Published online Dec 16, 2023. doi: 10.12998/wjcc.v11.i35.8364
Peer-review started: August 30, 2023
First decision: October 10, 2023
Revised: October 21, 2023
Accepted: December 4, 2023
Article in press: December 4, 2023
Published online: December 16, 2023
Processing time: 105 Days and 6.2 Hours
Although superior vena cava (SVC) syndrome has also been reported as a late complication of pacemaker (PM) implantation, acute onset of SVC syndrome caused by disdialysis syndrome in patients with PM implantation is very rare. There are no specific therapies or guidelines.
A 96-year-old woman receiving dialysis was implanted with a PM due to sick sinus syndrome. She was referred to our facility for chest discomfort experienced during dialysis. Upon examination, unilateral pleural effusion on the right side was cloudy with a foul odour. The patient was diagnosed with pyothorax and treated with antibiotics. After the effusion was reduced, it gradually reaggravated and remained cloudy. In this case, SVC syndrome, which is generally considered a late complication after PM implantation, rapidly developed following the bacteraemia, resulting in impaired venous return, chylothorax, and disdialysis syndrome. After catheter intervention for SVC stenosis, the patient’s symptoms promptly improved. The patient has been recurrence-free for a year.
Acute SVC syndrome can cause dysdialysis in PM-implanted patients. Catheter intervention alone has improved this condition for a traceable period.
Core Tip: While superior vena cava (SVC) syndrome has also been reported as a late complication after pacemaker (PM) implantation, our case is novel because, to the best of our knowledge, no instances of ‘acute’ SVC syndrome resulting in disdialysis in a patient on maintenance haemodialysis after PM implantation has been reported. We wish to emphasize the following key points: (1) Acute-onset SVC syndrome should be considered when acute disdialysis is encountered in patients with a PM on dialysis; and (2) the efficacy of treatment using percutaneous old balloon angioplasty alone, which has demonstrated long-term effectiveness.
- Citation: Yamamoto S, Kamezaki M, Ooka J, Mazaki T, Shimoda Y, Nishihara T, Adachi Y. Balloon venoplasty for disdialysis syndrome due to pacemaker-related superior vena cava syndrome with chylothorax post-bacteraemia: A case report. World J Clin Cases 2023; 11(35): 8364-8371
- URL: https://www.wjgnet.com/2307-8960/full/v11/i35/8364.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i35.8364
Superior vena cava (SVC) syndrome is caused by stenosis or obstruction of the SVC due to various factors, resulting in upper body oedema, precordial superficial vein distention associated with collateral circulation, cough, dyspnoea, fainting, dizziness, and headache. Although lung cancer is the most common cause, it has also been reported as a complication of pacemaker (PM) implantation and central venous catheters[1]. Treatment options include anticoagulation, catheter intervention, and surgical intervention; however, there are no specific therapies or guidelines. SVC syndrome has been reported as a late complication of PM implantation; however, we report a case of disdialysis syndrome caused by the acute onset of SVC syndrome with refractory chylothorax following bacteraemia and successful percutaneous balloon venoplasty, which led to dramatic remission.
The patient complained of malaise and dyspnoea.
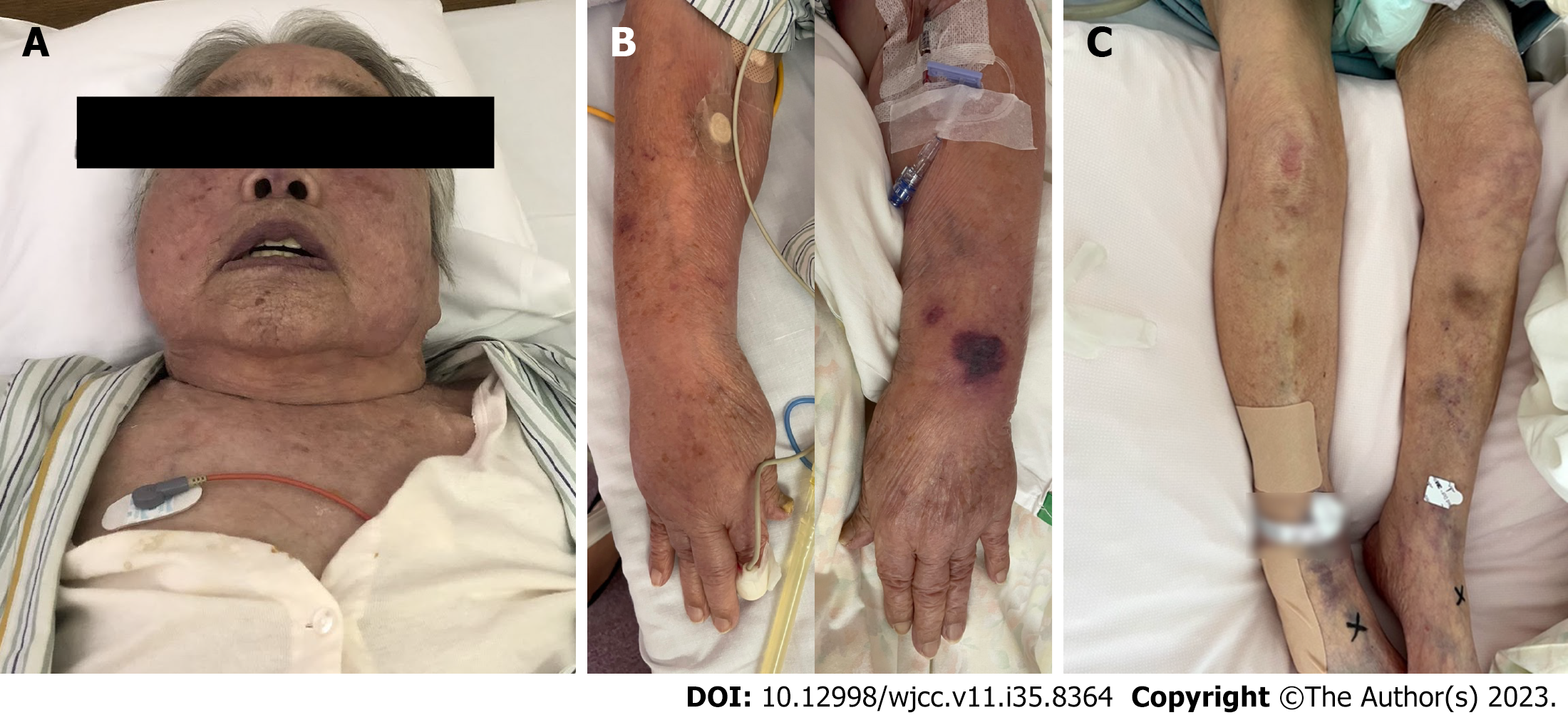
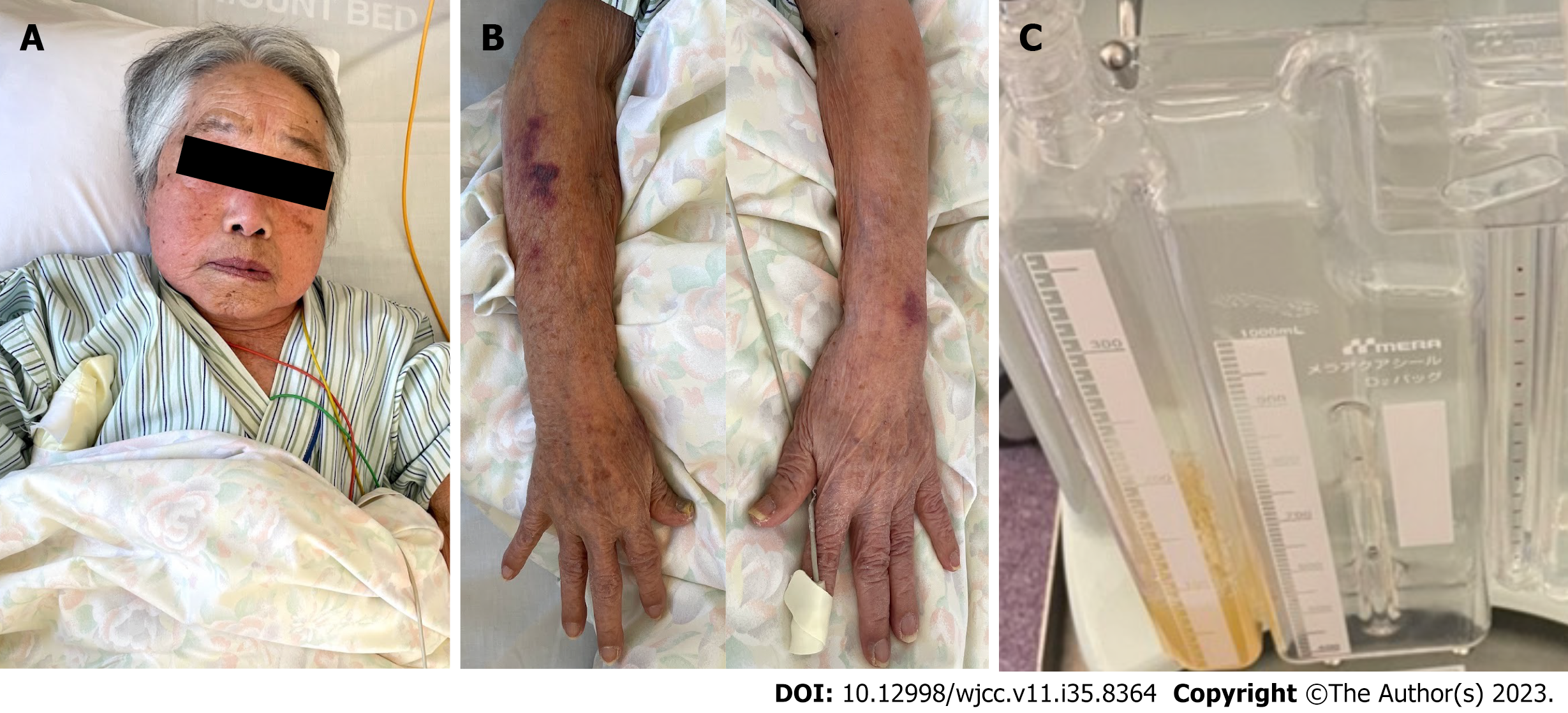
A 96-year-old woman was referred to our hospital for malaise and dyspnoea during dialysis, which had not been present previously. During hospitalization, edema in the upper extremities and face worsened (Figure 1), and although we attempted to recommend dehydration, we were unable to perform dialysis adequately due to hypotension during dialysis.
The patient was implanted with a dual-chamber PM due to sick sinus syndrome eight years earlier. The right ventricular (RV) lead was dislodged the day after implantation. Fortunately, the patient lived without symptoms in the AAI mode. Three years ago, she was referred for dialysis treatment for end-stage renal failure of an unknown aetiology. A shunt was created in the right upper extremity by anastomosing the brachial artery and the median mesodermal vein.
No family history was available.
The body temperature was elevated to 37.5 °C, and her SpO2 was 94% (oxygen consumption: 5 L/min). No murmurs or lung rales were noted during auscultation, and the breath sounds decreased on the right dorsal side. Visual inspection revealed no oedema of the extremities.
An infection was suspected due to the slightly elevated white blood cell count (11400/μL) and C-reactive protein level (1.4 mg/dL). Thoracentesis further revealed a foul-smelling, cloudy pleural effusion. Extended-spectrum beta-lactamase-producing Escherichia coli (ESBL E. coli) was detected in the pleural effusion and blood cultures. The patient was diagnosed with pyothorax and bacteraemia caused by ESBL E. coli; both were successfully treated by placement of a chest drainage tube and by administration of intravenous meropenem and clindamycin for 11 d. The pleural effusion decreased gradually, and a negative blood culture of ESBL E. coli was confirmed.
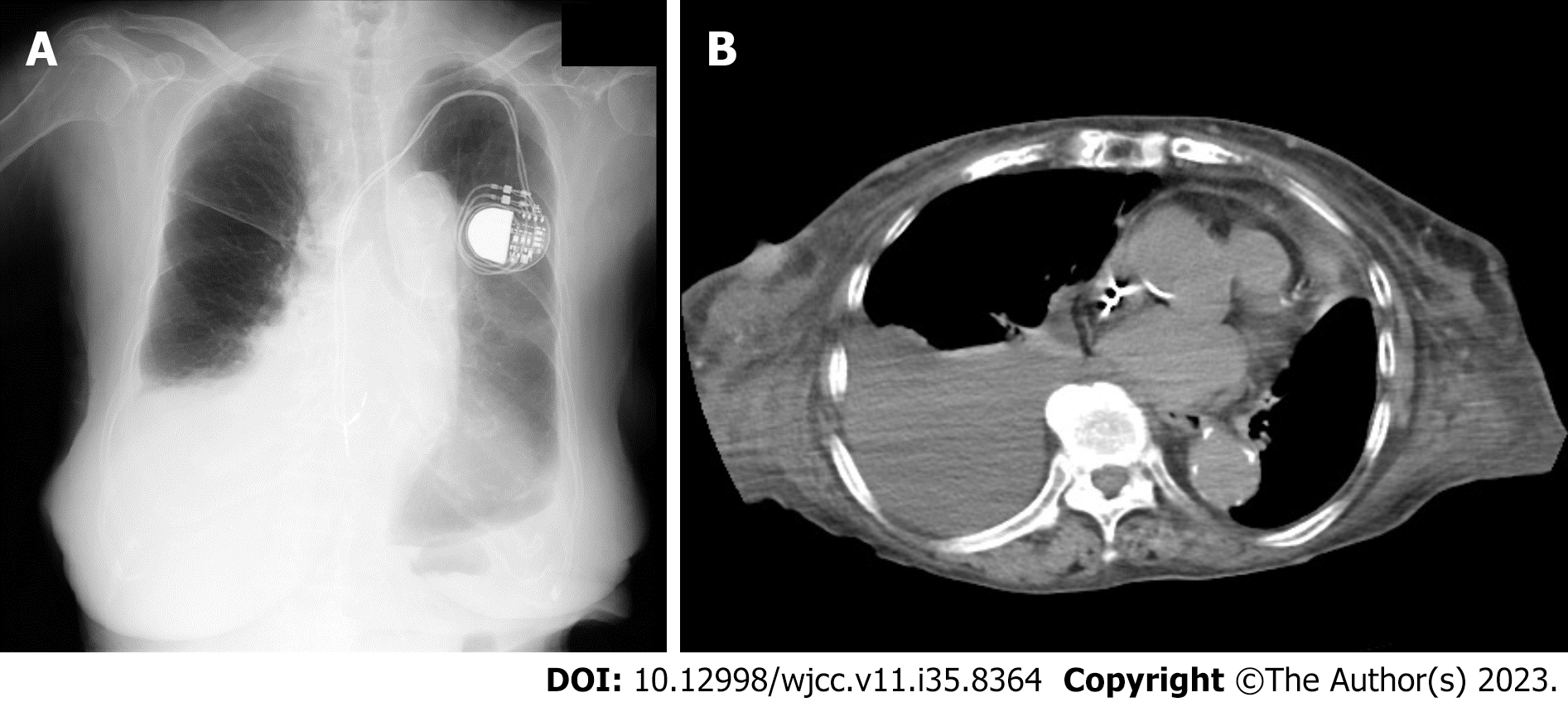
Chest radiography and computed tomography (CT) revealed a massive right pleural effusion (Figure 2). Echocardiography revealed an ejection fraction of 66.3% without asynergy, a trans-tricuspid valve regurgitant pressure gradient of 11.3 mmHg, no significant valvular disease, and no vegetation. Thus, acute heart failure and infectious endocarditis were ruled out.
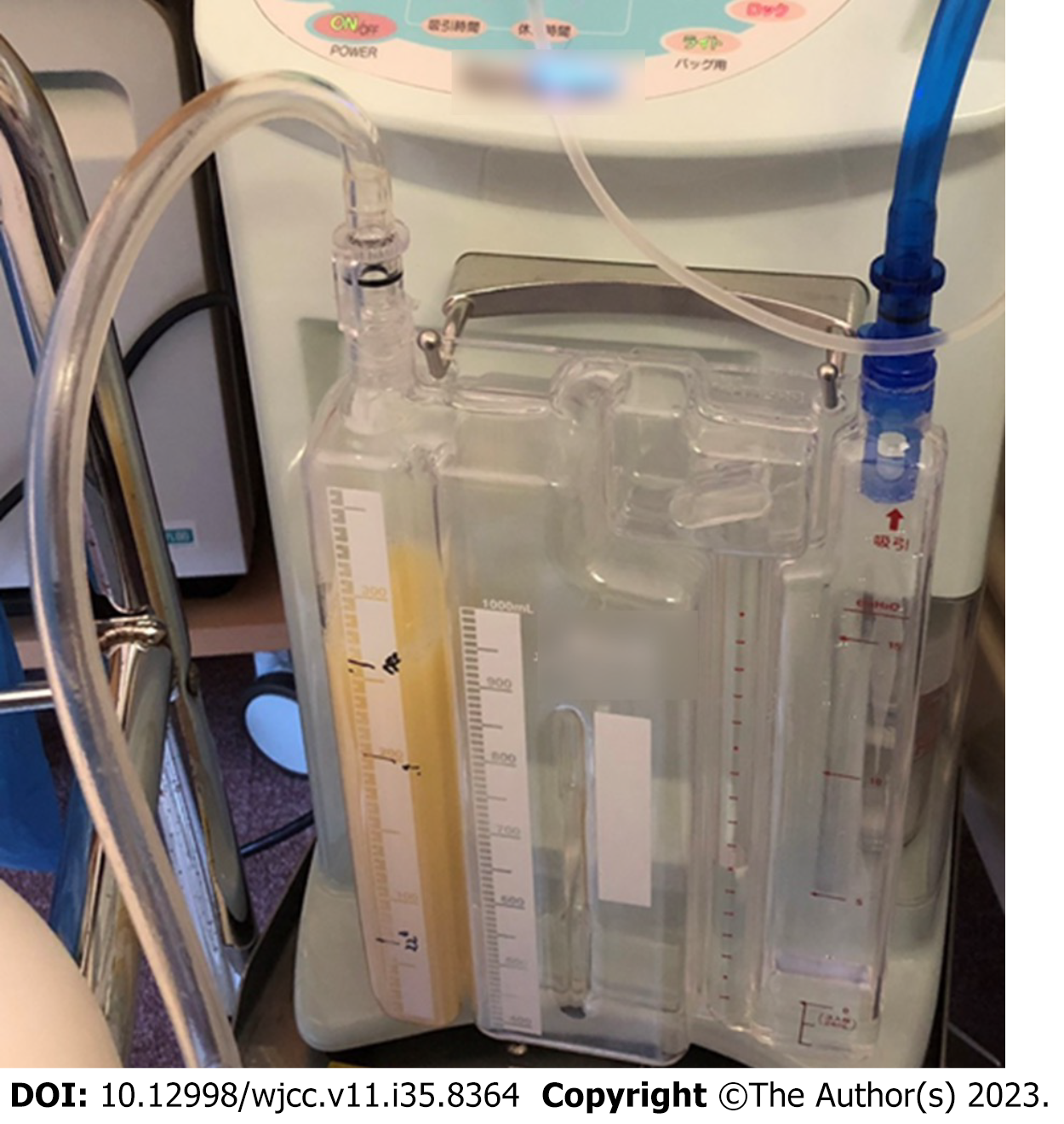
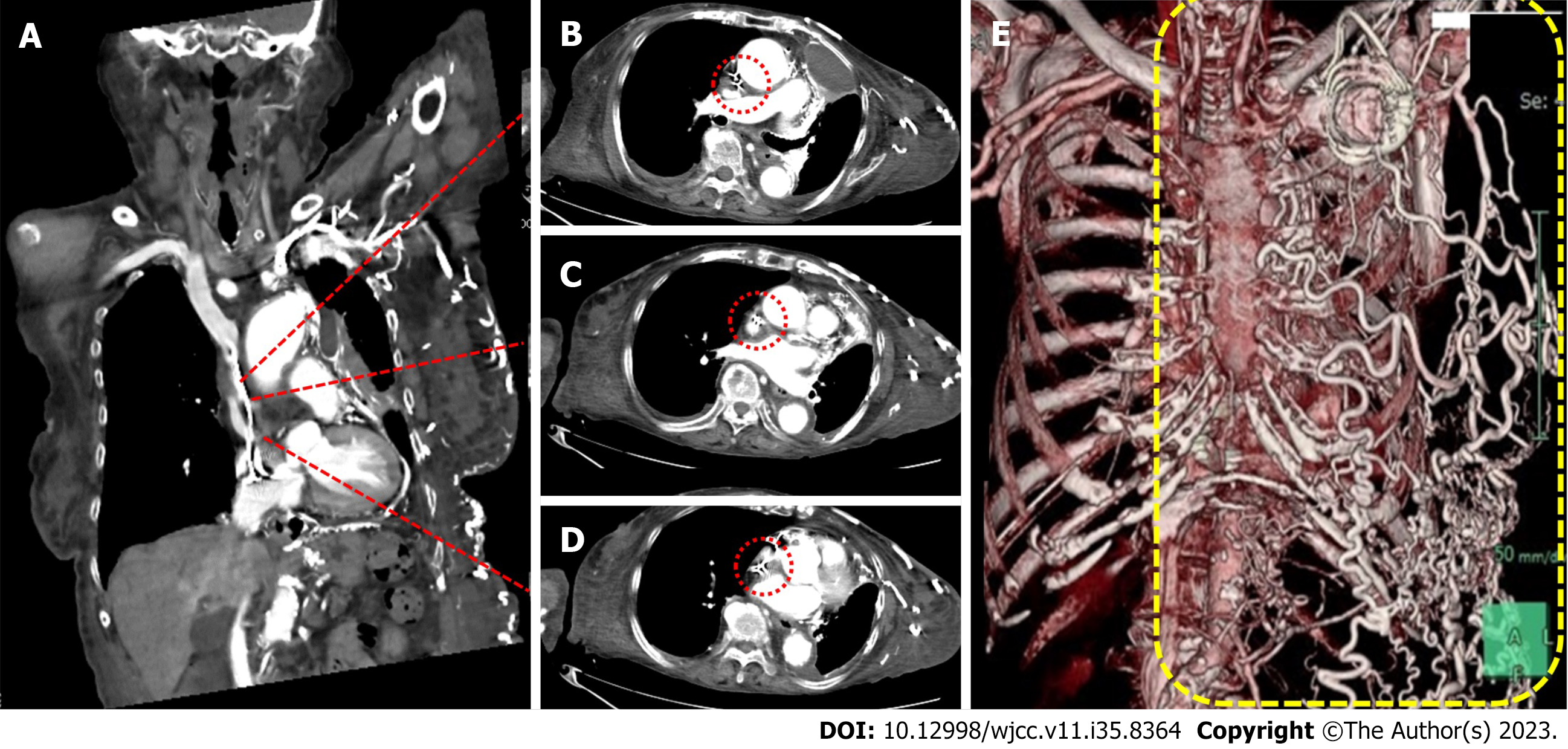
The differential diagnoses included transudative pleural effusion, exudative pleural effusion, haemothorax, prothorax, and chylothorax. However, the effusion was milky white without any foul odour (Figure 3), and the bacterial culture was negative; thus, the patient was diagnosed with chylothorax. The causes of chylothorax may be traumatic or non-traumatic; however, the patient had no history of trauma and had not traveled overseas. Furthermore, she had no history of tuberculosis, and cavitary lesions were absent on CT. Systemic findings were not suggestive of sarcoidosis or amyloidosis. Contrast-enhanced CT revealed no malignant disease, even near the thoracic duct, but revealed severe stenosis of the SVC, and a highly developed collateral circulation in the abdominal wall vein (Figure 4). Echocardiography revealed no evidence of heart failure.
Therefore, chylothorax was considered to have been caused by the SVC syndrome.
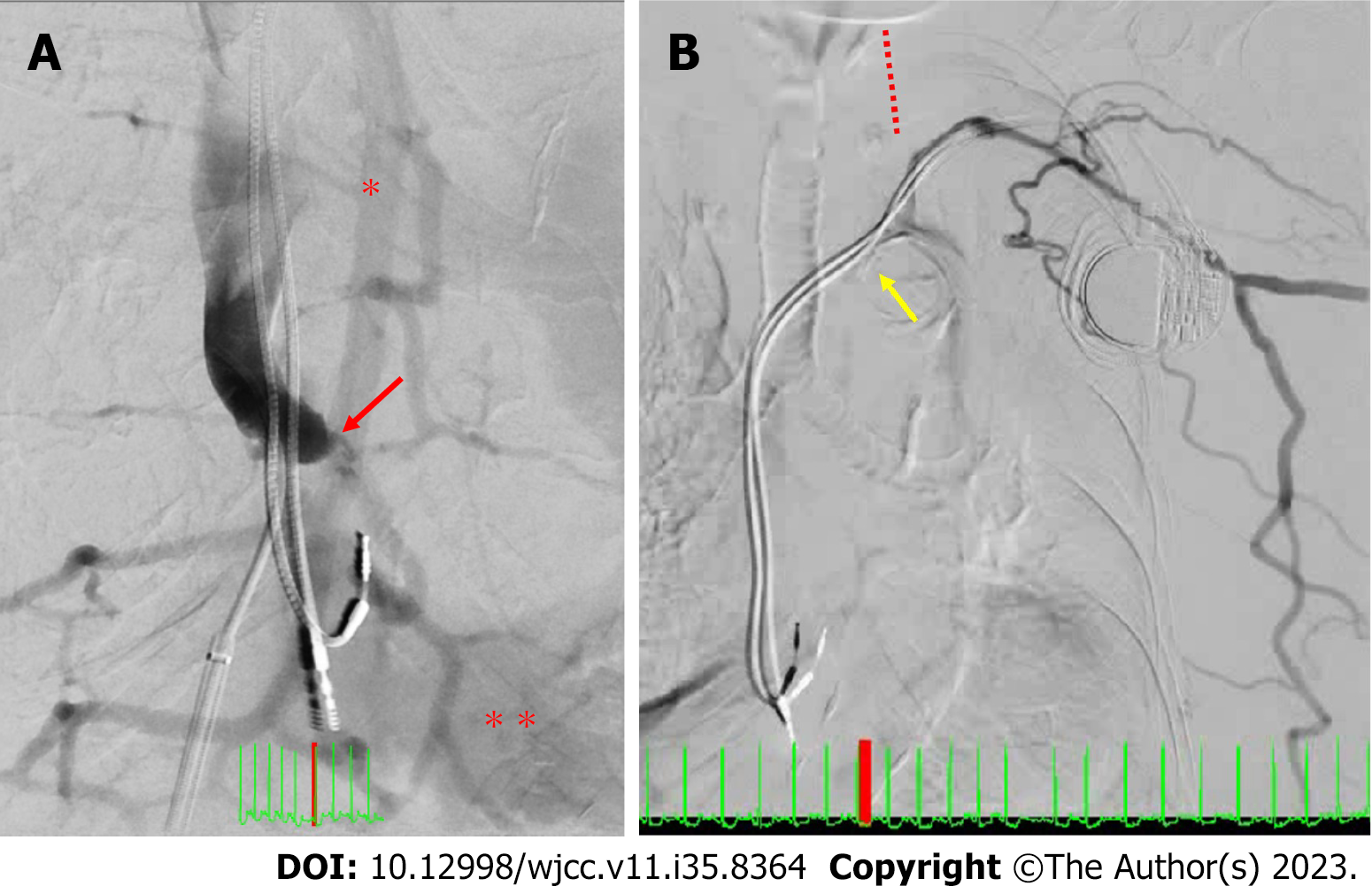
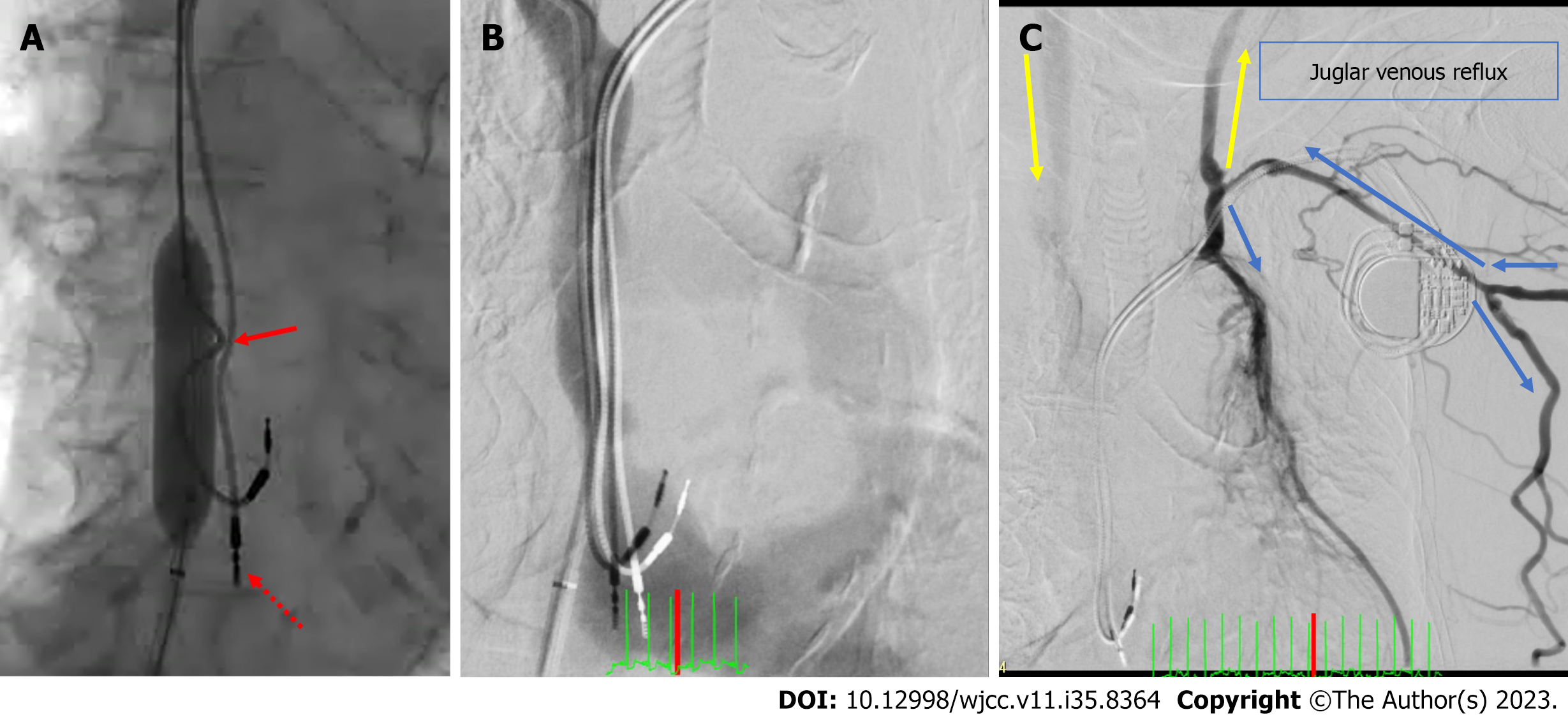
SVC recanalization by removal of the dislodged RV lead was considered; however, it was deemed too high-risk because the patient was of advanced age, and the RV lead implanted eight years ago could have adhered strongly to the innominate vein. Furthermore, thrombolysis was considered potentially ineffective because the patient had received anticoagulation therapy thrice a week during dialysis. Thus, we decided to perform a catheter intervention. A guide sheath was placed in the right atrium (RA). SVC venography was performed from the RA, but the SVC was not contrasted. SVC venography was also performed from the right internal shunt, and an SVC occlusion was noted. Subsequently, the RA was contrasted from the inferior vena cava via the developed azygos and hemiazygos veins (Figure 5A). SVC venography from the left forearm vein revealed innominate vein occlusion and a contrasted highly collateral venous circulation in the abdominal wall; however, the SVC and RA were not contrasted (Figure 5B). The RA pressure (RAP) was low despite the tendency for fluid retention (a/v/m: 1/-4/-2 mmHg). A guidewire was passed through the SVC obstruction, and an angiographic catheter was inserted into the SVC. The SVC pressure differed from the RAP and was extremely high (a/v/m: 30/26/28 mmHg). The localised upper body oedema, lower brain natriuretic peptide (BNP) level despite fluid retention, and chylothorax could be explained by an impaired venous return caused by the SVC occlusion. After confirming the vessel diameter with intravascular ultrasound, a balloon venoplasty was carefully performed (Figure 6A). During the procedure, the heart rhythm transitioned from sinus bradycardia to an atrial paced rhythm, potentially indicating a sinus node injury arising from the balloon venoplasty. However, a sinus rhythm was recovered a few minutes later. The SVC pressure (a/v/m: 17/15/16 mmHg) and RAP (a/v/m: 12/2/8 mmHg) dramatically decreased and increased, respectively, and each value was approximated. Though both pressures were still elevated, we considered the venous return to have improved successfully. When venography was performed from the SVC, the blood flowed into the RA directly (Figure 6B). SVC venography from the left forearm vein revealed that the blood flowing from the left subclavian vein returned to the SVC via the left internal jugular vein retrogradely into the intracranial vein and then through the right internal jugular vein in an antegrade manner (Figure 6C).
After catheter intervention, chylothorax, cyanosis, and upper body oedema improved immediately (Figure 7). Dialysis was performed stably without catecholamine administration; however, the BNP level markedly increased from 139 pg/mL to 1506 pg/mL due to the increased venous return, resulting in left-sided heart failure. Thus, the dry weight was lowered from 38.0 kg to 33.8 kg. The BNP level decreased to 356.6 pg/mL, and heart failure symptoms improved. She was discharged from our hospital on the 32nd day after undergoing rehabilitation.
SVC syndrome is most commonly caused by malignancy[2]. Alternatively, it may caused by benign aetiologies, such as PM leads, central venous ports, and vascular access catheters[3-8]. Some reports of chylothorax caused by the occlusion of the thrombotic subclavian vein, jugular vein, or innominate vein have also been reported[9,10]. SVC syndrome is considered a ‘late’ complication of PM implantation; however, in the present case, it was considered to have had an ‘acute’ onset because the symptoms occurred suddenly after the resolution of bacteraemia, although a highly developed collateral circulation was noted. Therefore, SVC obstruction might be caused by thrombosis or by biofilm formation in the PM lead during ESBL E. coli bacteraemia[11].
An acute disdialysis syndrome caused by SVC syndrome, as in the present case, is a very rare presentation. Acute SVC occlusion results in localised oedema in the upper body despite collateral circulation. Due to PM-associated innominate vein obstruction, venous return from the left upper body flows into the SVC through the collateral circulation in the abdominal wall and the jugular venous reflux (JVR). However, in the present case, due to the acute occlusion of the SVC, the development of the collateral circulation may not have occurred promptly; accordingly, the venous pressure in the right upper half of the body may have increased rapidly, making left JVR impossible. Consequently, the pressure around the left subclavian vein could have improved, impairing the lymphatic return and causing a rapid increase in the chylous pleural effusion[10]. If the SVC occlusion had a chronic course, a considerable increase in the SVC pressure would not have occurred; acute obstruction of the SVC may have contributed to chylous pleural effusion. Acute SVC occlusion resulted in a significant decrease in the cardiac preload. Moreover, dialysis caused a further reduction in the preload, resulting in an excessive blood pressure drop. The PM leads had no vegetation on echocardiography, and we did not remove and observe the leads directly; therefore, the exact cause of the sudden onset of the SVC syndrome remains unknown. Treatments for symptomatic catheter-related SVC syndrome include anticoagulation therapy, percutaneous angioplasty, intravenous stent placement, and surgical removal of the PM lead[12,13]. Some reports have described venous stent placement performed for up to 4 years without recurrence[14]. However, balloon venoplasty and stent placement may damage the PM leads; thus, removing the PM before treatment and reimplanting it thereafter is desirable. The risk of complications following lead removal in older adults has been reported to be relatively low[15]; however, it is essential to consider cases individually. In the present case, the RV lead was dislodged previously, and the atrial lead was bent strongly during venoplasty (Figure 6). The RA lead may have been dislodged and disconnected. In the worst possible case, this could lead to a cardiac arrest because of the simultaneous sinus node injury and RA pacing failure. Therefore, a PM should be placed temporarily before venoplasty.
Prompt revascularization due to the disdialysis syndrome was needed because of a worsening respiratory condition, and we decided to perform SVC revascularization without removing the lead. This treatment was safe and highly effective, considering the patient's age. Compared with the previously reported more invasive approach of PM removal, subsequent stenting, and PM reimplantation, treatment with percutaneous old balloon angioplasty (POBA) alone was less invasive and more successful in achieving a favorable prognosis during the follow-up period. In cases wherein Gram-negative bacteria are detected, PM removal is unnecessary if the antibiotic therapy is successful; however, when bacteraemia recurs, complete device and lead removal are recommended[16].
This report details a single case; nonetheless, our observations indicate that POBA alone may be less invasive and reduce medical costs. Acute SVC occlusion after PM implantation has been rarely reported, and its long-term outcomes are currently unknown. SVC syndrome may recur relatively shortly after treatment, and stent placement should be considered in such cases. Thus, it is necessary to conduct follow-up assessments for potential relapses in these cases. A year has passed since our patient was discharged from the hospital. The limitation of this report is that it is based on a single case, and one year is a short timeframe to definitively determine outcomes. Additional cases and long-term follow-up data are necessary to gain a more comprehensive understanding of the outcomes associated with the use of POBA as the sole treatment for PM-associated SVC syndrome.
We have reported a case of disdialysis syndrome secondary to an acute-onset SVC syndrome with chylothorax caused by a suspected PM lead infection. Venoplasty for SVC occlusion significantly improved the patient’s condition without postoperative complications. Future follow-up examinations are essential for the management of relapses. SVC syndrome should be included in the differential diagnoses of patients with disdialysis syndrome undergoing PM implantation or central venous catheterisation following bacteraemia.
| 1. | Riley RF, Petersen SE, Ferguson JD, Bashir Y. Managing superior vena cava syndrome as a complication of pacemaker implantation: a pooled analysis of clinical practice. Pacing Clin Electrophysiol. 2010;33:420-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Wilson LD, Detterbeck FC, Yahalom J. Clinical practice. Superior vena cava syndrome with malignant causes. N Engl J Med. 2007;356:1862-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 285] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 3. | Sfyroeras GS, Antonopoulos CN, Mantas G, Moulakakis KG, Kakisis JD, Brountzos E, Lattimer CR, Geroulakos G. A Review of Open and Endovascular Treatment of Superior Vena Cava Syndrome of Benign Aetiology. Eur J Vasc Endovasc Surg. 2017;53:238-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Mumoli N, Mazzone A, Evangelista I, Cei M, Colombo A. Superior vena cava syndrome after pacemaker implantation treated with direct oral anticoagulation. Thromb J. 2021;19:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Park HS, Choi J, Baik JH. Central venous disease in hemodialysis patients. Kidney Res Clin Pract. 2019;38:309-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Thapa S, Terry PB, Kamdar BB. Hemodialysis catheter-associated superior vena cava syndrome and pulmonary embolism: a case report and review of the literature. BMC Res Notes. 2016;9:233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Agarwal AK, Khabiri H, Haddad NJ. Complications of Vascular Access: Superior Vena Cava Syndrome. Am J Kidney Dis. 2017;69:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Aryana A, Sobota KD, Esterbrooks DJ, Gelbman AI. Superior vena cava syndrome induced by endocardial defibrillator and pacemaker leads. Am J Cardiol. 2007;99:1765-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Merza N, Lung J, Saadaldin M, Naguib T. Bilateral Chylothorax as a Unique Presentation of Pancreaticobiliary or Upper Gastrointestinal Cancer. Case Rep Pulmonol. 2019;2019:9387021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Thomas R, Christopher DJ, Roy A, Rose A, Chandy ST, Cherian RA, Rima J. Chylothorax following innominate vein thrombosis: a rare complication of transvenous pacemaker implantation. Respiration. 2005;72:546-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Beloin C, Roux A, Ghigo JM. Escherichia coli biofilms. Curr Top Microbiol Immunol. 2008;322:249-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 12. | Sotiriadis C, Volpi S, Douek P, Chouiter A, Muller O, Qanadli SD. Are Endovascular Interventions for Central Vein Obstructions due to Cardiac Implanted Electronic Devices Effective? Front Surg. 2018;5:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Kokotsakis J, Chaudhry UA, Tassopoulos D, Harling L, Ashrafian H, Vernandos M, Kanakis M, Athanasiou T. Surgical management of superior vena cava syndrome following pacemaker lead infection: a case report and review of the literature. J Cardiothorac Surg. 2014;9:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Teo N, Sabharwal T, Rowland E, Curry P, Adam A. Treatment of superior vena cava obstruction secondary to pacemaker wires with balloon venoplasty and insertion of metallic stents. Eur Heart J. 2002;23:1465-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Byrd CL, Wilkoff BL, Love CJ, Sellers TD, Reiser C. Clinical study of the laser sheath for lead extraction: the total experience in the United States. Pacing Clin Electrophysiol. 2002;25:804-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 235] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Kusumoto FM, Schoenfeld MH, Wilkoff BL, Berul CI, Birgersdotter-Green UM, Carrillo R, Cha YM, Clancy J, Deharo JC, Ellenbogen KA, Exner D, Hussein AA, Kennergren C, Krahn A, Lee R, Love CJ, Madden RA, Mazzetti HA, Moore JC, Parsonnet J, Patton KK, Rozner MA, Selzman KA, Shoda M, Srivathsan K, Strathmore NF, Swerdlow CD, Tompkins C, Wazni O. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14:e503-e551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 964] [Cited by in RCA: 906] [Article Influence: 100.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mo XM, China S-Editor: Yan JP L-Editor: A P-Editor: Yan JP