Published online Dec 16, 2023. doi: 10.12998/wjcc.v11.i35.8343
Peer-review started: July 14, 2023
First decision: August 30, 2023
Revised: September 14, 2023
Accepted: November 30, 2023
Article in press: November 30, 2023
Published online: December 16, 2023
Processing time: 152 Days and 20.6 Hours
Synchronous colorectal carcinomas (SCRC) are two or more primary colorectal carcinomas identified simultaneously or within 6 mo of the initial presentation in a single patient. Their incidence is low and the number of pathological types of SCRC is usually no more than two. It is very unusual that the pathological findings of a patient with SCRC show more than two different pathological subtypes. Here, we report a rare case of SCRC with three pathological subtypes.
A 75-year-old woman who had no previous medical history or family history was admitted to the hospital because of intermittent hematochezia for more than a month. Colonoscopy displayed an irregularly shaped neoplasm of the rectum, a tumor-like lesion causing intestinal stenosis in the descending colon, and a polypoidal neoplasm in the ileocecum. Subsequently, she underwent total colectomy, abdominoperineal resection for rectal cancer, and ileostomy. After operation, the pathological report showed three pathological subtypes including well-differentiated adenocarcinoma of the ascending colon, moderately differentiated adenocarcinoma of the descending colon, and mucinous adenocarcinoma of the rectum. She is now recovering well and continues to be closely monitored during follow-up.
Preoperative colonoscopy examination, imaging examination, and extensive intraoperative exploration play important roles in reducing the number of missed lesions.
Core Tip: In most cases, the number of pathologic types of synchronous colorectal carcinomas (SCRC) is limited to 1-2. It is very rare that the pathological findings of a patient with SCRC show more than two different pathological subtypes. Here, we report a rare case of SCRC with three different pathological subtypes without a family history of cancer or genetic predisposing factors.
- Citation: Li F, Zhao B, Zhang L, Chen GQ, Zhu L, Feng XL, Yao H, Tang XF, Yang H, Liu YQ. Rare synchronous colorectal carcinoma with three pathological subtypes: A case report and review of the literature. World J Clin Cases 2023; 11(35): 8343-8349
- URL: https://www.wjgnet.com/2307-8960/full/v11/i35/8343.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i35.8343
Colorectal cancer (CRC) is common malignant tumor of the gastrointestinal tract. According to the Global Cancer Statistics (GLOBOCAN 2020) published by the International Cancer Research Agency of the World Health Organization, the number of new cases of colorectal cancer and the number of deaths from colorectal cancer worldwide in 2020 were 1.93 million and 0.94 million, respectively, ranking third and second among all malignancies, and the incidence has been consistently increasing over the past 10 years[1]. At the same time, as a particular type of CRC, multiple primary colorectal carcinoma (MPCC) has increased in recent years and is increasingly concerning and considered in most areas worldwide[2]. MPCC, also called repeat colorectal cancer, occurs either synchronously or metachronoulsy in patients where there are two or more noncontinuous primary malignant tumors inside the large intestine[3]. The incidence of MPCC was reported at 0.6% to 12.3% during the same period of colorectal cancer review by Soldatkina in 2016[4]. According to the time of onset, MPCC can be divided into synchronous colorectal carcinoma (SCRC) and metachronous colorectal carcinoma. Synchronous carcinomas are exceptional and the frequency of SCRC with various pathological subtypes is even rarer, particularly in the Asian population. Due to missed diagnoses, inaccurate treatment appears frequently. Here, we report a rare case of SCRC with three pathological subtypes without a family history of colon cancer.
One-month history of hematochezia.
A 75-year-old woman was admitted to the Department of General Surgery in September 2022 due to a 1-mo history of hematochezia with no personal or familial history of any specific disease. There was no loss of appetite or general weakness. No fever was noted at home.
No remarkable history of past illness.
No personal or familial history of any specific disease.
Physical examination revealed tenderness in the right lower quadrant of the abdomen. A digital rectal examination could touch half of an annular mass in the anterior wall of the rectum 1-2 cm away from the anal sphincter. The boundary of the mass was clear, the range of motion of the mass was normal, and the intestinal canal was narrow, but a finger could pass through. After the examination, scarlet-colored blood was present on the gloves.
Laboratory investigations revealed a hematocrit value of 30%, hemoglobin of 9.6 g/dl, and albumin of 35 U/ml. Tumor markers including alpha-fetoprotein, carcinoembryonic antigen, and carbohydrate antigen 125 were within the normal reference range.
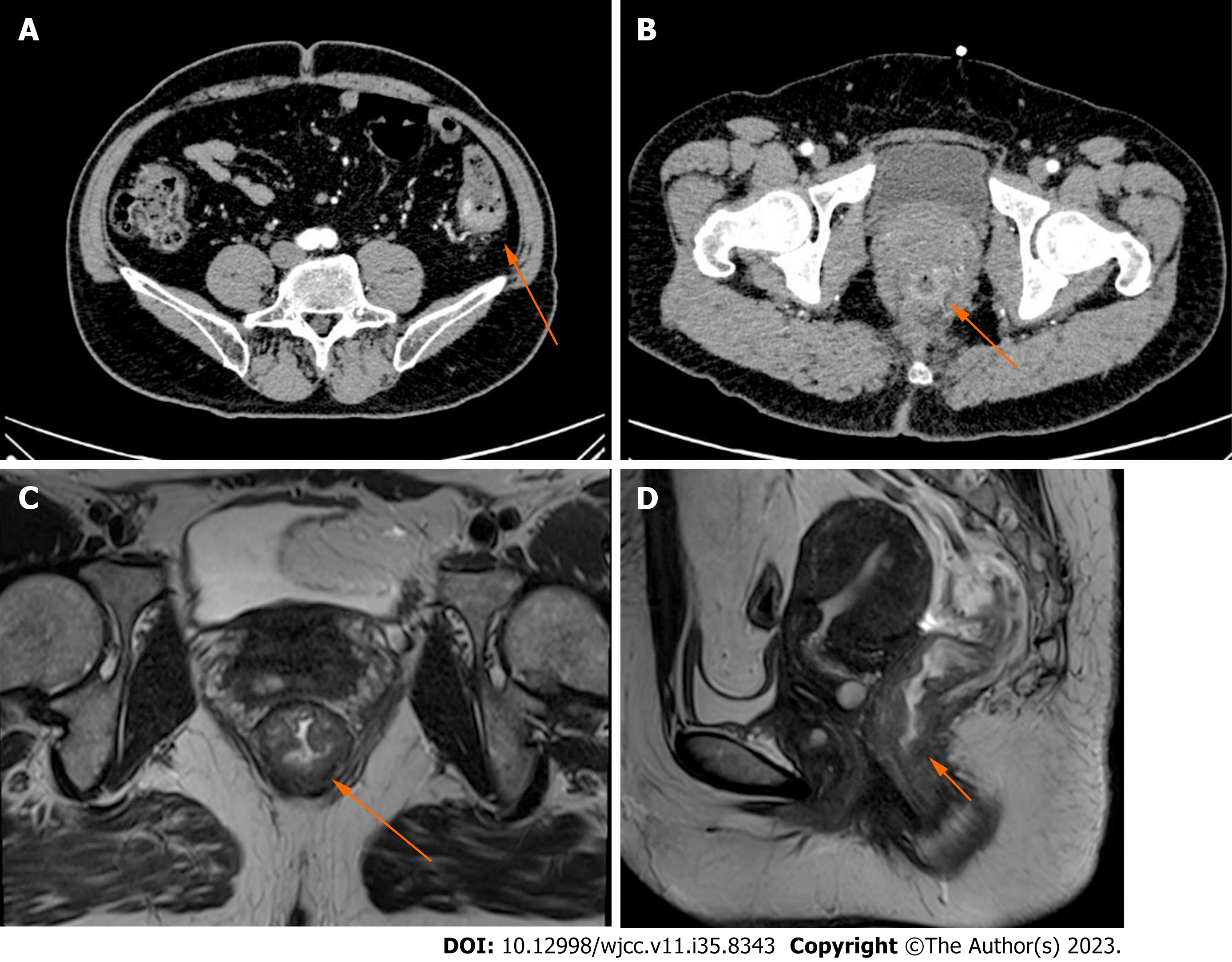
No anomalies were detected on chest X-ray, electrocardiogram, or fiberoptic gastroscopic examination. Computed tomography (CT) of the abdomen revealed intestinal stenosis in the splenic flexure of the colon (Figure 1A) and obvious bowel wall thickness in the lower portions of the rectum (Figure 1B). Magnetic resonance imaging (MRI) of the rectum depicted a sign of concentric tumor rings within the rectal wall and a tumor penetrating the layer of the intrinsic muscle into the perirectal mesenteric fat, and MRI staging was T3N1M0 (Figure 1C and D).
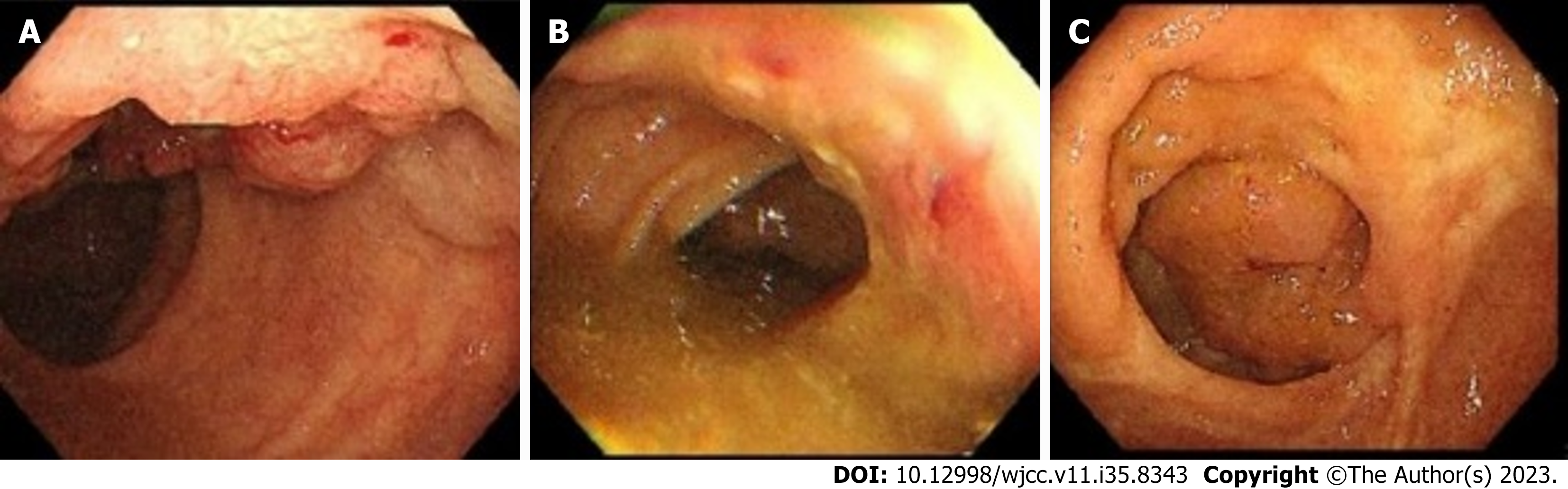
On colonoscopy, an irregularly shaped neoplasm was found in the right rectal wall that occupied over 1/3 of the rectum circumference approximately 2 cm from the anal margin (Figure 2A); a tumor-like lesion that occupied 1/2 of the colon circumference approximately 32 cm from the anal margin and caused intestinal stenosis in the descending colon (Figure 2B) and as well as a 2 cm-wide polypoidal neoplasm in the ileocecum (Figure 2C) were also found. Pathologic specimens were obtained during the colonoscopy procedure, and a preliminary histologic report showed adenocarcinoma in the descending colon and rectum and high-grade intraepithelial neoplasia in the ileocecum.
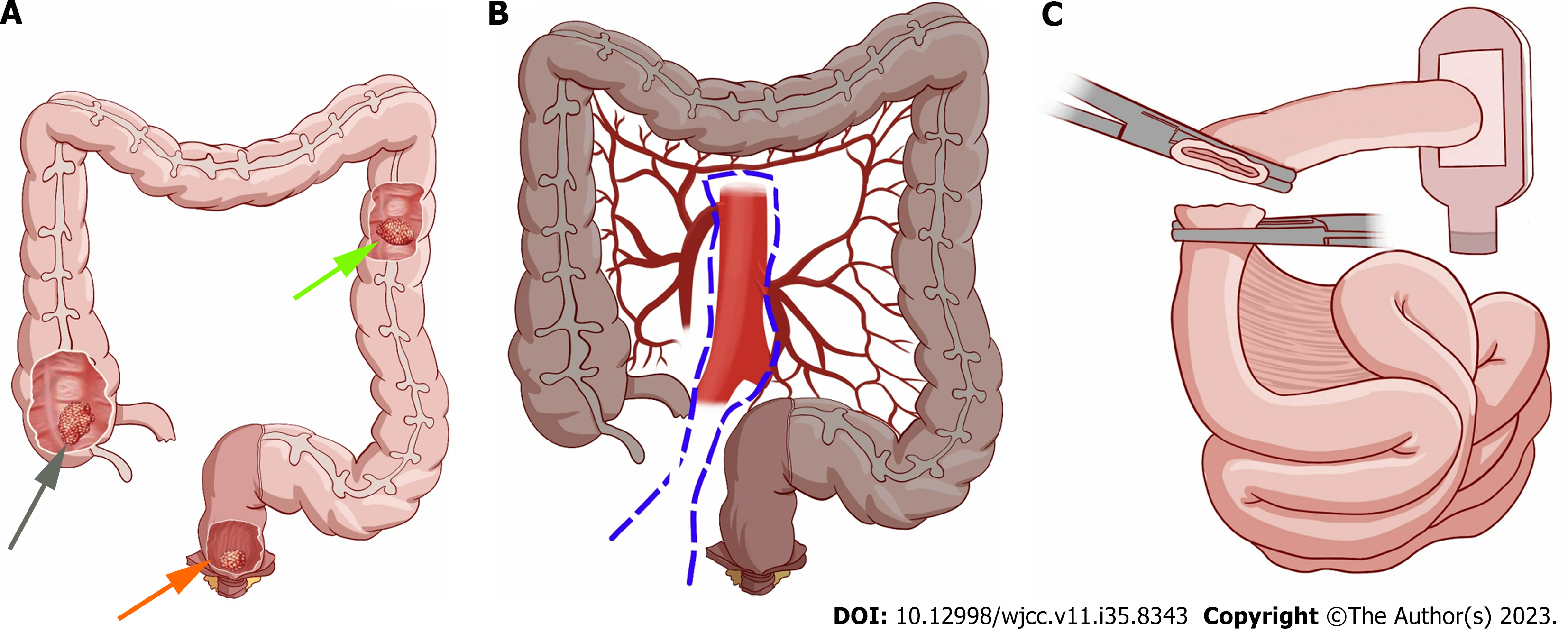
This is the first case of SCRC with three pathological subtypes in the southwest region of China. The patient had no family history of colon cancer or any other specific disease. The first identified tumor, with a diameter of 3 cm, was a mucinous adenocarcinoma located in the rectal region; the second identified tumor, which had a diameter of 4 cm, was diagnosed as a moderately differentiated adenocarcinoma located in the descending colon; and the third identified tumor, with a diameter of 2 cm, was a well-differentiated adenocarcinoma located in the ascending colon.
Total colectomy, abdominoperineal resection for rectal cancer, and ileostomy were performed. Chemotherapy with oxaliplatin and capecitabine was administered.
Exploratory laparotomy revealed a tumor with a diameter of 3 cm in the lower rectal region. A second tumor with a diameter of 4 centimeters was also found at the splenic flexure of the colon. A third tumor with a diameter of 2 centimeters was found in the ileocecum (Figure 3A). No metastatic tumors were detected in the abdominal cavity or liver. The patient underwent an operation including total colectomy, abdominoperineal resection for rectal cancer (Figure 3B) and ileostomy (Figure 3C).
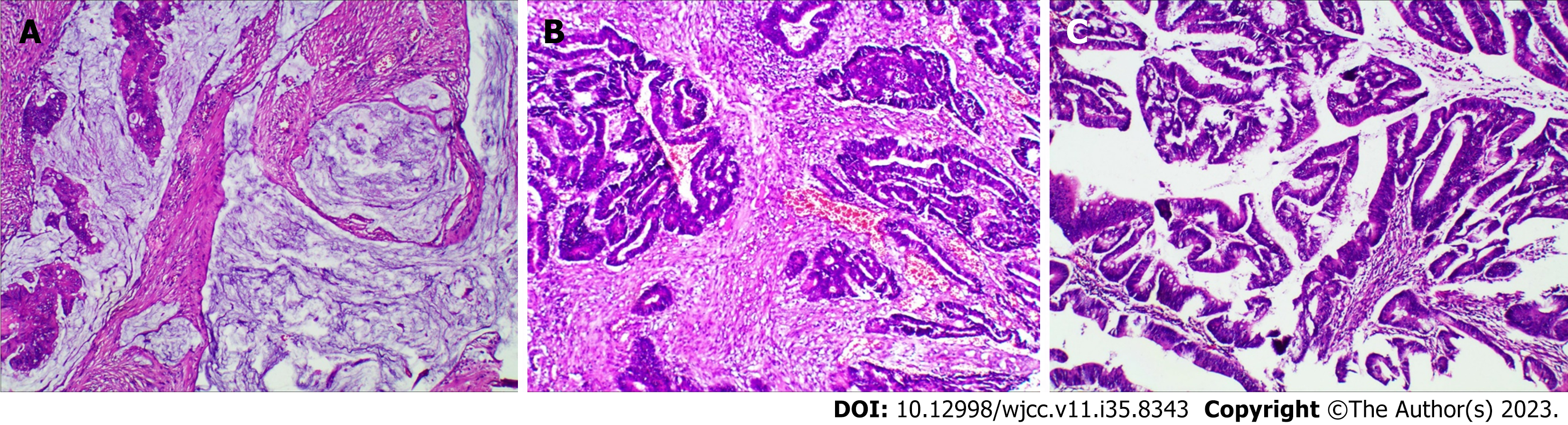
Histopathologic examination of the removed specimens revealed three different types of tumors. Postoperative pathology show rectal mucinous adenocarcinoma (Figure 4A), stage T3N2M0 (staging according to the American Joint Committee on Cancer staging system, 8th edition); moderately differentiated adenocarcinoma of the descending colon (Figure 4B), T4aN2M0; and well-differentiated adenocarcinoma of the ascending colon (Figure 4C), T1N0M0. The patient recovered without incident and was discharged on postoperative Day 9.
The patient is now recovering well and continues to be closely monitored during follow-up. She showed a carcinoembryonic antigen level of < 3.5 UI without tumor activity by CT. The ECOG score was 0 during a follow-up period of 6 mo. A follow-up examination in May 2023 (8 mo after surgery) revealed a well-nourished healthy older woman with no abnormal symptoms or complaints and normal stool evacuations without the need for laxatives or enemas.
A literature review shows that the prevalence of SCRC is approximately 3.5% among all CRCs. This disease has a male to female ratio of 1.8:1[5]. The incidence increases particularly in patients with a history of hereditary inflammatory bowel disease (ulcerative colitis and Crohn’s disease), adenomatosis polyposis, or nonpolyposis colorectal cancer[6,7]. Overlooking potential lesions can cause elevated morbidity and the possibility of advanced staging of the cancer. Therefore, full preoperative colonic evaluation with colonoscopy is recommended according to scholars in several studies[8-10]. In the present case, although no sign of a nidus in the ileocecal region was found by CT, a polypoidal neoplasm in this area was found by preoperative endoscopy. However, approximately 8% to 29% of patients with colorectal cancer present with acute malignant colonic obstruction[11], which prevents colonoscopists from examining the whole large bowel. Additionally, poor bowel preparation and technical limitations are also important reasons for the failure of preoperative colonoscopy of the entire colon. Intraoperative palpation is crucial for a comprehensive diagnosis. Heald et al[12] found that intraoperative palpation can decrease the rate of missed diagnosis of synchronous cancers by 69%.
SCRC refers to two or more primary CRCs identified simultaneously or within 6 mo of the initial presentation in a single patient[13,14]. SCRCs are normally at least 4 cm from each other with a well-defined histopathology of invasive cancer[15,16]. Most SCRCs consist of 2 lesions, but few patients have 3 or even 4 lesions. In an exceptional case, Kaibara et al[17] reported a patient with 7 simultaneous colon cancers. Notably, in most cases, the number of pathologic types of SCRC is limited to 1-2, and it is very unusual that the pathological findings of a patient with SCRC show more than two different pathological subtypes. Here, we report a rare case of SCRC with three different pathological subtypes without a family history of cancer or genetic predisposing factors based on genetic counselling. The patient underwent Lynch syndrome screening according to international guidelines. As a result, a diagnosis of Lynch syndrome was ruled out because there was no history of colorectal cancer in the patient's family, indicating failure to meet the 1999 Amsterdam II criteria, and the immunohistochemical results of the tumor tissue indicated that he patient had mismatch repair-proficient CRC. With the popularization of digestive endoscopy, imaging examinations and other diagnostic and treatment methods in clinical practice, the diagnosis rate of SCRC has increased in recent years. The etiology of SCRC remains unclear, and smoking, alcohol consumption, advanced age, male sex, hereditary cancer, hypertension, cirrhosis, and microsatellite instability have been identified as independent risk factors for the development of SCRC[18,19].
Comprehensive intestinal exploration during surgery is also important for the detection of a second cancer in patients with SCRC. The entire colon and rectum should be palpated in regular sequence. It is noted that the rectum and sigmoid colon are common sites of SCRC. Despite these considerations, sometimes minimal tumors may be overlooked. The use of intraoperative colonoscopy is recommended to confirm that there are no other tumors after surgical resection of the lesioned bowel.
However, not every investigator agrees on the effectiveness of intraoperative colonoscopy. In some studies, it has been reported that the incidence of cancer outside the surgical location is high and that intraoperative colonoscopy increases the risk of infection[20]. Additionally, some studies have shown that this procedure may increase the likelihood of peritoneal contamination and should not be routinely performed intraoperatively[21].
There is no universal standard of surgical treatment for SCRC. Several studies have suggested that total or subtotal colectomy should be performed to remove potential synchronous tumors or polyps that have not been detected and total colectomy may substantially improve the postoperative survival rate[22]. However, other studies recommend a more conservative surgical approach, and the main consideration is to maximize the preservation of patients' bowel function[23]. In our opinion, the operations for multiple primary colorectal carcinomas of the large intestine need to be customized for an individual on the basis of the tumor location, range of invasion, distant metastases, and patient’s health status.
Few prognostic studies of SCRC have been reported worldwide due to the rarity of such cases, and no common conclusion about survival has been reached. Oya et al[24] reported a lower postoperative survival rate for SCRC than for ordinary solitary colorectal cancer and suggested that it is probably related to a more frequent incidence rate of distant metastasis. Conversely, Passman et al[25] and Zhang et al[26] proposed no significant difference in survival between the two entities. We expect that with the continuous improvement of advanced medical examination methods, the level of surgical technology, and the close follow-up of patients with high-risk factors, the survival rate of SCRC will substantially be improved in the future.
To the best of our knowledge, this is the first case of SCRC with three pathological subtypes in the southwest region of China. The patient had no family history of colon cancer or any other specific disease. The first tumor measuring 3 cm in diameter was a rectal mucinous adenocarcinoma in the rectal region, the second tumor measuring 4 cm in diameter was a moderately differentiated adenocarcinoma in the descending colon, and the third tumor measuring 2 cm in diameter was a well-differentiated adenocarcinoma in the ascending colon. Preoperative colonoscopy examination, imaging examination, and extensive intraoperative exploration play important roles in reducing the number of missed lesions, which may increase the survival rate of patients with these cancers. Early diagnosis, radical resection of the tumor at early stages, and close postoperative follow-up can improve survival in these patients.
| 1. | Ferlay J, Ervik M, Lam F. Global cancer observatory: Cancer today [EB/OL]. Lyon, France: International Agency for Research on Cancer 2021 Jan 9. Available from: https://gco.iarc.fr/today. |
| 2. | Zhao Y, Wu J, Pei F, Zhang Y, Bai S, Shi L, Zhang X, Ma J, Zhao X, Ma T, Wang J, Huang M, Fan X, Huang J. Molecular Typing and Clinical Characteristics of Synchronous Multiple Primary Colorectal Cancer. JAMA Netw Open. 2022;5:e2243457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 3. | Huang Q, He X, Qin H, Fan X, Xie M, Lian L. Triple primary malignancies in a patient with colorectal adenocarcinoma: A case report. Int J Surg Case Rep. 2018;42:34-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Soldatkina NV, Kit OI, Gevorkyan YA, Milakin AG. [Multiple primary colorectal cancer: Clinical aspects]. Ter Arkh. 2016;88:53-58. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Lam AK, Chan SS, Leung M. Synchronous colorectal cancer: clinical, pathological and molecular implications. World J Gastroenterol. 2014;20:6815-6820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 118] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 6. | Arriba M, Sánchez R, Rueda D, Gómez L, García JL, Rodríguez Y, Pajares JA, Pérez J, Urioste M, Sarmiento RG, Perea J. Toward a Molecular Classification of Synchronous Colorectal Cancer: Clinical and Molecular Characterization. Clin Colorectal Cancer. 2017;16:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Papadopoulos V, Michalopoulos A, Basdanis G, Papapolychroniadis K, Paramythiotis D, Fotiadis P, Berovalis P, Harlaftis N. Synchronous and metachronous colorectal carcinoma. Tech Coloproctol. 2004;8 Suppl 1:s97-s100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Yeh CC, Hsi SC, Chuu CP, Kao YH. Synchronous triple carcinoma of the colon and rectum. World J Surg Oncol. 2013;11:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Ridolfi TJ, Valente MA, Church JM. Achieving a complete colonic evaluation in patients with incomplete colonoscopy is worth the effort. Dis Colon Rectum. 2014;57:383-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Askew A, Ward M, Cowen A. The influence of colonoscopy on the operative management of colorectal cancer. Med J Aust. 1986;145:254-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Deans GT, Krukowski ZH, Irwin ST. Malignant obstruction of the left colon. Br J Surg. 1994;81:1270-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 365] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 12. | Heald RJ, Bussey HJ. Clinical experiences at St. Mark's Hospital with multiple synchronous cancers of the colon and rectum. Dis Colon Rectum. 1975;18:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 116] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Kato T, Alonso S, Muto Y, Noda H, Miyakura Y, Suzuki K, Tsujinaka S, Saito M, Perucho M, Rikiyama T. Clinical characteristics of synchronous colorectal cancers in Japan. World J Surg Oncol. 2016;14:272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Huang CS, Yang SH, Lin CC, Lan YT, Chang SC, Wang HS, Chen WS, Lin TC, Lin JK, Jiang JK. Synchronous and Metachronous Colorectal Cancers: Distinct Disease Entities or Different Disease Courses? Hepatogastroenterology. 2015;62:838-842. [PubMed] |
| 15. | Tziris N, Dokmetzioglou J, Giannoulis K, Kesisoglou I, Sapalidis K, Kotidis E, Gambros O. Synchronous and metachronous adenocarcinomas of the large intestine. Hippokratia. 2008;12:150-152. [PubMed] |
| 16. | Şavlovschi C, Comandaşu M, Şerban D. Specifics of diagnosis and treatment in synchronous colorectal cancers (SCC). Chirurgia (Bucur). 2013;108:43-45. [PubMed] |
| 17. | Kaibara N, Koga S, Jinnai D. Synchronous and metachronous malignancies of the colon and rectum in Japan with special reference to a coexisting early cancer. Cancer. 1984;54:1870-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Chin CC, Kuo YH, Chiang JM. Synchronous colorectal carcinoma: predisposing factors and characteristics. Colorectal Dis. 2019;21:432-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Drew DA, Nishihara R, Lochhead P, Kuchiba A, Qian ZR, Mima K, Nosho K, Wu K, Wang M, Giovannucci E, Fuchs CS, Chan AT, Ogino S. A Prospective Study of Smoking and Risk of Synchronous Colorectal Cancers. Am J Gastroenterol. 2017;112:493-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Torralba JA, Robles R, Parrilla P, Lujan JA, Liron R, Piñero A, Fernandez JA. Subtotal colectomy vs. intraoperative colonic irrigation in the management of obstructed left colon carcinoma. Dis Colon Rectum. 1998;41:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Ikeda Y, Saku M, Kawanaka H, Muranaka T, Sugimachi K. Distribution of synchronous and metchronous multiple colorectal cancers. Hepato-Gastroen-terology. 2004;51:443-466. [DOI] [Full Text] |
| 22. | Easson AM, Cotterchio M, Crosby JA, Sutherland H, Dale D, Aronson M, Holowaty E, Gallinger S. A population-based study of the extent of surgical resection of potentially curable colon cancer. Ann Surg Oncol. 2002;9:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Tsantilas D, Ntinas A, Petras P, Zambas N, Al Mogrambi S, Frangandreas G, Spyridis C, Gerasimidis T. Metachronous colorectal adenocarcinomas. Tech Coloproctol. 2004;8 Suppl 1:s202-s204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Oya M, Takahashi S, Okuyama T, Yamaguchi M, Ueda Y. Synchronous colorectal carcinoma: clinico-pathological features and prognosis. Jpn J Clin Oncol. 2003;33:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Passman MA, Pommier RF, Vetto JT. Synchronous colon primaries have the same prognosis as solitary colon cancers. Dis Colon Rectum. 1996;39:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Zhang L, Zhang Z, Zhang Y, Zhou D. [An analysis of 35 cases of multiple gastrointestinal cancers]. Zhonghua Nei Ke Za Zhi. 1999;38:88-90. [PubMed] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lim YC, Brunei Darussalam; Maffeis V, Italy; Augustin G, Croatia S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Cai YX