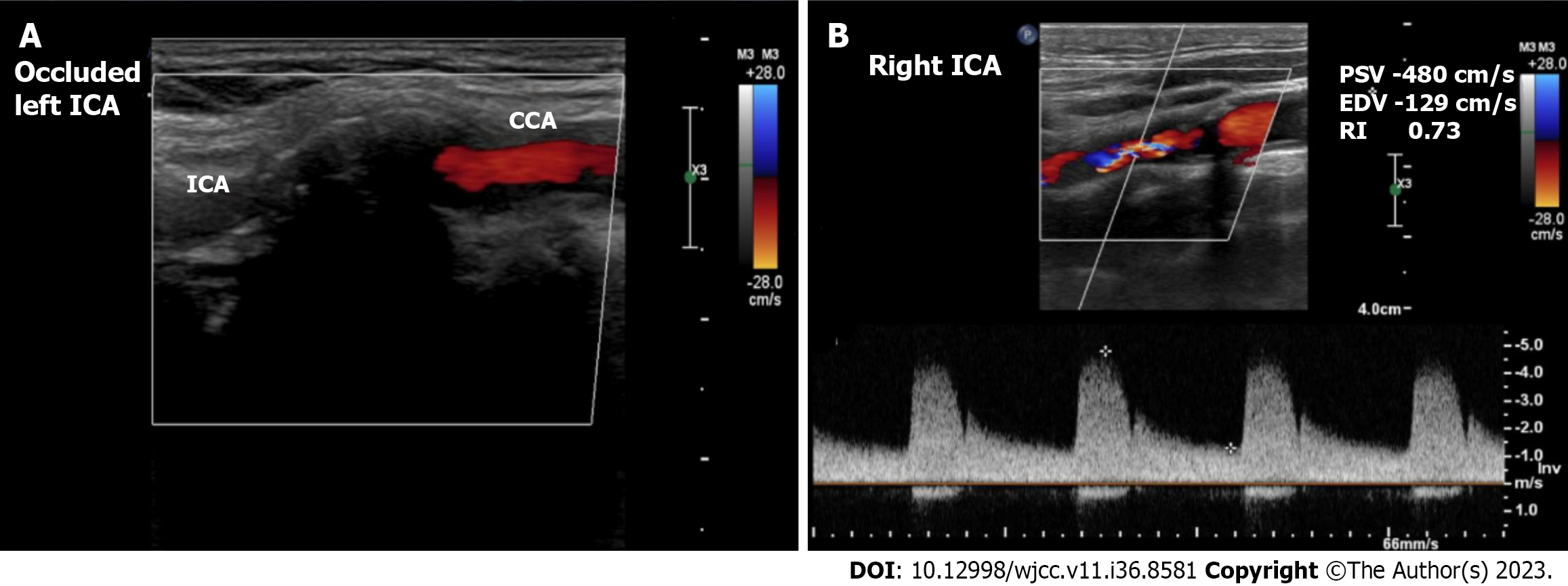
Case 2: Echocardiography showed a > 50% LVEF with mild mitral regurgitation. CAG showed triple vessel disease. Carotid Doppler showed total occlusion of the left ICA and > 90% stenosis of the right ICA (Figure 2).
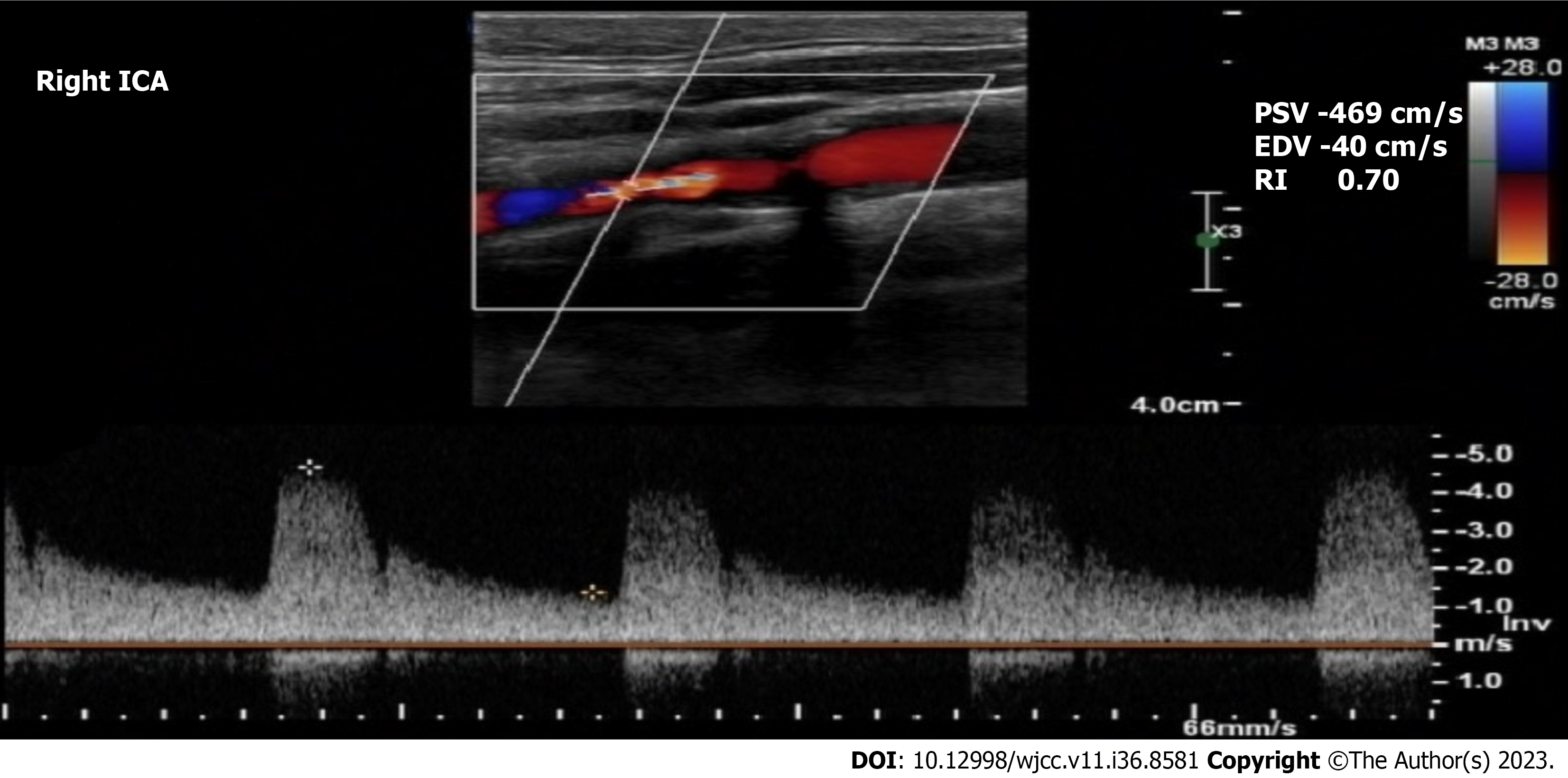
Case 3: Echocardiography showed a 30% LVEF with mild aortic regurgitation. CAG showed left main coronary artery stenosis (> 50%). Carotid Doppler showed 90%-99% stenosis of the right ICA and normal left ICA (Figure 3).
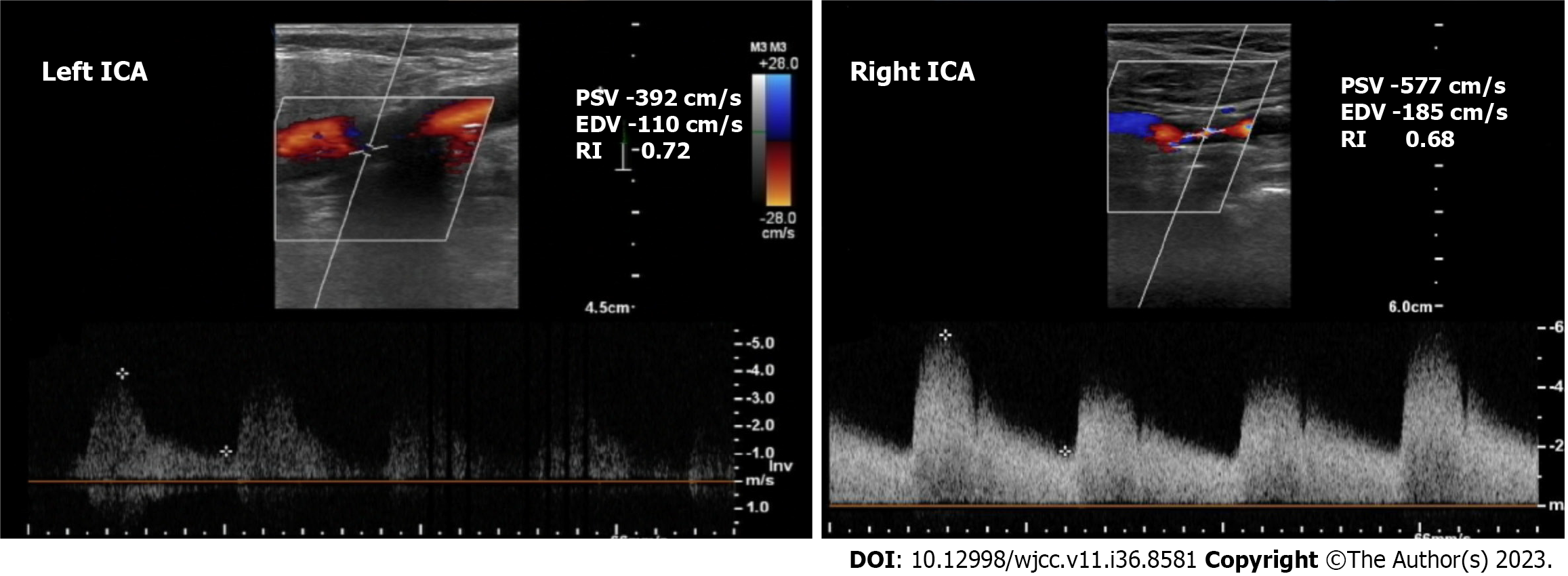
Case 4: Echocardiography a 35%-40% LVEF with mild mitral regurgitation. CAG showed significant triple vessel disease. Carotid Doppler showed 90% stenosis of the left ICA and > 90% stenosis of the right ICA (Figure 4).
Case 1: The patient was diagnosed with triple vessel coronary artery disease with low ejection fraction and total occlusion of the right ICA and 90% stenosis of the left ICA.
Case 2: The patient was diagnosed with triple vessel coronary artery disease with preserved ejection fraction and total occlusion of the left ICA and > 90% stenosis of the right ICA.
Case 3: The patient was diagnosed with left main coronary artery stenosis with reduced LVEF, NSTEMI, and > 90% stenosis of the right ICA.
Case 4: The patient was diagnosed with triple vessel coronary artery disease presenting as NSTEMI with reduced ejection fraction and bilateral > 90% ICA stenosis.
Case 1: The patient underwent left CEA and on-pump CABG.
Case 2: The patient underwent right CEA and on-pump CABG.
Case 3: The patient underwent right CEA and on-pump CABG.
Case 4: The patient underwent left CEA and on-pump CABG, with planned right CEA 12 mo after the CABG.
Case 1: The patient’s postoperative course was uneventful and was discharged 1 wk after her surgery. Her last follow-up was 5 years after the surgery. She did not experience stroke or myocardial infarction (MI) during the follow-up period.
Case 2: The patient’s postoperative course was uneventful, he was discharged after 2 wk. His last follow up was 4 years after the surgery. He did not experience stroke or MI during the follow-up period.
Case 3: The patient had no complications postoperatively and was discharged 5 d after the surgery. His last follow up was 1 year after the surgery. He did not experience stroke or MI during the follow-up period.
Case 4: The patient’s intensive care unit stay was uneventful and he was transferred to the ward 4 d after the surgery and discharged on the 6th d postoperatively. His last follow-up was 1 year after the surgery. He did not experience stroke or MI during the follow-up period.
The risk of perioperative stroke in patients who are treated with CABG is around 2%[2]. CS is considered an independent predictor for the risk of perioperative stroke in CABG patients[3]. There are multiple causes of perioperative stroke in patients undergoing CABG, including CS, aortic embolism during manipulation, cannulation and decannulation, graft anastomosis to the aorta, platelet aggregation on cardiopulmonary bypass, hypercoagulable states, postoperative arrhythmias, and hemodynamic instability[4]. CS > 80% is found in 7% of CABG patients[2]. The risk of perioperative stroke in CABG patients with > 50% and > 80% CS is 7% and 9%, respectively[5]. The impact of perioperative stroke on patient survival is significant when compared to the survival of cardiac surgery patients with no stroke. In a meta-analysis of 174000 cardiac operations, the operative mortality of patients who suffered perioperative stroke was 29.0% vs 2.4% in patients who did not suffer from perioperative stroke (P < 0.001)[6].
The routine screening of CS in patients who are candidates for cardiac surgery is controversial. Most of the international societies support selective screening for high-risk patients[4,7]. The European Society of Cardiology (ESC) recommends screening patients who are undergoing CABG with duplex ultrasound if they have a history of recent (< 6 mo) transient ischemic attack (TIA) or stroke[4]. They also recommend screening patients with no history of recent TIA or stroke but are ≥ 70-years-old, have multivessel coronary artery disease, have concomitant peripheral arterial disease, or have carotid bruit on examination[4]. The ESC does not recommend screening patients if they require urgent CABG and have no history of recent TIA/stroke[4]. The European Society for Vascular Surgery (ESVS) recommends screening patients who are aged > 70-years-old, have a history of TIA or stroke, carotid bruit, or left mainstem disease[7].
Prophylactic carotid intervention in patients with carotid artery stenosis is controversial. There is no strong evidence to support carotid intervention in all CABG patients with asymptomatic CS[4]. Select patients may benefit significantly from carotid intervention because it could reduce their risk of stroke-related morbidity and mortality and of a prolonged hospital stay[2]. Patients who could benefit are at a high risk of postoperative stroke, such as patients with asymptomatic severe (70%-99%) bilateral stenosis, asymptomatic severe stenosis with contralateral occlusion, or a history of prior stroke or TIA[4,7].
The surgical and endovascular options for patients are staged CEA then CABG, staged CABG then CEA, synchronous CEA plus CABG, staged carotid artery stenting (CAS) then CABG, and same day CAS then CABG. Most of the data comparing these options are from observational studies and meta-analyses. In a meta-analysis that involved 25021 patients who had undergone either combined or staged CEA and CABG, there was no difference between the two approaches in early mortality (relative risk: 1.36; 95% confidence interval: 0.78-2.36; P = 0.27) and postoperative stroke (relative risk: 1.14; 95% confidence interval: 0.99-1.31; P = 0.07)[8].
There are only two randomized controlled trials that evaluated synchronous or staged CEA in CABG patients with unilateral asymptomatic CS. Illuminati et al[9] randomized 185 patients with severe unilateral asymptomatic CS to one of three groups: CEA synchronous with CABG; staged CEA before CABG; or isolated CABG then delayed CEA. They concluded that staged CEA then CABG or synchronous CEA and CABG prevented stroke better than delayed CEA. The CABACS (i.e., Coronary Artery Bypass graft surgery in patients with Asymptomatic Carotid Stenosis) trial randomized 129 CABG patients from 17 centers. The enrolled patients had unilateral asymptomatic severe (80%-99%) CS. They were randomized to either synchronous CEA and CABG or CABG alone. The 30-d death/stroke rate among patients who underwent the synchronous procedure was 18.5% vs 9.7% in patients with isolated CABG[10]. Unfortunately, this trial was terminated prematurely after funding withdrawal. However, the authors concluded that the very high rate of perioperative stroke does not justify the synchronous approach in patients with severe asymptomatic CS[10].
One of the options to manage this population of patients is either same-day or staged CAS. This modality may be beneficial because it is less invasive compared to conventional CEA. However, CAS can complicate the management of CABG patients because dual-antiplatelet therapy is needed after CAS. This could increase the risk of bleeding during the CABG procedure or increase the risk of MI if CABG was delayed for the administration of dual-antiplatelets. In a systematic review that included 11 studies that evaluated the outcome of 760 staged or same-day CAS plus CABG procedures, the majority of the patients (87%) were asymptomatic. The overall mortality rate was 5.5%, and the risk of suffering any stroke was 4.2%[11]. This review also observed that CABG performed within 48 h of a CAS procedure was not associated with a significant risk compared to CABG performed 2 wk after a CAS procedure[11].
The most recent guidelines emphasize that the management of patients should be individualized and determined by a multidisciplinary team[4]. The ESC recommends that patients who will be treated with a CABG procedure and who have a recent history of stroke or TIA should be considered for carotid revascularization if there is 50%-99% CS, without specifying the means for revascularization[4]. They also recommend that prophylactic carotid revascularization may be considered in a patient with bilateral 70%-99% CS or 70%-99% CS and contralateral occlusion[4]. On the other hand, the most recent ESVS guidelines were more specific in regard to the modality and timing of revascularization[7]. The ESVS recommends staged or synchronous CEA and CABG in patients who have a recent history of TIA or stroke with ipsilateral 50%-99% CS[7]. It also recommends staged or synchronous CAS/CEA and CABG in patients who are asymptomatic and who have either bilateral 70%-99% CS or 70%-99% CS and contralateral occlusion[7].
In conclusion, CS is prevalent among patients with coronary artery disease who are undergoing CABG. Screening for CS is indicated in patients with high-risk features, such as old age and left mainstem disease. The indications, modality, and timing for carotid revascularization in CABG patients is still controversial, and more evidence is needed to decide on the best management plan for this patient population.
| 1. | Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J Am Coll Cardiol. 2022;80:2361-2371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1272] [Reference Citation Analysis (0)] |
| 2. | Naylor AR, Mehta Z, Rothwell PM, Bell PR. Carotid artery disease and stroke during coronary artery bypass: a critical review of the literature. Eur J Vasc Endovasc Surg. 2002;23:283-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 243] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | Poi MJ, Echeverria A, Lin PH. Contemporary Management of Patients with Concomitant Coronary and Carotid Artery Disease. World J Surg. 2018;42:272-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, Collet JP, Czerny M, De Carlo M, Debus S, Espinola-Klein C, Kahan T, Kownator S, Mazzolai L, Naylor AR, Roffi M, Röther J, Sprynger M, Tendera M, Tepe G, Venermo M, Vlachopoulos C, Desormais I; ESC Scientific Document Group. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39:763-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 2379] [Article Influence: 339.9] [Reference Citation Analysis (0)] |
| 5. | Naylor AR, Bown MJ. Stroke after cardiac surgery and its association with asymptomatic carotid disease: an updated systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2011;41:607-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Gaudino M, Rahouma M, Di Mauro M, Yanagawa B, Abouarab A, Demetres M, Di Franco A, Arisha MJ, Ibrahim DA, Baudo M, Girardi LN, Fremes S. Early Versus Delayed Stroke After Cardiac Surgery: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2019;8:e012447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 7. | Naylor R, Rantner B, Ancetti S, de Borst GJ, De Carlo M, Halliday A, Kakkos SK, Markus HS, McCabe DJH, Sillesen H, van den Berg JC, Vega de Ceniga M, Venermo MA, Vermassen FEG; Esvs Guidelines Committee; Antoniou GA, Bastos Goncalves F, Bjorck M, Chakfe N, Coscas R, Dias NV, Dick F, Hinchliffe RJ, Kolh P, Koncar IB, Lindholt JS, Mees BME, Resch TA, Trimarchi S, Tulamo R, Twine CP, Wanhainen A, Document Reviewers, Bellmunt-Montoya S, Bulbulia R, Darling RC 3rd, Eckstein HH, Giannoukas A, Koelemay MJW, Lindström D, Schermerhorn M, Stone DH. Editor's Choice - European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur J Vasc Endovasc Surg. 2023;65:7-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 506] [Article Influence: 168.7] [Reference Citation Analysis (0)] |
| 8. | Sharma V, Deo SV, Park SJ, Joyce LD. Meta-analysis of staged versus combined carotid endarterectomy and coronary artery bypass grafting. Ann Thorac Surg. 2014;97:102-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Illuminati G, Ricco JB, Caliò F, Pacilè MA, Miraldi F, Frati G, Macrina F, Toscano M. Short-term results of a randomized trial examining timing of carotid endarterectomy in patients with severe asymptomatic unilateral carotid stenosis undergoing coronary artery bypass grafting. J Vasc Surg. 2011;54:993-9; discussion 998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Weimar C, Bilbilis K, Rekowski J, Holst T, Beyersdorf F, Breuer M, Dahm M, Diegeler A, Kowalski A, Martens S, Mohr FW, Ondrášek J, Reiter B, Roth P, Seipelt R, Siggelkow M, Steinhoff G, Moritz A, Wilhelmi M, Wimmer-Greinecker G, Diener HC, Jakob H, Ose C, Scherag A, Knipp SC; CABACS Trial Investigators. Safety of Simultaneous Coronary Artery Bypass Grafting and Carotid Endarterectomy Versus Isolated Coronary Artery Bypass Grafting: A Randomized Clinical Trial. Stroke. 2017;48:2769-2775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Naylor AR, Mehta Z, Rothwell PM. A systematic review and meta-analysis of 30-day outcomes following staged carotid artery stenting and coronary bypass. Eur J Vasc Endovasc Surg. 2009;37:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Saudi Arabia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lakusic N, Croatia; Ueda H, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Zhao S