Published online Nov 26, 2023. doi: 10.12998/wjcc.v11.i33.8013
Peer-review started: August 14, 2023
First decision: October 9, 2023
Revised: October 19, 2023
Accepted: November 2, 2023
Article in press: November 2, 2023
Published online: November 26, 2023
Processing time: 101 Days and 20 Hours
Surgical site infection (SSI) is one of the most common complications after gastric cancer (GC) surgery. The occurrence of SSI can lead to a prolonged postoperative hospital stay and increased medical expenses, and it can also affect postoperative rehabilitation and the quality of life of patients. Subcutaneous fat thickness (SFT) and abdominal depth (AD) can be used as predictors of SSI in patients under
To explore the potential relationship between SFT or AD and SSI in patients undergoing elective radical resection of GC.
Demographic, clinical, and pre- and intraoperative information of 355 patients who had undergone elective radical resection of GC were retrospectively collected from hospital electronic medical records. Univariate analysis was performed to screen out the significant parameters, which were subsequently analyzed using binary logistic regression and receiver-operating characteristic curve analysis.
The prevalence of SSI was 11.27% (40/355). Multivariate analyses revealed that SFT [odds ratio (OR) = 1.150; 95% confidence interval (95%CI): 1.090–1.214; P < 0.001], AD (OR = 1.024; 95%CI: 1.009–1.040; P = 0.002), laparoscopic-assisted surgery (OR = 0.286; 95%CI: 0.030–0.797; P = 0.017), and operation time (OR = 1.008; 95%CI: 1.001–1.015; P = 0.030) were independently associated with the incidence of SSI after elective radical resection of GC. In addition, the product of SFT and AD was a better potential predictor of SSI in these patients than either SFT or AD alone.
SFT and AD are independent risk factors and can be used as predictors of SSI in patients undergoing radical resection of GC.
Core Tip: Surgical site infection (SSI) is one of the most common complications after gastric cancer (GC) surgery. We identified subcutaneous fat thickness (SFT) and abdominal depth (AD) as independent risk factors that can be used as predictors of SSI in patients undergoing radical resection of GC. Our findings may assist clinicians in evaluating the risk of SSI in patients with higher SFT and AD values.
- Citation: Yu KY, Kuang RK, Wu PP, Qiang GH. Subcutaneous fat thickness and abdominal depth are risk factors for surgical site infection after gastric cancer surgery. World J Clin Cases 2023; 11(33): 8013-8021
- URL: https://www.wjgnet.com/2307-8960/full/v11/i33/8013.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i33.8013
Gastric cancer (GC) is the fifth most common type of cancer worldwide and the third most fatal cancer[1]. Surgical resection accompanied by systemic adjuvant chemotherapy is still the best treatment for potentially treatable GC[2].
Surgical site infection (SSI) is the most common among all healthcare-associated infections[3]. SSI frequently occurs after gastric surgery, with an incidence ranging from 3.9% to 18.7%[4-7]. In addition to increasing hospital costs, SSI clearly prolongs hospital stay and results in long-term disability[8]. Prolonged operative duration[9], higher body mass index (BMI)[10], total gastrectomy, open surgery, and intraoperative blood transfusion[11] have been proven to be predictors of the development of SSI after elective gastrectomy.
As abdominal anatomical characteristics, subcutaneous fat thickness (SFT) and abdominal depth (AD) vary dramatically among individuals. Recently, SFT has been reported to be an independent risk factor for the development of SSI in intestinal resection [odds ratio (OR) = 2.519; 95% confidence interval (95%CI): 1.350–4.698; P = 0.004][12] and open appendectomy (OR = 3.52; 95%CI: 1.75–7.08; P < 0.001)[13]. Teppa et al[14] showed that when the SFT increases by more than 2.5 cm, the risk of SSI increases in abdominal surgeries. In Zhang et al’s study of patients undergoing radical resection of colorectal cancer, the complication group had a greater AD (9.24 ± 2.91 vs 7.77 ± 2.08, P < 0.001) compared to the non-complication group, and they thus concluded that a greater AD is associated with an increased risk of short-term postoperative complications for these patients[15]. Another study revealed a correlation between AD and heightened SSI risk following elective radical resection for colon cancer[16].
However, there are few studies on SFT, AD, and SSI after GC surgery. Therefore, we conducted a retrospective study to explore the relationship between SFT or AD and the incidence of SSI after elective radical resection of GC.
Patients who had undergone GC surgery at our hospital between January 2015 and April 2023 were screened for eligibility. The exclusion criteria encompassed: (1) Emergent radical resection of GC; (2) reoperation for the recurrence of GC; (3) the presence of concomitant abdominal infectious diseases before the operation; and (4) incomplete clinical data (Figure 1). The eligible patients were those who underwent gastrectomy for GC and were histopathologically diagnosed with GC. The study cohort comprised 355 patients who had undergone elective radical resection of GC. Of these, 40 patients developed an SSI.
The patients’ data, including demographic information, clinical data, preoperative laboratory results, surgical information, and pathological diagnosis, were collected from the electronic medical record system of the hospital. Computed tomography (CT) was performed to measure SFT and AD. SFT and AD were both measured at the level of the umbilicus in supine CT images (Figure 2). SFT was defined as the maximum sagittal distance between the parietal and visceral sides of the subcutaneous fat. AD was defined as the sagittal distance between the bottom of the umbilicus and the top of the vertebra. These parameters were measured by three independent operators, and the mean value was further analyzed.
We assessed whether there was an infection at the surgical site in line with the World Health Organization criteria[17]. SSIs were classified into three groups, namely, superficial, deep, and organ/space infections. Briefly, an SSI occurred at the surgical site within 30 d of surgery, and was characterized by at least one of the following conditions: Purulent drainage from the surgical site, organisms isolated from an aseptically obtained culture of fluid or tissue from the surgical site, and/or incisional inflammation, including pain or tenderness, redness, and localized swelling. Based on the outcome, we divided all eligible patients into either an SSI or a non-SSI group.
A second-generation cephalosporin was administered intravenously as a prophylactic antibiotic in all of the patients within 30 min before the surgery, and an additional dose was administered every 3 h during the surgery. Quinolones or penicillin were administered to the patients allergic to cephalosporins. The duration of prophylactic antibiotic administration was generally 3–5 d after the surgery. This study was approved by the Medical Ethics Committee of the Nanjing Jiangbei Hospital. The need for informed consent was waived because of the retrospective nature of the study.
Continuous data were evaluated for normality using the Shapiro–Wilk test. Normally distributed data were analyzed using an unpaired t-test and are represented as the mean and standard deviation (mean ± SD). Non-normally distributed data were analyzed using the Mann–Whitney U test and are represented as the median and interquartile range. Categorical data were analyzed using Fisher’s exact probability test and are expressed as frequencies and percentages. Univariate and multivariate logistic regression analyses were used to identify the risk factors for SSI. Multivariate logistic regression analysis was performed only on variables with P < 0.05 in the univariate analysis. Receiver-operating characteristic (ROC) curve analysis was performed to evaluate the predictive ability and optimal cutoff value of all biomarkers. All statistical analyses were performed using SPSS software (version 24.0; SPSS Inc., Chicago, IL, United States). All tests were two-tailed. Statistical significance was set at P < 0.05.
We analyzed 355 patients who had undergone elective radical resection of GC. Their mean age was 65.6 years (range, 29–91 years), and 71.9% of the patients were male. The characteristics of the patients with and without SSI are compared in Table 1. While no statistically significant difference in age, gender, smoking, alcohol use, diabetes, hypertension, type of resection, blood loss, history of laparotomy, albumin, prealbumin, or tumor-node-metastasis stage was observed between the SSI and non-SSI groups, there were statistically significant differences in BMI (P < 0.001), SFT (P < 0.001), AD (P < 0.001), laparoscopic-assisted surgery (P = 0.042), and operation time (P = 0.013).
| Variable | Surgical site infection | P value | |
| Absent (n = 315) | Present (n = 40) | ||
| Age (yr) | 65.3 ± 9. 6 | 66.4 ± 9.8 | 0.529 |
| Male | 227 (72.10) | 28 (70.00) | 0.785 |
| BMI (kg/m2) | 22.68 ± 3.22 | 25.12 ± 2.78 | < 0.001a |
| SFT (mm) | 14.20 (10.00–18.40) | 20.45 (17.00–26.63) | < 0.001a |
| AD (mm) | 64.90 (50.60–78.20) | 82.50 (68.43–97.00) | < 0.001a |
| Smoking | 87 (27.60) | 15 (37.50) | 0.193 |
| Alcohol use | 66 (21.00) | 11 (27.50) | 0.344 |
| Diabetes | 40 (12.70) | 9 (22.50) | 0.090 |
| Laparoscopic-assisted surgery | 96 (30.5) | 6 (15.00) | 0.042a |
| Hypertension | 95 (30.2) | 12 (30.0) | 0.984 |
| Type of resection | 0.371 | ||
| Total gastrectomy | 206 (65.40) | 29 (34.60) | |
| Partial gastrectomy | 109 (35.98) | 11 (27.50) | |
| Operation time (min) | 210.00 (180.00–240.00) | 240.00 (209.25–270.00) | 0.013a |
| Blood loss (mL) | 150.00 (100.00–200.00) | 150.00 (112.00–200.00) | 0.099 |
| History of laparotomy | 47 (14.90) | 7 (17.50) | 0.669 |
| Albumin (g/dL) | 39.37 ± 4.99 | 40.29 ± 3.55 | 0.258 |
| Prealbumin (g/dL) | 188.25 ± 59.06 | 194.92 ± 63.09 | 0.509 |
| TNM stage | 0.304 | ||
| I | 96 (29.2) | 10 (25.0) | |
| II | 92 (30.5) | 17 (42.5) | |
| III | 127 (40.3) | 13 (32.5) |
To study the relationship between the individual risk factors and the incidence of SSI, we performed multivariate analysis on the factors with P < 0.05 in the univariate analysis. As shown in Table 2, the univariate analysis revealed that the incidence of SSI was significantly associated with BMI (P < 0.001), SFT (P < 0.001), AD (P < 0.001), the product of SFT and AD (SFT × AD) (P < 0.001), laparoscopic-assisted surgery (P = 0.048), and operation time (P = 0.010). The multivariate analysis revealed that SFT (OR = 1.150; 95%CI: 1.090–1.214; P < 0.001), AD (OR = 1.024; 95%CI: 1.009–1.040; P = 0.002), laparoscopic-assisted surgery (OR = 0.322; 95%CI: 0.119–0.870; P = 0.025), and operation time (OR = 1.008; 95%CI: 1.001–1.015; P = 0.026) were independently associated with the incidence of SSI.
| Variable | Univariate analysis | P value | Multivariate analysis | P value |
| OR (95%CI) | OR (95%CI) | |||
| Age (yr) | 1.011 (0.977–1.047) | 0.528 | ||
| Male | 0.905 (0.440–1.858) | 0.785 | ||
| BMI (kg/m2) | 1.259 (1.133–1.398) | < 0.001 | - | - |
| SFT (mm) | 1.137 (1.186–1.192) | < 0.001 | 1.150 (1.090–1.214) | < 0.001a |
| AD (mm) | 1.031 (1.017–1.046) | < 0.001 | 1.024 (1.009–1.040) | 0.002a |
| Smoking | 1.572 (0.792–3.123) | 0.196 | ||
| Alcohol use | 1.431 (0.679–3.015) | 0.346 | ||
| Diabetes | 1.996 (0.885–4.500) | 0.096 | ||
| Laparoscopic-assisted surgery | 0.403 (0.164–0.991) | 0.048 | 0.286 (0.03–0.797) | 0.017a |
| Hypertension | 0.992 (0.484–2.034) | 0.984 | ||
| Type of resection | ||||
| Total gastrectomy | 1.395 (0.671–2.900) | 0.373 | ||
| Operation time (min) | 1.008 (1.002–1.015) | 0.010 | 1.008 (1.001–1.015) | 0.030a |
| Blood loss (mL) | 1.000 (0.999–1.002) | 0.659 | ||
| History of laparotomy | 1.210 (0.505–2.894) | 0.669 | ||
| Albumin (g/dL) | 1.041 (0.971–1.116) | 0.258 | ||
| Prealbumin (g/dL) | 1.002 (0.996–1.001) | 0.508 | ||
| TNM stage | ||||
| I | – | – | ||
| II | 1.629 (0.709–3.743) | 0.250 | ||
| III | 0.942 (0.396–2.241) | 0.892 |
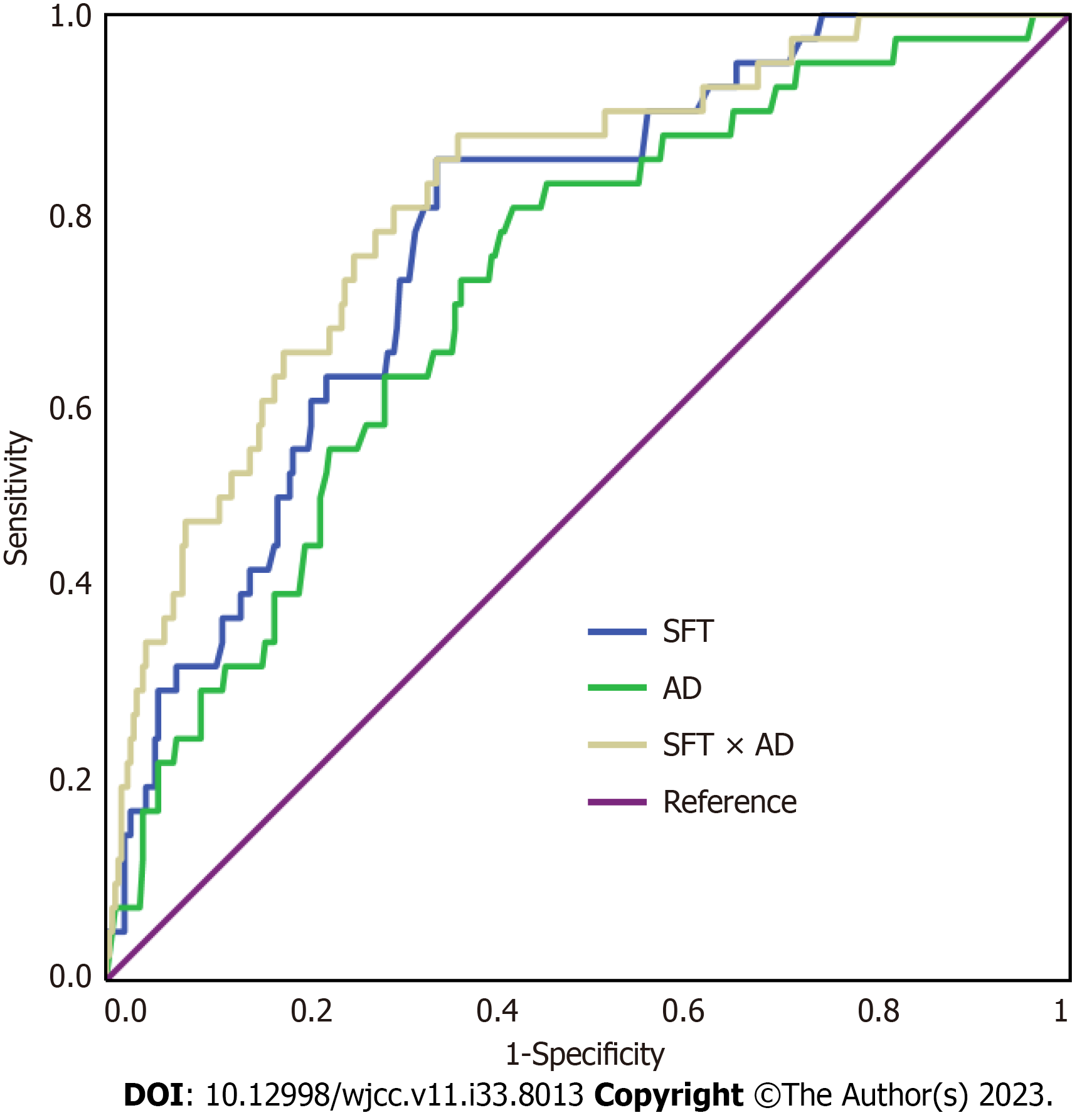
ROC analysis was performed to determine the predictive value of SFT, AD, and SFT × AD value for SSI in patients who underwent elective radical resection of GC (Table 3 and Figure 3). The optimum cutoff values for SFT, AD, and SFT × AD were 16.55, 67.85, and 11.11, respectively. The area under the ROC (AUC) values of SFT and AD were 0.770 (95%CI: 0.700–0.839) and 0.715 (95%CI: 0.635–0.795), respectively. The use of SFT × AD (AUC = 0.810; 95%CI: 0.740–0.879) demonstrated higher diagnostic value than the use of either SFT or AD alone.
| Cutoff value | Sensitivity | Specificity | +LR | −LR | AUC | |
| SFT | 16.55 | 0.850 | 0.657 | 2.479 | 0.228 | 0.770 (0.700–0.839) |
| AD | 67.85 | 0.800 | 0.578 | 1.895 | 0.346 | 0.715 (0.635–0.795) |
| SFT × AD | 11.11 | 0.875 | 0.635 | 2.397 | 0.197 | 0.810 (0.740–0.879) |
By comparing 315 non-SSI and 40 SSI patients who had received elective radical resection of GC, we found that the two groups significantly differed in BMI, SFT, AD, laparoscopic-assisted surgery, and operation time; these variables may be risk factors for SSI. In addition, SFT, AD, laparoscopic-assisted surgery, and operation time were independently associated with SSI as evidenced by the logistic regression analysis. Further diagnostic power analysis identified that SFT × AD was a better potential predictor of SSI in these patients than either SFT or AD alone. These findings may assist clinicians in evaluating the risk of SSI in patients with a higher SFT × AD value.
SSI remains a significant cause of morbidity after gastric surgery. SSI prolongs the length of hospital stay and increases the risk of incisional hernias. Although various measures to prevent the occurrence of SSI have recently been reported, the incidence of infection has not dropped below a negligible level. In this study, we explored the relationship between abdominal anatomical characteristics, including SFT, AD, and SFT × AD, and the rate of SSI following gastric surgery.
Thicker subcutaneous fat can increase the tension of the suture at the incision site, thereby reducing the blood supply to the incision site, which increases the risk of incision liquefaction and delayed healing. As previously reported, SFT is an independent risk factor for SSI in a variety of surgical procedures, including surgery for Crohn’s disease[12], acute appendicitis surgery[13], elective colorectal surgery[18], and posterior cervical fusion surgery[19]. However, Liu et al[16] found that although the SFT was higher in patients with SSI after colorectal surgery than in non-SSI patients, it was not an independent risk factor for SSI. In our study, SFT was positively associated with the rate of SSI after elective radical resection of GC.
A previous study reported that the sagittal abdominal diameter was closely associated with general and visceral obesity[20]. Sur et al revealed that visceral obesity was related to SSI in patients undergoing surgery for colon cancer[21]. For patients with a deeper abdomen, surgical exposure is usually more difficult and the operation time is longer, which increases the risk of postoperative SSI. In the study by Liu et al[16], involving 55 SSI-afflicted patients juxtaposed against 55 propensity-score-matched counterparts without SSI, both groups having experienced elective radical resection for colon cancer, elevated AD value emerged as a potential risk factor for SSI. This observation is consistent with the conclusion drawn in the current research.
Increased BMI as a biomarker to measure obesity has been reported to be an incremental and independent risk factor for SSI in patients undergoing colorectal surgery[22]. The same was confirmed in patients undergoing gastric surgery[23]. Our univariate model data revealed that the patients in the SSI group exhibited high BMI values compared with those in the non-SSI group, but the significance was lost in the multivariate model, so BMI could not be used as a biomarker for SSI prediction.
A meta-analysis has shown that prolonged operative duration bears an increased risk of SSI after various surgical procedures, such as colorectal surgery, urological surgery, plastic and maxillofacial surgery, obstetrics and gynecology surgery, and orthopedic surgery[24]. According to Michael et al, increased operative duration is associated with an increased risk of SSI after unicompartmental knee arthroplasty, and the authors believe that the operative duration is a surgeon-dependent and potentially modifiable risk factor, which may indicate the complexity and difficulty of the operation[25]. In the present study, the longer operation time was an independent risk factor for the rate of SSI after GC surgery.
To the best of our knowledge, this study is the first to investigate the relationship between SFT or AD and SSI after elective radical resection of GC. Our findings reveal the relationship between these two abdominal anatomical indicators and the development of SSI in patients undergoing elective radical gastrectomy, which can help clinicians in the early identification and treatment of postoperative SSI.
The present study has several limitations. First, this was a single-center retrospective study with some inevitable recall and selection biases, which may limit its generalizability. Second, this study did not assess other known potential predictors related to SSI, such as intraoperative hypothermia, anemia, and inadequate oxygenation. Third, the present study did not investigate the mechanism by which abdominal anatomical features affect the risk of SSI. Therefore, a multicenter prospective study is warranted to confirm the accuracy of the results and to provide strategies to prevent SSI in patients with GC.
Our results suggest that preoperative SFT, AD, and operation time are independent risk factors for SSI after GC surgery, while laparoscopic-assisted surgery is a protective factor. The multiplied value of SFT and AD can be used as a predictor of SSI in patients after elective radical resection of GC.
Surgical site infection (SSI) is one of the most common complications after gastric cancer (GC) surgery. The occurrence of SSI has an adverse impact on the prognosis of patients. There are very few studies that focus on the effect of subcutaneous fat thickness (SFT) and abdominal depth (AD) on postoperative SSI.
In this study, the authors sought to identify ways to assist clinicians in the early identification and treatment of postoperative SSI after GC surgery.
To explore the potential relationship between SFT or AD and SSI in patients after elective radical resection of GC.
Demographic, clinical, and pre- and intraoperative information of 355 patients who had undergone elective radical resection of GC were retrospectively collected from hospital electronic medical records. Univariate and multivariate logistic regression analyses were used to screen for the risk factors contributing to SSI incidence. Furthermore, the receiver-operating characteristic (ROC) curve method was employed to evaluate the predictive power and best cutoff value for the biomarkers under consideration.
The prevalence of SSI was 11.27% (40/355). Multivariate analyses revealed that SFT, AD, laparoscopic-assisted surgery, and operation time were independently associated with the incidence of SSI after elective radical resection of GC. The area under the ROC curve values of SFT, AD, and the product of SFT and AD (SFT × AD) were 0.770 [95% confidence interval (95%CI): 0.700–0.839], 0.715 (95%CI: 0.635–0.795), and 0.810 (95%CI: 0.740–0.879), respectively.
Our results suggest that preoperative SFT, AD, and operation time are independent risk factors for SSI after GC surgery, while laparoscopic-assisted surgery is a protective factor. In addition, SFT × AD is a better potential predictor of SSI in these patients than either SFT or AD alone.
In the future, we will increase the sample size used to construct the model and conduct a multicenter study.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56702] [Article Influence: 7087.8] [Reference Citation Analysis (135)] |
| 2. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1417] [Article Influence: 283.4] [Reference Citation Analysis (2)] |
| 3. | Ban KA, Minei JP, Laronga C, Harbrecht BG, Jensen EH, Fry DE, Itani KM, Dellinger EP, Ko CY, Duane TM. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J Am Coll Surg. 2017;224:59-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 712] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 4. | Qiao YQ, Zheng L, Jia B, Wang WH, Zheng XH, Fan LL, Xie YB, Tian YT. Risk factors for surgical-site infections after radical gastrectomy for gastric cancer: a study in China. Chin Med J (Engl). 2020;133:1540-1545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Hirao M, Tsujinaka T, Imamura H, Kurokawa Y, Inoue K, Kimura Y, Shimokawa T, Furukawa H; Osaka Gastrointestinal Cancer Chemotherapy Study Group (OGSG). Overweight is a risk factor for surgical site infection following distal gastrectomy for gastric cancer. Gastric Cancer. 2013;16:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Morikane K, Honda H, Suzuki S. Factors Associated With Surgical Site Infection Following Gastric Surgery in Japan. Infect Control Hosp Epidemiol. 2016;37:1167-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Olmez T, Gulmez S, Karakose E, Ofluoglu CB, Senger AS, Bozkurt H, Duman M, Polat E. Relation between Sarcopenia and Surgical Site Infection in Patients Undergoing Gastric Cancer Surgery. Surg Infect (Larchmt). 2021;22:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Allegranzi B, Zayed B, Bischoff P, Kubilay NZ, de Jonge S, de Vries F, Gomes SM, Gans S, Wallert ED, Wu X, Abbas M, Boermeester MA, Dellinger EP, Egger M, Gastmeier P, Guirao X, Ren J, Pittet D, Solomkin JS; WHO Guidelines Development Group. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016;16:e288-e303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 575] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 9. | Jeong SJ, Ann HW, Kim JK, Choi H, Kim CO, Han SH, Choi JY, Peck KR, Kang CI, Yeom JS, Choi YH, Lim SK, Song YG, Choi HJ, Yoon HJ, Kim HY, Kim YK, Kim MJ, Park YS, Kim JM. Incidence and risk factors for surgical site infection after gastric surgery: a multicenter prospective cohort study. Infect Chemother. 2013;45:422-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Ruiz-Tovar J, Oller I, Llavero C, Arroyo A, Muñoz JL, Calero A, Diez M, Zubiaga L, Calpena R. Pre-operative and early post-operative factors associated with surgical site infection after laparoscopic sleeve gastrectomy. Surg Infect (Larchmt). 2013;14:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Kosuga T, Ichikawa D, Komatsu S, Kubota T, Okamoto K, Konishi H, Shiozaki A, Fujiwara H, Otsuji E. Clinical and surgical factors associated with organ/space surgical site infection after laparoscopic gastrectomy for gastric cancer. Surg Endosc. 2017;31:1667-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Cai X, Shen W, Guo Z, Li Y, Cao L, Gong J, Zhu W. Thickness of Subcutaneous Fat Is a Predictive Factor of Incisional Surgical Site Infection in Crohn's Disease Surgery: A Retrospective Study. Gastroenterol Res Pract. 2018;2018:1546075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Thapa B, Sutanto E, Bhandari R. Thickness of subcutaneous fat is a risk factor for incisional surgical site infection in acute appendicitis surgery: a prospective study. BMC Surg. 2021;21:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Teppa R, Sude NS, Karanam VPK, Mallipudi BVP. Relevance of Subcutaneous Fat Thickness as a Risk Factor for Surgical Site Infections in Abdominal Surgeries. Cureus. 2022;14:e20946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Zhang X, Yang Z, Meng C, Gao J, Liu Y, Shi B, Sun L, Wu G, Yao H, Zhang Z. Abdomen anatomic characteristics on CT scans as predictive markers for short-term complications following radical resection of colorectal cancer. Front Surg. 2022;9:899179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Liu S, Wang M, Lu X, Feng M, Wang F, Zheng L, Guan W. Abdomen Depth and Rectus Abdominis Thickness Predict Surgical Site Infection in Patients Receiving Elective Radical Resections of Colon Cancer. Front Oncol. 2019;9:637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Allegranzi B, Bischoff P, de Jonge S, Kubilay NZ, Zayed B, Gomes SM, Abbas M, Atema JJ, Gans S, van Rijen M, Boermeester MA, Egger M, Kluytmans J, Pittet D, Solomkin JS; WHO Guidelines Development Group. New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016;16:e276-e287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 539] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 18. | Fujii T, Tsutsumi S, Matsumoto A, Fukasawa T, Tabe Y, Yajima R, Asao T, Kuwano H. Thickness of subcutaneous fat as a strong risk factor for wound infections in elective colorectal surgery: impact of prediction using preoperative CT. Dig Surg. 2010;27:331-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Donnally CJ, Henstenburg JM, Pezzulo JD, Farronato D, Patel PD, Sherman M, Canseco JA, Kepler CK, Vaccaro AR. Increased Surgical Site Subcutaneous Fat Thickness Is Associated with Infection after Posterior Cervical Fusion. Surg Infect (Larchmt). 2022;23:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 20. | Saad MAN, Jorge AJL, de Ávila DX, Martins WA, Dos Santos MMS, Tedeschi LT, Cavalcanti IL, Rosa MLG, Filho RADC. Sagittal abdominal diameter as a marker of visceral obesity in older primary care patients. J Geriatr Cardiol. 2020;17:279-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Sur MD, Namm JP, Hemmerich JA, Buschmann MM, Roggin KK, Dale W. Radiographic Sarcopenia and Self-reported Exhaustion Independently Predict NSQIP Serious Complications After Pancreaticoduodenectomy in Older Adults. Ann Surg Oncol. 2015;22:3897-3904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Wahl TS, Patel FC, Goss LE, Chu DI, Grams J, Morris MS. The Obese Colorectal Surgery Patient: Surgical Site Infection and Outcomes. Dis Colon Rectum. 2018;61:938-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Kambara Y, Yuasa N, Takeuchi E, Miyake H, Nagai H, Yoshioka Y, Okuno M, Miyata K. Overweight or Obesity is an Unfavorable Long-Term Prognostic Factor for Patients who Underwent Gastrectomy for Stage II/III Gastric Cancer. World J Surg. 2019;43:1766-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Cheng H, Clymer JW, Po-Han Chen B, Sadeghirad B, Ferko NC, Cameron CG, Hinoul P. Prolonged operative duration is associated with complications: a systematic review and meta-analysis. J Surg Res. 2018;229:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 623] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 25. | Held MB, Boddapati V, Sarpong NO, Cooper HJ, Shah RP, Geller JA. Operative Duration and Short-Term Postoperative Complications after Unicompartmental Knee Arthroplasty. J Arthroplasty. 2021;36:905-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aydin S, Turkey S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Xu ZH