Published online Nov 26, 2023. doi: 10.12998/wjcc.v11.i33.8058
Peer-review started: September 3, 2023
First decision: October 24, 2023
Revised: November 1, 2023
Accepted: November 10, 2023
Article in press: November 10, 2023
Published online: November 26, 2023
Processing time: 81 Days and 21.6 Hours
Intravascular large B-cell lymphoma (IVLBCL) is a rare subtype of extranodal lymphoma. In particular, the Asian variant of IVLBCL is characterized by hemo
A 67-year-old female patient complained of gradually worsening cognitive impairment. While hospitalized, she developed a high fever and showed marked bicytopenia. Intracranial imaging revealed a suspected leptomeningeal disease. Although no malignant cells were found in the cerebrospinal fluid (CSF), the protein and lactate dehydrogenase levels in CSF were increased. Bone marrow examination revealed an increased number of hemophagocytic histiocytes, and
Suspicion of IVLBCL and immediate diagnosis lead to timely treatment. More
Core Tip: Intravascular large B-cell lymphoma (IVLBCL) is a rare but clinically aggressive lymphoproliferative disease. Given its aggressive nature, immediate diagnosis of IVLBCL and timely treatment are critical for better clinical outcomes. As central nervous system (CNS) involvement adversely affects prognosis if IVLBCL, active CNS examination is required at diagnosis. In addition, along with conventional pathology, targeted sequencing contributes to diagnosis and provides a basis for use of targeted agents. Here, we report a case of Asian variant IVLBCL with highly suspected CNS involvement. We first describe the clinical course of disease and then discuss the genetic aberrations found in the patient.
- Citation: Lee YP, Son SM, Kwon J. Asian variant intravascular large B-cell lymphoma with highly suspected central nervous system involvement: A case report. World J Clin Cases 2023; 11(33): 8058-8064
- URL: https://www.wjgnet.com/2307-8960/full/v11/i33/8058.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i33.8058
Intravascular large B-cell lymphoma (IVLBCL), characterized by growth of lymphoma cells within the lumen of blood vessels, is a rare type of lymphoid malignancy[1]. According to the World Health Organization classification, IVLBCL is divided into classic, hemophagocytic syndrome–associated, and isolated cutaneous variants. In addition, classification into Asian and Western variants according to the clinical manifestation and geographic distribution is also practiced[2,3]. The Asian variant of IVLBCL predominantly involves the liver, spleen, and bone marrow and often accompanies hemo
A 67-year-old female patient visited the department of neurology for deteriorating cognitive function.
The patient was able to walk with a cane and take care of herself. However, upon presentation, her cognitive function had deteriorated, she could not recognize her neighbors, and she had difficulty walking unassisted.
She had been diagnosed with cerebellar ataxia a few years prior and was on the medications such as cilostazol and atorvastatin.
She had no personal or family history.
At the time of examination, she had a mild fever of 37.8°C, but her other vital signs were stable, and she reported no symptoms other than deteriorated cognitive function. There was no sensory deficit, and motor power was intact, although her coordination was poor.
Laboratory testing confirmed bicytopenia (hemoglobin, 8.5 g/dL; platelet count, 77 × 103/μL) and elevated C-reactive protein (5.26 mg/dL) and lactate dehydrogenase (LDH) (891 IU/L) levels (Table 1). She was confirmed to have a urinary tract infection caused by Escherichia coli.
| Sex/age (yr) | ECOG | Disease involvement sites | Ann Arbor stage | IPI | Cell of origin | WBC (103/μL) | Hb (g/dL) | Plt | LDH (IU/L) | CRP (mg/dL) | Ferritin (ng/mL) |
| F/67 | 4 | Right ethmoid sinus, liver, spleen, bilateral adrenal glands | IV | 5 | Non-GCB | 5.35 | 8.5 | 77 | 891 | 5.26 | 835 |
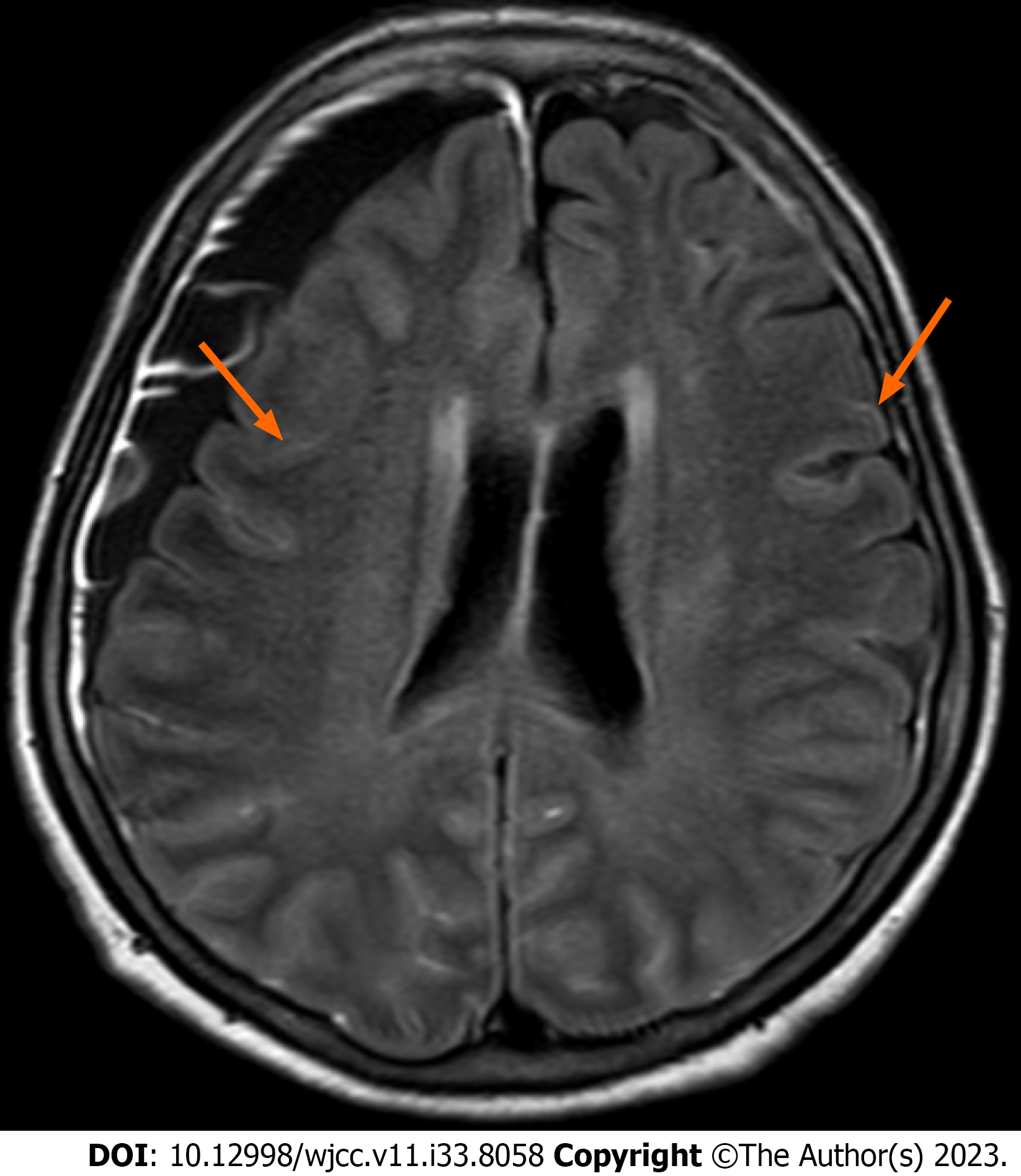
Following brain magnetic resonance imaging (MRI), a focal diffusion restrictive lesion in the left parietal lobe and chronic subdural hemorrhage in the right frontal convexity were observed. In addition, pachymeningeal enhancement of the bilateral frontoparietal convexities was noted, suggesting leptomeningeal disease (Figure 1).
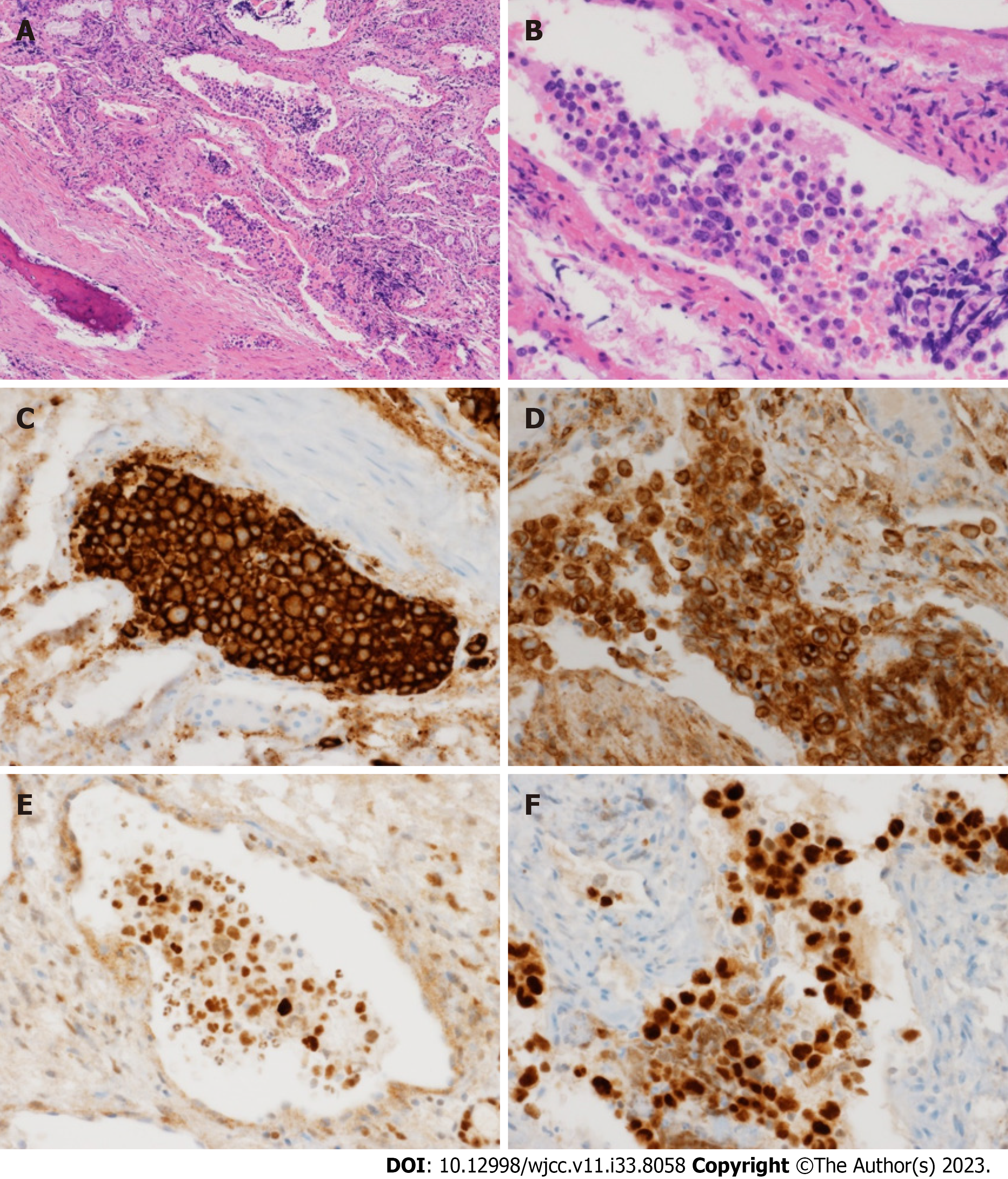
Three consecutive lumbar punctures were performed, and a consistent increase in protein and LDH levels in cerebrospinal fluid (CSF) was observed. No malignant cells were observed and the CSF pressure was within the normal range (7.5 cmH2O). Abdominal CT showed bilateral enlargement of adrenal glands along with hepatomegaly and splenomegaly. Meanwhile, her cognitive function and the results of blood tests were worsening (e.g., exacerbation of cytopenia, elevation of ferritin and triglyceride levels), and, despite improved urinalysis findings after antibiotic therapy, she developed a high fever up to 39°C. Thus, the patient was referred to a hematologist who performed immediate bone marrow exam. Increased numbers of hemophagocytic histiocytes were found without malignant cells, suggesting secondary HLH. PET-CT was performed to identify the underlying disease. An increased FDG uptake was observed in both adrenal glands, the liver, and the right ethmoid sinus (Figure 2), and a biopsy of the right ethmoid sinus was ordered. Histological examination revealed atypical large lymphoid cells with prominent nucleoli in the vessels (Figure 3A and B). Immunohistochemical analysis showed that the tumor cells were positive for CD20, BCL2, BCL6, and IRF4/MUM1 (Figure 3C-F) but negative for CD3 and CD10. In addition, MYD88, TET2, and PIM1 mutations were identified by targeted sequencing using tissues (Table 2).
| Tier 1 variants | Tier 2 variants | Tier 3 variants | |||||||||
| Gene | DNA | Protein | VAF | Gene | DNA | Protein | VAF | Gene | DNA | Protein | VAF |
| MYD88 | c.755T>C | p.Leu252Pro | 54.8% | PIM1 | c.237G>C | p.Glu79Asp | 30.77% | NOTCH1 | c.6283C>T | p.Arg2095Cys | 37.7% |
| ETV6 | c.1123G>A | p.Gly375Arg | 14.4% | ||||||||
| TET2 | c.3280A>T | p.Lys1094Ter | 16.0% | BTG2 | c.97C>T | p.Gln33Ter | 19.6% | HIST1H1E | c.367G>A | p.Ala123Thr | 15.6% |
| TBL1XR1 | c.848G>A | p.Ser283Asn | 16.5% | ||||||||
The Asian variant of IVLBCL was diagnosed. In addition, although there was no cytological confirmation, the IVLBCL was considered to be accompanied by CNS involvement based on the findings of brain MRI and CSF analysis as well as her clinical manifestation.
Intravenous methylprednisolone administration at a dose of 1 mg/kg was started immediately after the biopsy, and, following the final diagnosis, immunochemotherapy including rituximab and CNS-directed therapy with methotrexate (MTX) was considered. However, due to her poor performance status and economic issues, she decided to receive only steroid therapy and best supportive care.
The patient deteriorated and passed away two weeks after her diagnosis.
Despite a quantum leap of cancer diagnostic technology, the diagnosis of IVLBCL remains challenging due to the ambiguous signs and symptoms that do not precisely reflect the characteristics of the disease. In particular, approximately 20%-30% of Asian variant IVLBCL cases have CNS involvement at diagnosis[3,7], which is associated with poor prognosis[8]. However, since malignant lymphocytes are rarely found in CSF and there are no previously described pathognomonic findings on MRI[9], auxiliary diagnostic tools may often be required. Recently introduced less-invasive diagnostic methods using peripheral blood or CSF, such as liquid biopsy[10], or mutation detection using circulating tumor DNA[11,12] can play a complementary role in diagnosing IVLBCL. Therefore, when diagnosing IVLBCL, a multidisciplinary approach and an integrated diagnostic process are needed to analyze each symptom according to involved organ, including active CNS examination.
Malignant lymphoma derived from T-cells or natural killer cells is one of the leading causes of HLH in adults[13,14], but B-cell origin lymphoproliferative disease can also provoke HLH[14]. Indeed, the Asian variant of IVLBCL is commonly accompanied by HLH[2,3,6]. Therefore, in adult patients suspected of secondary HLH, systemic evaluation and biopsy based on PET-CT scan should be performed promptly. However, as opposed to the nodal diffuse large B-cell lymphoma (DLBCL), IVLBCL mainly involves extranodal sites and shows various levels (usually mild to moderate) of FDG uptake in PET-CT[5], and in general, the selection of PET-CT-based biopsy lesions may be difficult under these circumstances. Nonetheless, in diagnosing IVLBCL, when infectious or inflammatory diseases are excluded, such findings may help to select the appropriate biopsy site[5]. Therefore, despite some limitations, PET-CT may play an important role in the diagnosis of IVLBCL.
The absence of a prospective study due to the rarity of the disease hindered the establishment of standard of care for IVLBCL. Thus, IVLBCL has been managed by adapting the treatment strategy of DLBCL, where several immuno
In conclusion, given the aggressive nature of IVLBCL, suspicion of the disease and subsequent immediate and accurate diagnosis lead to timely treatment, which results in better clinical outcomes. In addition, considering its poor prognosis, careful examination of CNS involvement at diagnosis is recommended. Even if CNS invasion of IVLBCL is not confirmed, active CNS-directed therapy is required when highly suspected.
| 1. | Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4245] [Cited by in RCA: 5649] [Article Influence: 564.9] [Reference Citation Analysis (1)] |
| 2. | Murase T, Yamaguchi M, Suzuki R, Okamoto M, Sato Y, Tamaru J, Kojima M, Miura I, Mori N, Yoshino T, Nakamura S. Intravascular large B-cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood. 2007;109:478-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 305] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 3. | Yoon SE, Kim WS, Kim SJ. Asian variant of intravascular large B-cell lymphoma: a comparison of clinical features based on involvement of the central nervous system. Korean J Intern Med. 2020;35:946-956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Ponzoni M, Arrigoni G, Gould VE, Del Curto B, Maggioni M, Scapinello A, Paolino S, Cassisa A, Patriarca C. Lack of CD 29 (beta1 integrin) and CD 54 (ICAM-1) adhesion molecules in intravascular lymphomatosis. Hum Pathol. 2000;31:220-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 192] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Lim CH, Yoon SE, Kim WS, Lee KH, Kim SJ. Imaging Features and Prognostic Value of FDG PET/CT in Patients with Intravascular Large B-Cell Lymphoma. Cancer Manag Res. 2021;13:7289-7297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Hong JY, Kim HJ, Ko YH, Choi JY, Jung CW, Kim SJ, Kim WS. Clinical features and treatment outcomes of intravascular large B-cell lymphoma: a single-center experience in Korea. Acta Haematol. 2014;131:18-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Shimada K, Murase T, Matsue K, Okamoto M, Ichikawa N, Tsukamoto N, Niitsu N, Miwa H, Asaoku H, Kosugi H, Kikuchi A, Matsumoto M, Saburi Y, Masaki Y, Yamamoto K, Yamaguchi M, Nakamura S, Naoe T, Kinoshita T; IVL Study Group in Japan. Central nervous system involvement in intravascular large B-cell lymphoma: a retrospective analysis of 109 patients. Cancer Sci. 2010;101:1480-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Fonkem E, Dayawansa S, Stroberg E, Lok E, Bricker PC, Kirmani B, Wong ET, Huang JH. Neurological presentations of intravascular lymphoma (IVL): meta-analysis of 654 patients. BMC Neurol. 2016;16:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks. Blood. 2018;132:1561-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 198] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 10. | Baraniskin A, Schroers R. Liquid Biopsy and Other Non-Invasive Diagnostic Measures in PCNSL. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Rimelen V, Ahle G, Pencreach E, Zinniger N, Debliquis A, Zalmaï L, Harzallah I, Hurstel R, Alamome I, Lamy F, Voirin J, Drénou B. Tumor cell-free DNA detection in CSF for primary CNS lymphoma diagnosis. Acta Neuropathol Commun. 2019;7:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Yoon SE, Kim YJ, Shim JH, Park D, Cho J, Ko YH, Park WY, Mun YC, Lee KE, Cho D, Kim WS, Kim SJ. Plasma Circulating Tumor DNA in Patients with Primary Central Nervous System Lymphoma. Cancer Res Treat. 2022;54:597-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383:1503-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 1024] [Article Influence: 85.3] [Reference Citation Analysis (0)] |
| 14. | La Rosée P, Horne A, Hines M, von Bahr Greenwood T, Machowicz R, Berliner N, Birndt S, Gil-Herrera J, Girschikofsky M, Jordan MB, Kumar A, van Laar JAM, Lachmann G, Nichols KE, Ramanan AV, Wang Y, Wang Z, Janka G, Henter JI. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133:2465-2477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 731] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 15. | Ferreri AJ, Dognini GP, Bairey O, Szomor A, Montalbán C, Horvath B, Demeter J, Uziel L, Soffietti R, Seymour JF, Ambrosetti A, Willemze R, Martelli M, Rossi G, Candoni A, De Renzo A, Doglioni C, Zucca E, Cavalli F, Ponzoni M; International Extranodal Lymphoma Study Group. The addition of rituximab to anthracycline-based chemotherapy significantly improves outcome in 'Western' patients with intravascular large B-cell lymphoma. Br J Haematol. 2008;143:253-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Shimada K, Matsue K, Yamamoto K, Murase T, Ichikawa N, Okamoto M, Niitsu N, Kosugi H, Tsukamoto N, Miwa H, Asaoku H, Kikuchi A, Matsumoto M, Saburi Y, Masaki Y, Yamaguchi M, Nakamura S, Naoe T, Kinoshita T. Retrospective analysis of intravascular large B-cell lymphoma treated with rituximab-containing chemotherapy as reported by the IVL study group in Japan. J Clin Oncol. 2008;26:3189-3195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 17. | Wang J, Alhaj Moustafa M, Kuhlman JJ, Seegobin K, Jiang L, Gupta V, Tun HW. Intravascular Large B Cell Lymphoma with CNS Involvement Successfully Treated with High-Dose Methotrexate and High-Dose Ara-C Based CNS-Directed Chemoimmunotherapy Alternating with Anthracycline Based Chemoimmunotherapy. Blood Lymphat Cancer. 2022;12:47-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Takahashi H, Nishimaki H, Nakanishi Y, Hamada T, Nakagawa M, Iizuka K, Uchino Y, Iriyama N, Miura K, Nakayama T, Masuda S, Hatta Y, Nakamura H. Clinical impact of central nervous system-directed therapies on intravascular large B-cell lymphoma: A single institution's experience. EJHaem. 2022;3:467-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Schrader AMR, Jansen PM, Willemze R, Vermeer MH, Cleton-Jansen AM, Somers SF, Veelken H, van Eijk R, Kraan W, Kersten MJ, van den Brand M, Stevens WBC, de Jong D, Abdul Hamid M, Tanis BC, Posthuma EFM, Nijland M, Diepstra A, Pals ST, Cleven AHG, Vermaat JSP. High prevalence of MYD88 and CD79B mutations in intravascular large B-cell lymphoma. Blood. 2018;131:2086-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Somers S, Jansen P, Veelken H, Vermeer M, Kraan W, Schrader A-R, Posthuma W, Nijland M, Diepstra A, Kersten MJ, Pals S, Cleven A, Vermaat J. High Prevalence of Oncogenic MYD88 and CD79B Mutations in Intravascular Large B-Cell Lymphoma: Implication for Therapy with Bruton's Kinase Inhibitors? Blood. 2017;130 Suppl 1:4000. [DOI] [Full Text] |
| 21. | Weissinger SE, Dugge R, Disch M, Barth TF, Bloehdorn J, Zahn M, Marienfeld R, Viardot A, Möller P. Targetable alterations in primary extranodal diffuse large B-cell lymphoma. EJHaem. 2022;3:688-697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Wilson WH, Young RM, Schmitz R, Yang Y, Pittaluga S, Wright G, Lih CJ, Williams PM, Shaffer AL, Gerecitano J, de Vos S, Goy A, Kenkre VP, Barr PM, Blum KA, Shustov A, Advani R, Fowler NH, Vose JM, Elstrom RL, Habermann TM, Barrientos JC, McGreivy J, Fardis M, Chang BY, Clow F, Munneke B, Moussa D, Beaupre DM, Staudt LM. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21:922-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 949] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 23. | Zhang Y, Jia C, Wang W, Zhang L, Cao X, Li J, Zhang W, Zhou D. The Interim Analysis from a Prospective Single-Center Phase 2 Study of Zanubrutinib Plus R-CHOP in Treat-Naïve Intravascular Large B Cell Lymphoma. Blood. 2021;138 Suppl 1:3563. [RCA] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Roganovic J, Croatia S-Editor: Liu JH L-Editor: A P-Editor: Xu ZH