Published online Nov 26, 2023. doi: 10.12998/wjcc.v11.i33.8030
Peer-review started: September 14, 2023
First decision: September 28, 2023
Revised: October 9, 2023
Accepted: November 13, 2023
Article in press: November 13, 2023
Published online: November 26, 2023
Processing time: 70 Days and 23.3 Hours
Hepatic cysts are common benign liver tumors that are typically asymptomatic. However, larger cysts, particularly giant liver cysts, can potentially induce symptoms. If the diameter of the cyst exceeds 10 cm, it can exert pressure on adjacent organs, leading to manifestations of corresponding symptoms. Here, we report the case of a complex giant hepatic cyst that caused pseudocystitis.
A 16-year-old girl was admitted to our hospital with frequent and urgent uri
Our patient presented with symptoms suggestive of pseudocystitis, stressing the need for considering possibilities of other etiologies and differential diagnoses.
Core Tip: Giant hepatic cysts can cause symptoms upon reaching a significant size. We present a case of a complex giant liver cyst causing pseudocystitis in a 16-year-old girl with frequent and urgent urination. Imaging revealed a large cystic lesion in the liver. Laparoscopic resection with partial liver resection was performed, resulting in the resolution of the urinary symptoms. Therefore, clinicians should consider the possibility of giant liver cysts in patients presenting with similar symptoms.
- Citation: Li S, Tang J, Ni DS, Xia AD, Chen GL. Giant complex hepatic cyst causing pseudocystitis: A case report. World J Clin Cases 2023; 11(33): 8030-8037
- URL: https://www.wjgnet.com/2307-8960/full/v11/i33/8030.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i33.8030
Liver cysts are common asymptomatic benign tumors with an incidence of 4.5%–7.0%[1,2] and a female preponderance (1.5:1)[3,4]. Liver cysts are mostly diagnosed in adults aged around 40 years and are usually detected incidentally during imaging using computed tomography and/or ultrasonography[5-7]. Frequent and urgent micturition in women, especially married women, is mostly caused by urinary tract and gynecological pathologies. Liver cysts do not cause symptoms related to other abdominal organs unless they expand in size, and cause compression on adjacent structures and symptoms subsequent to that[8,9]. Pseudocystitis resulting from a liver cyst is rare. Here, we report a case of pseudocystitis caused by a giant liver cyst.
Frequent and urgent urination and discovery of an abdominal mass.
A 16-year-old girl was admitted to our hospital with frequent and urgent urination. She had no nausea, vomiting, abdominal pain, or feeling of bloating. Gynecological examination using ultrasonography revealed no obvious uterine adnexal abnormalities. A hypoechoic cystic mass measuring approximately 173 mm × 84 mm × 138 mm with clear boundaries and an unclear blood flow signal was observed in the abdominal cavity, extending from the lower edge of the left liver lobe to the upper edge of the bladder.
The patient was in good health condition with no history of acute or chronic infectious diseases, no history of drug or food allergies, no history of surgery or trauma, and no history of blood transfusion. She was fully vaccinated per societal schedules.
The patient had a history of pancreatitis in the previous year, no history of hypertension or diabetes, and no family history of liver or renal cysts. Her family members had no similar medical history. Furthermore, she denied any history of familial hereditary diseases.
Physical examination revealed the following: Temperature, 36.8°C; heart rate, 108 beats/min; respiratory rate, 18 beats/min; and blood pressure, 114/82 mmHg. There was no yellowing of the skin or sclera, or swelling of superficial lymph nodes throughout the body. The neck was soft, and the chest was symmetrical, with no obvious abnormalities heard during cardiac and pulmonary auscultation. Her abdomen was flat. A mass measuring approximately 12 cm × 10 cm was palpated in the lower abdomen. The mass was soft in texture, with smooth surface, rounded edges, and clear boundaries, had a range of motion, and could be pushed forward without tenderness. It did not extend to the liver or spleen under the ribs, and Murphy’s sign was negative. There was no pain on percussion in the renal area and no bilateral lower limb edema. Her examination was negative for shifting dullness, and bowel sounds were normal.
Laboratory examination revealed the following: White blood cell count, 3.93 × 109/L; neutrophil percentage, 36.00%; neutrophil count, 1.421 × 109/L; hemoglobin concentration, 109.00 g/L; serum C-reactive protein level, 0.23 mg/L; β-human chorionic gonadotropin level, 0.23 mIU/mL; serum albumin level, 38.6 g/L; total bilirubin level, 21.0 μmol/L; and serum alanine aminotransferase level, 12.4 U/L. After 2 d of bladder fluid cultivation, no bacterial or fungal growth was observed. The remaining findings are shown in Tables 1-5.
| Parameter | Patient’s value | Reference value |
| White blood cell count (109/L) | 3.93 | 3.50–9.50 |
| C-reactive protein (mg/L) | 0.23 | 0–6 |
| Neutrophils (%) | 36.00 | 40–75 |
| Lymphocytes (%) | 55.70 | 20–50 |
| Monocytes (%) | 5.20 | 3–10 |
| Hemoglobin (g/L) | 109.00 | 115.0–150.0 |
| Platelet count (109/L) | 172 | 125–350 |
| Activated thromboplastin time (s) | 13.50 | 9.4-12.5 |
| Activated partial thromboplastin time (s) | 11.0 | 11–14 |
| Albumin (g/L) | 38.6 | 40.0–55.0 |
| Total protein (g/L) | 62.8 | 65.0–85.0 |
| Total bilirubin (μmol/L) | 21.0 | 0.0–21.0 |
| Aspartate aminotransferase (U/L) | 15.1 | 13.0–35.0 |
| Alanine aminotransferase (U/L) | 12.4 | 7.0–40.0 |
| Blood creatinine (μmol/L) | 54.5 | 41.0–81.0 |
| Parameter | Patient’s value | Reference value |
| Carcinoembryonic antigen (ng/mL) | 1.36 | 0–5.0 |
| Alpha-fetoprotein (ng/mL) | 1.71 | < 9.01 |
| CA-199 (U/mL) | 3.40 | 0–25 |
| β-human chorionic gonadotropin (mIU/mL) | 0.24 | 0–35 |
| CA-125 (U/mL) | 15.17 | 0–5 |
| CA-15-3 (U/mL) | 3.10 | 0–14 |
| CA-50 (U/mL) | 3.91 | < 25 |
| CA-72-4 (U/mL) | 4.02 | 0–10 |
| CA-24-2 (U/mL) | 4.27 | < 25 |
| Squamous cell carcinoma-associated antigen (ng/mL) | 0.78 | 0–1.5 |
| Cytokeratin 19 fragment (ng/mL) | 1.59 | < 3.3 |
| Neuron specific enolase (ng/mL) | 9.93 | 0–20 |
| Parameter | Patient’s value | Reference value |
| Color | Yellow | |
| Solidification | No solidification | |
| Pellucidity | Slightly turbid | |
| Proportion | 1.010 | Leakage fluid < 1.015 Exudate > 1.018 |
| Rivalta test | + | Leakage fluid: - Exudate: + |
| White blood cell count (109/L) | 0.04 | Leakage fluid < 0.1 Exudate > 0.5 |
| Red blood cell count (109/L) | 0.40 | 0 |
| Other | Cholesterol crystals detected |
| Parameter | Patient’s value |
| TG (mmol/L) | 0.12 |
| GLU (mmol/L) | 0.37 |
| CH (mmol/L) | 0.67 |
| AMY (U/L) | 55 |
| ADA (U/L) | 25.6 |
| LDH (U/L) | 58.6 |
| ACE (U/L) | 6.1 |
| Total protein (g/L) | 66.4 |
| Albumin (g/L) | 42.4 |
Abdominal contrast-enhanced computed tomography revealed a giant cystic mass in the abdominal and pelvic cavities, possibly originating from the liver. Furthermore, a small amount of free fluid was observed in the pelvic cavity (Figure 1). Magnetic resonance imaging revealed a large cystic mass in the abdominal and pelvic cavities, with features suggesting a benign lesion (Figure 2).
Bile duct derived complex liver cyst.
The patient underwent laparoscopic resection of the giant liver cyst along with partial liver resection. Her presenting symptoms of frequent or urgent urination were completely relieved post-surgery and she was discharged on the sixth postoperative day.
The patient recovered well, with no symptoms of frequent or urgent urination, and no specific discomfort was observed during follow-up at 0.5, 1, and 3 mo after discharge.
Liver cysts are a benign disease with genetic characteristics[10,11]. Simple liver cysts are typical cystic thin-walled masses that originate from bile duct cells that form abnormally during embryonic development[5]. In most cases, cysts occur only in the liver and patients generally have no obvious clinical symptoms. However, in some patients, the expansion of liver cysts can cause abdominal symptoms, mainly due to a series of corresponding clinical symptoms caused by compression of the surrounding tissues or organs caused by oversized liver cysts[12]. Asymptomatic simple liver cysts usually do not require treatment. The treatments for liver cysts with obvious clinical symptoms include percutaneous puncture, aspiration, sclerotherapy, and surgery[5].
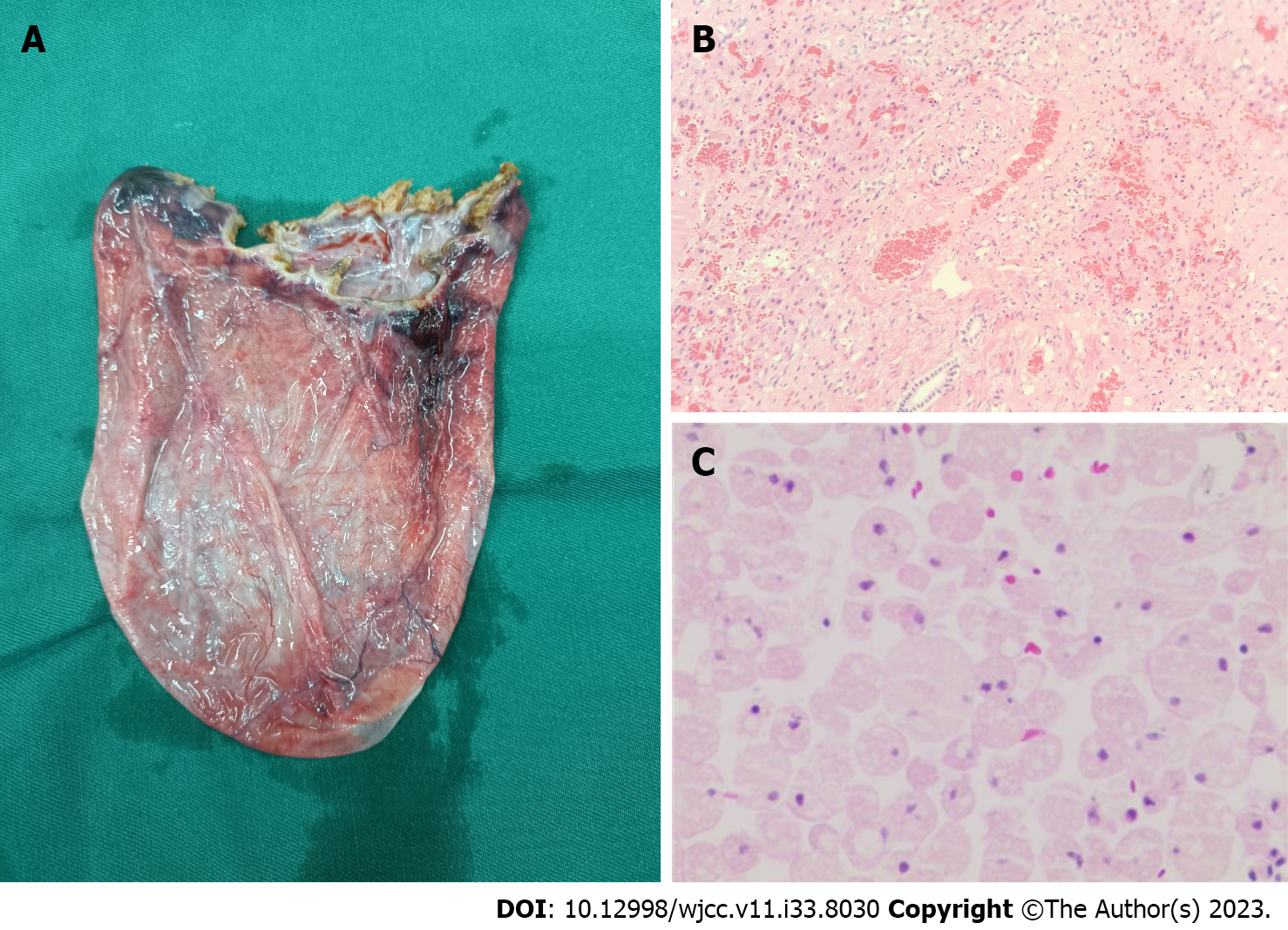
Herein, we report a case of pseudocystitis caused by a giant complex liver cyst that was pathologically suggestive of a bile duct derived liver cyst and was different from a conventional liver cyst (Figure 3). There are no reports in the literature of complex liver cysts causing symptoms of pseudocystitis. Liver cysts are typically asymptomatic. An increase in cyst size, particularly in giant liver cysts, can initiate symptoms. If the diameter of the cyst exceeds 10 cm, it can expand and cause pressure effect on adjacent organs and corresponding symptoms may appear[8], including abdominal pain, nausea, vomiting, obstructive jaundice, superior vena cava thrombosis, acute pulmonary embolism, and acute pancreatitis[6,13-15]. Giant liver cysts should be differentiated from Caroli disease[16], giant mesenchymal hamartomas of the liver[17], teratomas[18], and other diseases[19-21]. Our patient had previously experienced symptoms of pancreatitis. Therefore, we assumed that the pain was caused by a liver cyst. At the time of presentation, the liver cyst caused symptoms in distant organs that disappeared postoperatively. Thus, it was confirmed that the giant liver cyst caused the pain. These cysts usually require surgical intervention. In our patient, the giant cyst was located at the edge of the liver; thus, the liver was stretched and deformed under the influence of gravity. During the surgical process, we obtained biopsy samples of adjacent tissues to determine the possibility of further deterioration of liver architecture, and to rule out possible recurrence, which the liver cysts are prone to.
Our case report highlights that the diagnosis and treatment of complex giant liver cysts that cause pseudocystitis should be comprehensive and multidimensional. The differential diagnosis of such abdominal masses should be considered before treatment. The patient was a young, unmarried girl, and a detailed plan was specified to minimize major trauma and achieve the best treatment outcomes. These patients require close follow-up because liver cysts are prone to recurrence.
| 1. | Regev A, Reddy KR, Berho M, Sleeman D, Levi JU, Livingstone AS, Levi D, Ali U, Molina EG, Schiff ER. Large cystic lesions of the liver in adults: a 15-year experience in a tertiary center. J Am Coll Surg. 2001;193:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Gomez A, Wisneski AD, Luu HY, Hirose K, Roberts JP, Hirose R, Freise CE, Nakakura EK, Corvera CU. Contemporary Management of Hepatic Cyst Disease: Techniques and Outcomes at a Tertiary Hepatobiliary Center. J Gastrointest Surg. 2021;25:77-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Xu WP, Wang XH, Wu SP, Shi PM, Yuan ZL, Guo YB, Zeng X, Xie WF. The prevalence and associated factors of simple hepatic cysts in Shanghai: a population-based cross-sectional study. Chin Med J (Engl). 2021;134:1248-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Cowles RA, Mulholland MW. Solitary hepatic cysts. J Am Coll Surg. 2000;191:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 5. | Shimizu T, Yoshioka M, Kaneya Y, Kanda T, Aoki Y, Kondo R, Takata H, Ueda J, Kawano Y, Hirakata A, Matsushita A, Taniai N, Mamada Y, Yoshida H. Management of Simple Hepatic Cyst. J Nippon Med Sch. 2022;89:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Miyamoto M, Oka M, Izumiya T, Nagaoka T, Ishihara Y, Ueda K, Enomoto S, Yanaoka K, Arii K, Tamai H, Shimizu Y, Ichinose M. Nonparasitic solitary giant hepatic cyst causing obstructive jaundice was successfully treated with monoethanolamine oleate. Intern Med. 2006;45:621-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Tsuruya K, Nishizaki Y, Tatemichi M, Mishima Y, Shimma Y, Arase Y, Hirose S, Shiraishi K, Kagawa T. The prevalence and natural history of hepatic cysts examined by ultrasound: a health checkup population retrospective cohort study. Sci Rep. 2022;12:12797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Fantola G, Barth X, Monneuse O. Education and imaging. Hepatobiliary and pancreatic: Pancreatitis associated with a large hepatic cyst. J Gastroenterol Hepatol. 2010;25:1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Panchal M, Alansari A, Wallack M, Visco F, Williams S, Sy AM. Hepatic Cyst Compressing The Right Atrial and Ventricular Inflow Tract: An Uncommon Cardiac Complication. Ann Hepatol. 2018;17:165-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Choi BY, Nguyen MH. The diagnosis and management of benign hepatic tumors. J Clin Gastroenterol. 2005;39:401-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Lantinga MA. Evaluation of hepatic cystic lesions. World J Gastroenterol. 2013;19:3543-3554. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 119] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (3)] |
| 12. | Temmerman F, Missiaen L, Bammens B, Laleman W, Cassiman D, Verslype C, van Pelt J, Nevens F. Systematic review: the pathophysiology and management of polycystic liver disease. Aliment Pharmacol Ther. 2011;34:702-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Wijnands TF, Görtjes AP, Gevers TJ, Jenniskens SF, Kool LJ, Potthoff A, Ronot M, Drenth JP. Efficacy and Safety of Aspiration Sclerotherapy of Simple Hepatic Cysts: A Systematic Review. AJR Am J Roentgenol. 2017;208:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Wijnands TF, Neijenhuis MK, Kievit W, Nevens F, Hogan MC, Torres VE, Gevers TJ, Drenth JP. Evaluating health-related quality of life in patients with polycystic liver disease and determining the impact of symptoms and liver volume. Liver Int. 2014;34:1578-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Torzilli G, Santambrogio R, Vellini S, Palmisano A, Donadon M, Cornalba G, Montorsi M. Inferior vena cava thrombosis: an unusual complication of a large simple non-parasitic liver cyst requiring an integrated approach. Hepatogastroenterology. 2003;50:2188-2191. [PubMed] |
| 16. | Almohtadi A, Ahmed F, Mohammed F, Sanhan M, Ghabisha A, Al-Moliki L. Caroli's disease incidentally discovered in a 16-years-old female: a case report. Pan Afr Med J. 2022;41:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Bezabih YS, Tessema WA, Getu ME. Giant biliary mucinous cystadenoma mimicking mesenchymal hamartoma of the liver in a child: A case report. Int J Surg Case Rep. 2021;88:106523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Lešková J, Kříž JT, Štichhauer R. Bilateral Mature Ovarian Teratoma with Torsion in a Premenarchal Girl. Acta Medica (Hradec Kralove). 2022;65:33-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Matsuo H, Katayama K, Hayasaki A, Iizawa Y, Endo M, Murata T, Mizuno S, Dohi K. Biliary peritonitis due to liver cyst rupture in autosomal dominant polycystic kidney disease. BMC Gastroenterol. 2021;21:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Cnossen WR, Drenth JP. Polycystic liver disease: an overview of pathogenesis, clinical manifestations and management. Orphanet J Rare Dis. 2014;9:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 21. | Gevers TJ, Drenth JP. Diagnosis and management of polycystic liver disease. Nat Rev Gastroenterol Hepatol. 2013;10:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kodama T, Japan S-Editor: Lin C L-Editor: Wang TQ P-Editor: Lin C