Published online Nov 6, 2023. doi: 10.12998/wjcc.v11.i31.7663
Peer-review started: August 27, 2023
First decision: September 26, 2023
Revised: October 7, 2023
Accepted: October 27, 2023
Article in press: October 27, 2023
Published online: November 6, 2023
Processing time: 71 Days and 5.5 Hours
Salivary carcinosarcoma is an extremely rare tumor containing both malignant epithelial and mesenchymal constituents. This article reports a rare case of carcinosarcoma with salivary duct carcinoma and osteosarcoma as the tumor components. The clinicopathological characteristics, treatment, and prognosis are discussed in conjunction with the literature.
A 48-year-old man presented with a complaint of a mass in the right parotid region. Osteosarcoma was first considered for assessment by fine-needle aspiration cytology. Physical examination revealed a mass measuring approximately 4 cm × 3.5 cm × 3 cm. The mass, the whole lobe of the right parotid gland, and the right mandible were completely removed during surgery. Postoperative histopathology confirmed carcinosarcoma of the salivary gland.
A definite diagnosis of salivary gland carcinosarcoma can only be obtained after complete surgical resection.
Core Tip: Carcinosarcoma is a highly rare tumor with less than 100 reported cases, and only 13 cases of carcinosarcoma with an osteosarcoma component have been reported so far. Here, a rare case of carcinosarcoma with salivary duct carcinoma and osteosarcoma as the tumor components is reported. The clinicopathological characteristics, treatment, and prognosis are discussed in conjunction with the literature.
- Citation: Tang YY, Zhu GQ, Zheng ZJ, Yao LH, Wan ZX, Liang XH, Tang YL. Carcinosarcoma of the deep lobe of the parotid gland in the parapharyngeal region: A case report. World J Clin Cases 2023; 11(31): 7663-7672
- URL: https://www.wjgnet.com/2307-8960/full/v11/i31/7663.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i31.7663
Malignant pleomorphic adenoma (MPA) is an uncommon malignant tumor of the salivary gland that makes up 4% of all salivary gland tumors and not more than 0.2% of all malignant salivary gland tumors[1,2]. MPAs of the head and neck were classified into three types, carcinoma ex pleomorphic adenoma (CAxPA), carcinosarcoma, and metastasizing pleomorphic adenoma, by the World Health Organization in 2017[3]. Kirklin et al[4] first described carcinosarcoma in 1951, a true malignant mixed tumor that can occur de novo or arise from pleomorphic adenomas[5,6]. Carcinosarcoma is a highly rare tumor with fewer than 100 reported cases[7], and only 13 cases of carcinosarcoma with an osteosarcoma component have been reported thus far[8-11]. Here, a rare case of carcinosarcoma with salivary duct carcinoma and osteosarcoma as the tumor components is reported. The clinicopathological characteristics, treatment, and prognosis are discussed in conjunction with the literature.
A 48-year-old man presented with a 1-mo history of a mass in the right parotid region.
One month prior, the patient had developed a right facial mass without obvious cause, accompanied by mild facial swelling, pain, dizziness, etc. The patient had no fever or facial paralysis. The patient had treated himself with oral anti-inflammatory drugs, and the pain was relieved. However, the mass was still present and had slowly enlarged. Since his illness, he had lost weight.
The patient had no past illness.
The patient had no history of living in epidemic foci and local epidemic areas, no history of exposure to toxic and radioactive substances, and no habit of smoking and alcohol. There was no disease in his family that was similar to his present illness, and there was no chromosomal or genetic inherited disease.
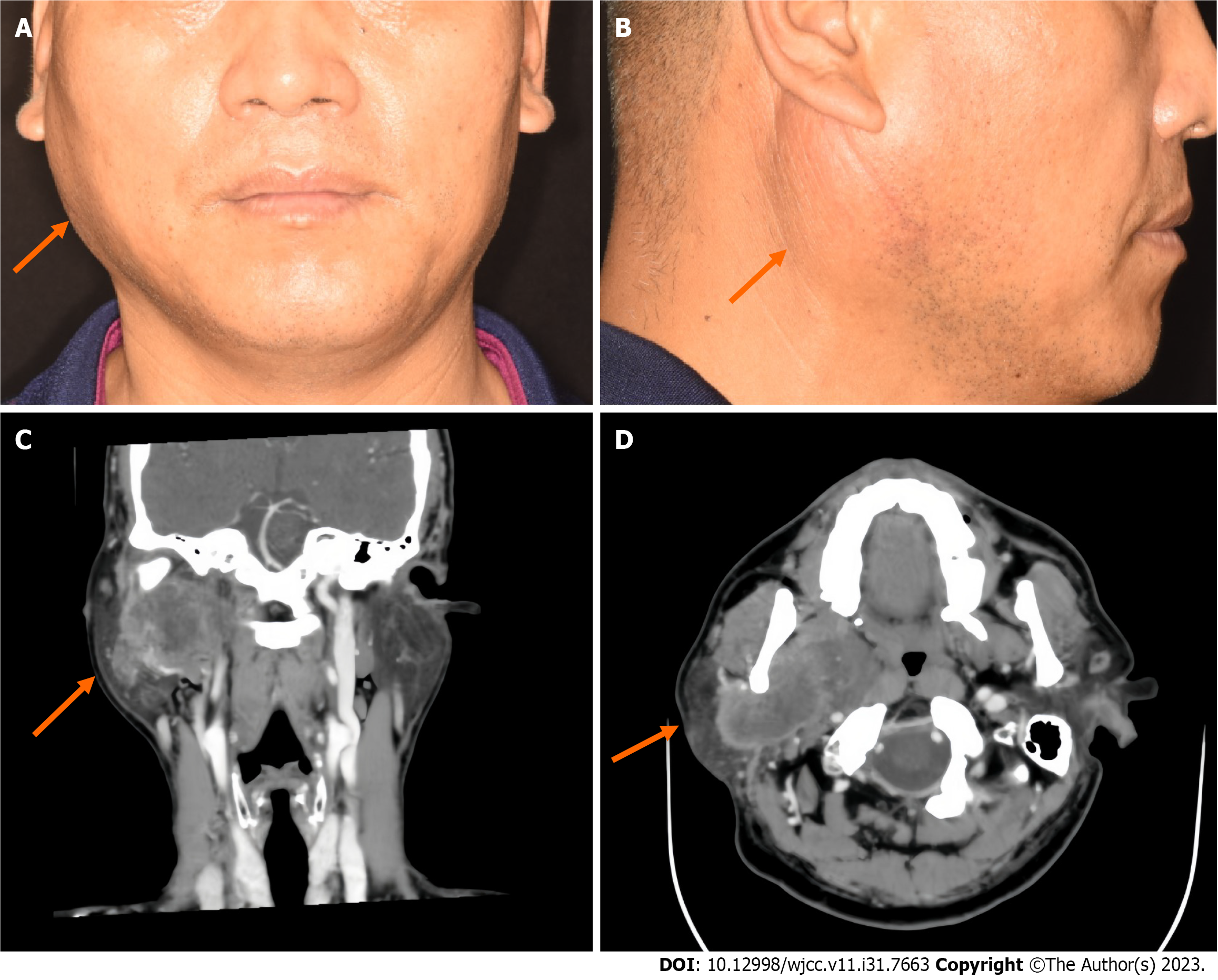
The patient had an asymmetric facial shape, swelling of the right side of the face, and an obvious eminence in the right parotid gland region. The surface of the mass was complete, and there was no redness or facial paralysis (Figure 1A and B). On palpation, a mass measuring 4 cm × 3.5 cm × 3 cm was palpable. The surface of the mass was smooth, qualitatively medium, with unclear borders, and generally mobile. The right external jugular vein was thickened. There were no significantly enlarged lymph nodes in the neck.
No special laboratory tests were performed.
Contrast-enhanced computed tomography (CT) revealed a large mass measuring 6.9 cm × 3.5 cm in cross section in the deep lobe of the right parotid gland, extending into the parapharyngeal region. There was obvious uneven enhancement, local ring enhancement, and a low-density liquefying zone and partition. The medial and lateral pterygoid muscles, pterygoid plate bone, and right external jugular vein were invaded. There was no obvious invasion of the skull base bone (Figure 1C and D).
The results of the above physical and imaging examinations led the patient to be diagnosed with MPA of the right skull base. The differential diagnosis included osteosarcoma and squamous cell carcinoma.
Fine needle aspiration cytology revealed a tumor with extensive necrosis, and immunohistochemical staining was recommended. Immunohistochemically, the tumor cells were positive for special AT-rich sequence-binding protein 2, mouse double minute 2 homolog, cyclin dependent kinase 4, and vimentin. These results combined with the microscopic morphology and immunohistochemistry suggested that the tumor was malignant with necrosis. Osteosarcoma was the first consideration, and further diagnosis after complete resection of the tumor was recommended.
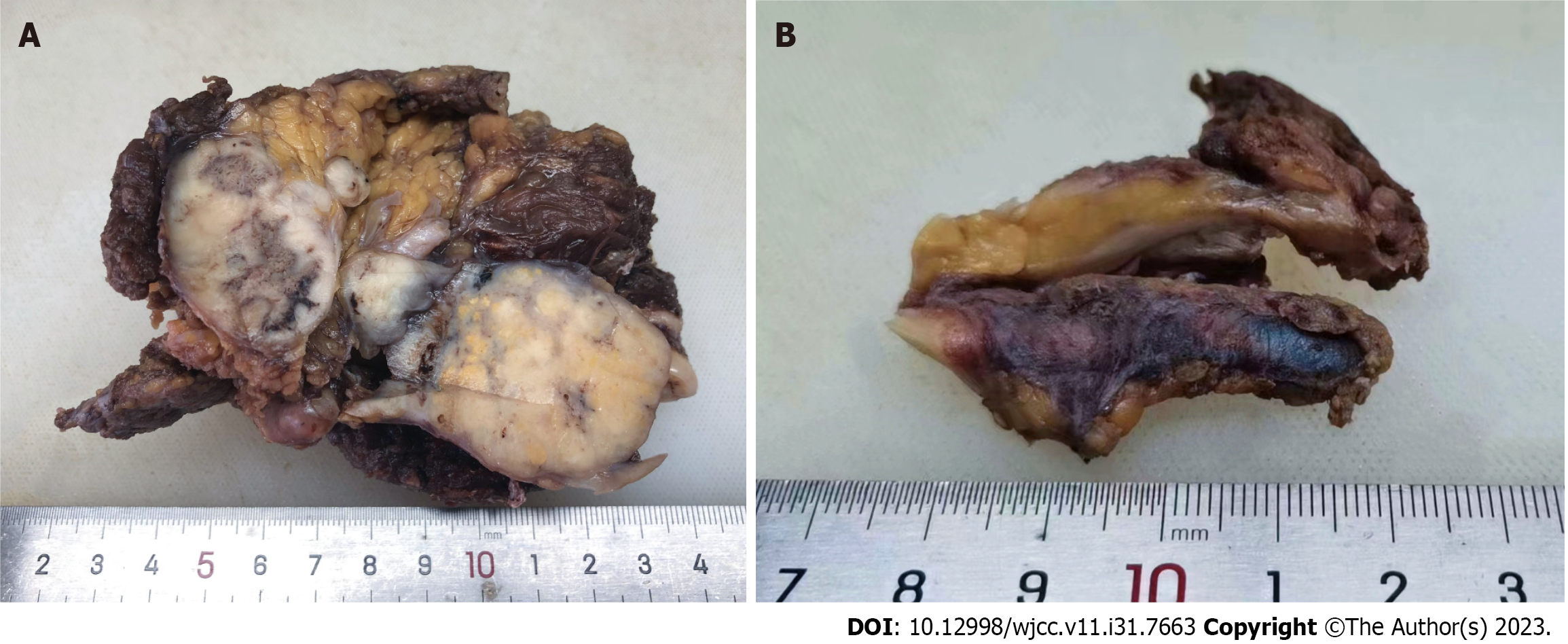
Gross examination showed that the tumor size was approximately 6 cm × 4.5 cm × 4.5 cm, with a nodular surface and partial capsule. The cut surface was gray-white, solid, and tough. There was also thickened venous tissue, measuring approximately 4.5 cm in length (Figure 2).
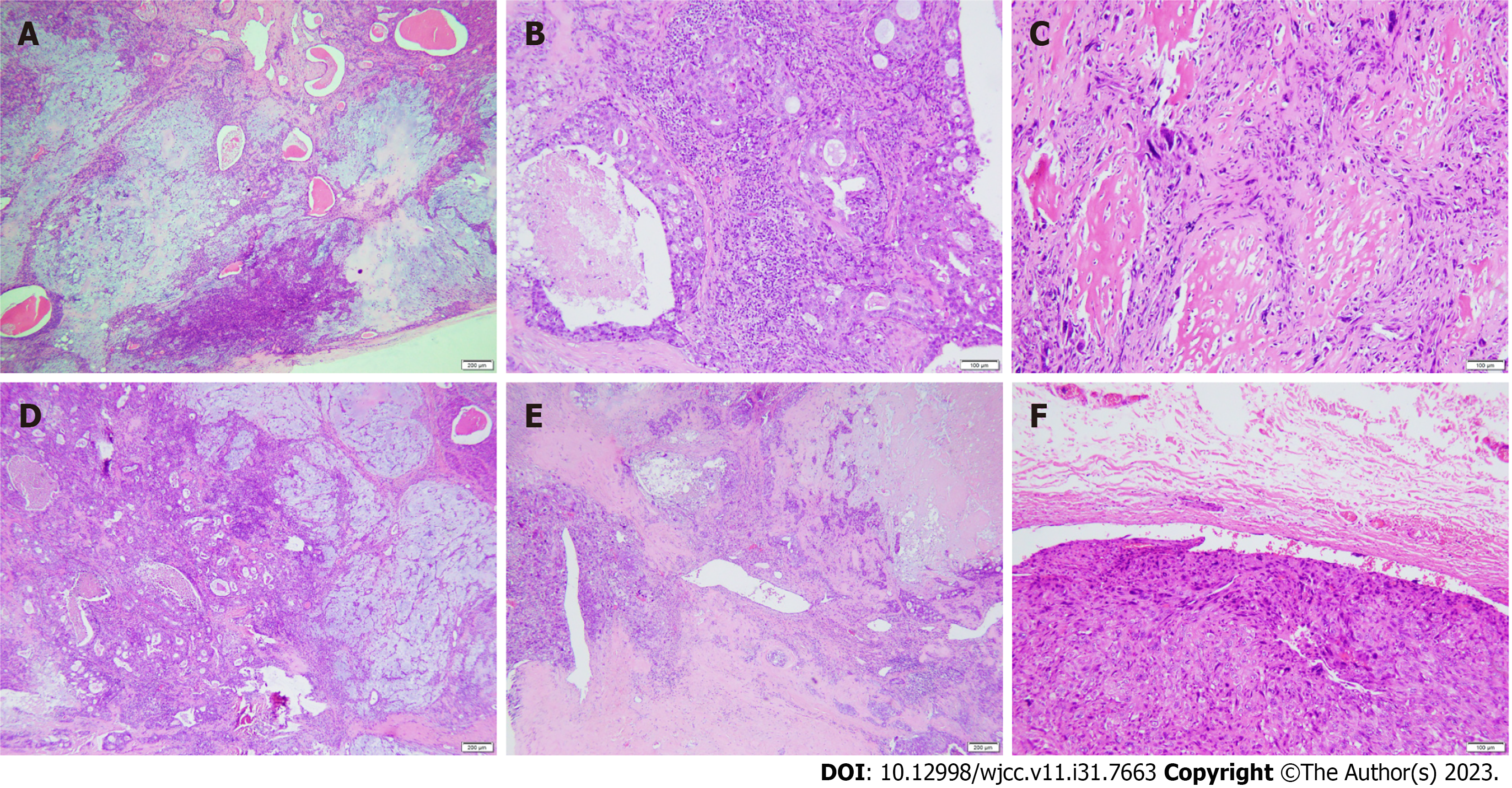
Microscopic examination revealed diverse tumor components consisting of a benign pleomorphic adenoma component, a carcinomatous component, and a sarcomatous component, with all three components intermingled (Figure 3A, D and E). The regional tissue structure of pleomorphic adenoma is complex and composed of abundant glandular epithelial and myoepithelial components. The stromal elements were myxoid and chondromatous (Figure 3A). Salivary duct carcinoma made up the carcinoma component of the tumor, while osteosarcoma composed the sarcoma component. The epithelial components were arranged into solid epithelial clumps, ethmoids, and small nests. Comedo-like necrosis was observed in the center of the solid epithelial mass. The tumor cells were large, cubic, rich in cytoplasm, and eosinophilic, with large nuclei and atypical mitoses (Figure 3B). The sarcoma area was composed of malignant spindle cells with obvious cell atypia, abundant cytoplasm, and abundant lace-like bone matrix formation around the cells (Figure 3C). A tumor thrombus was found in the venous cavity (Figure 3F). There was no metastasis in the lymph nodes around the mass.
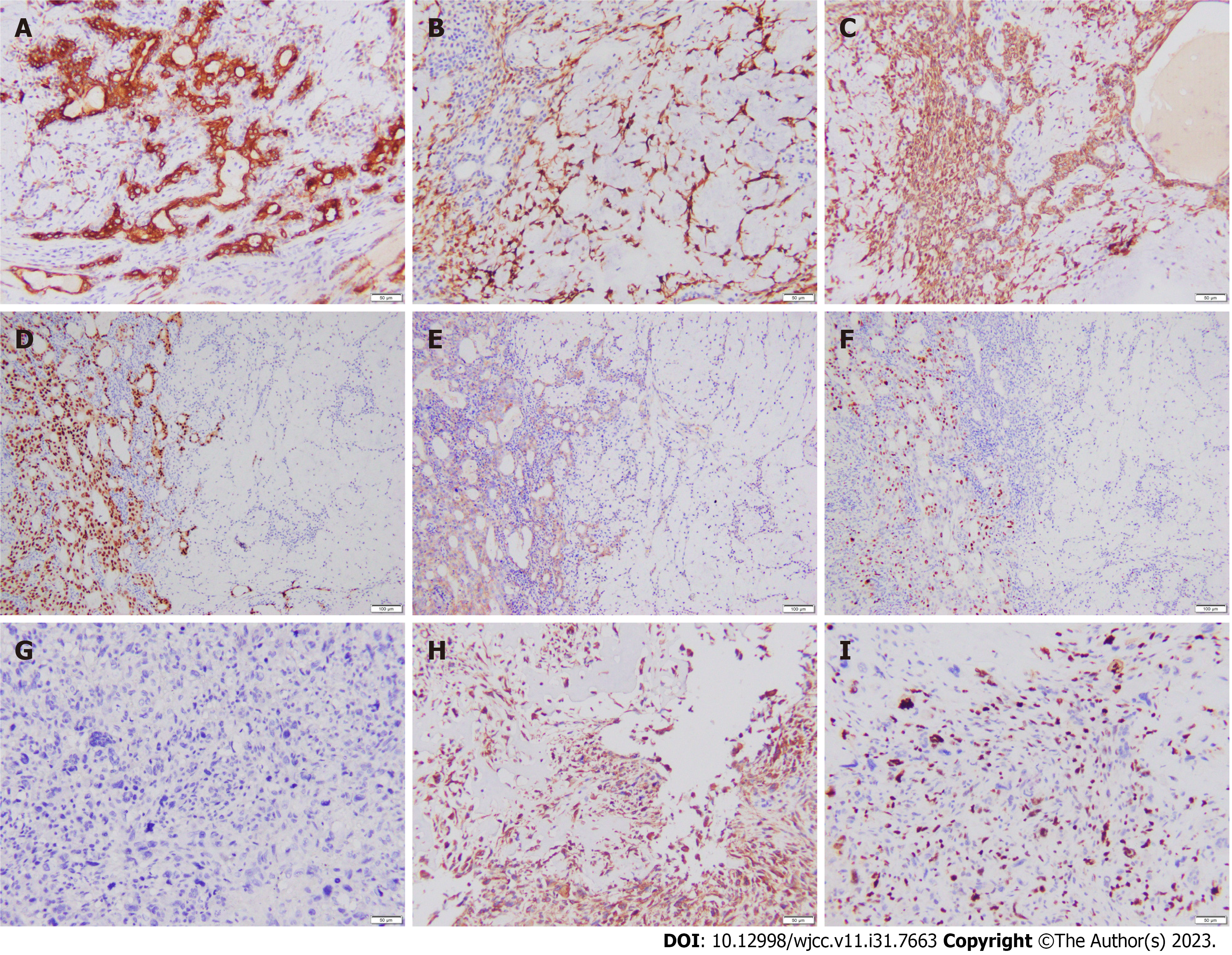
Immunohistochemistry showed that the pleomorphic adenoma area was positive for cytokeratin 7 (CK7), p63, p40, smooth muscle actin (SMA), cytokeratin 5/6 (CK5/6), calponin, and S-100 but negative for androgen receptor (AR) and human epithelial growth factor receptor 2 (HER2) (Figure 4A-C). The Ki-67 proliferation index was < 1%. The salivary duct carcinoma area was CK7, CK5/6, AR, and HER2 positive but p63, SMA, and S-100 negative (Figure 4D and E). The proliferation index of Ki-67 was approximately 30% (Figure 4F). Vimentin was positive in the osteosarcoma area, but pan-cytokeratin, CK7, and CK5/6 were negative (Figure 4G and H). The Ki-67 proliferation index was approximately 50% (Figure 4I).
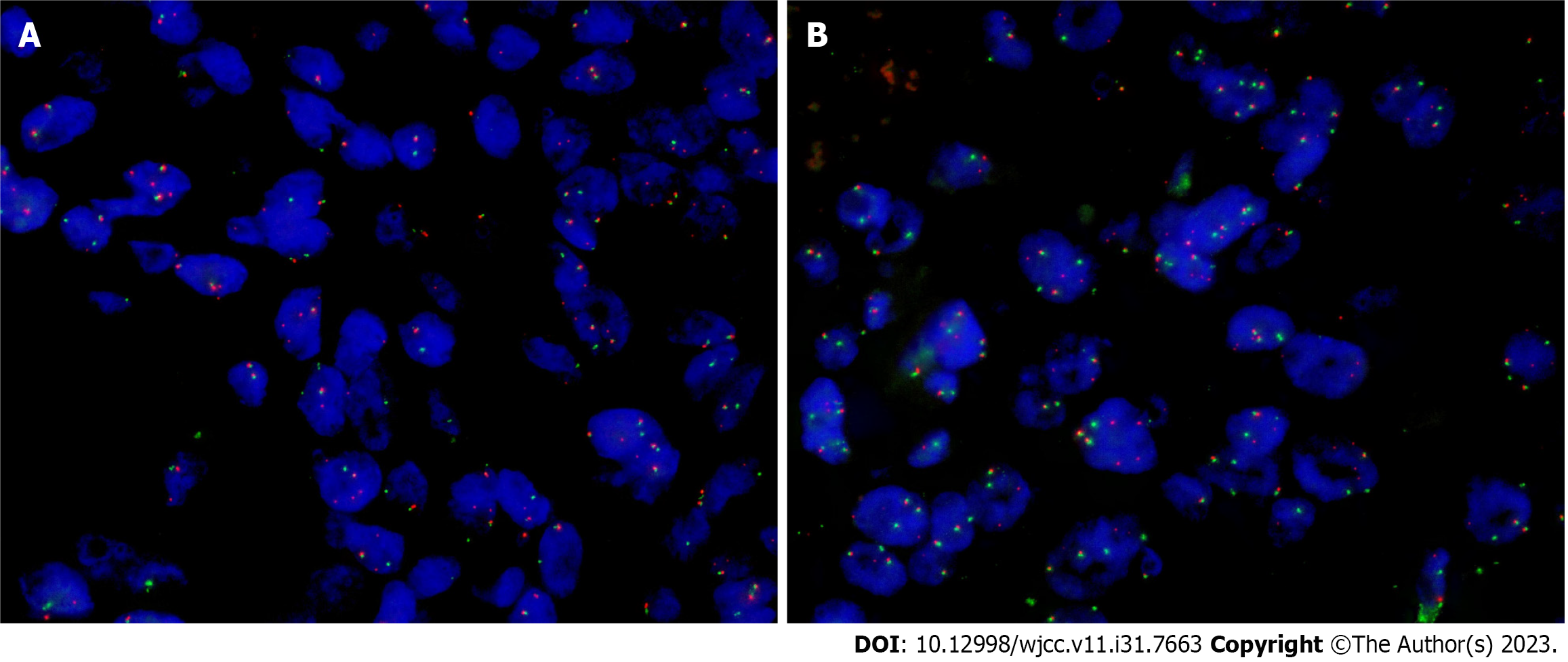
Fluorescence in situ hybridization (FISH) for pleiomorphic adenoma gene 1 (PLAG1), high mobility group A2
According to the above imaging examination, surgical resection of the mass, and pathological examination data, the final diagnosis was carcinosarcoma of the salivary gland.
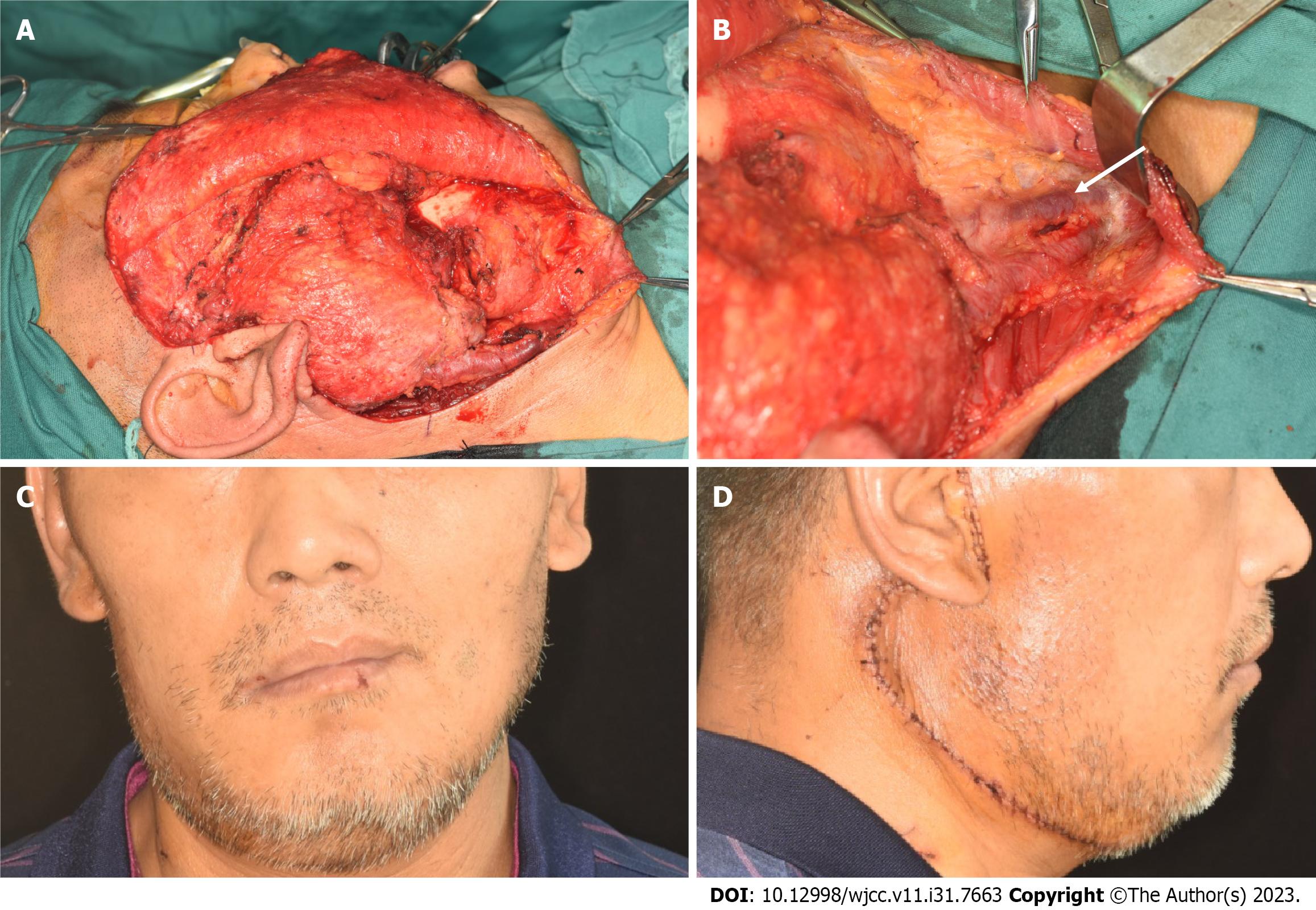
After a comprehensive preoperative examination, the patient underwent extended resection of the malignant tumor in the right skull base, right parotidectomy, and right mandibulectomy. During the operation, the external jugular vein was found to be thickened and hardened, and the boundary between the mass and the parotid gland was not clear. It was located in the deep surface of the parotid gland and adhered to the mandible (Figure 6A and B). After complete resection of the tumor along with the whole lobe of the parotid gland and the right mandible, vascular anastomosis, nerve anastomosis, facial elevation, and fascial tissue flap formation were performed, and the patient's facial shape recovered well after the operation (Figure 6C and D).
Following surgery, the patient received 33 cycles of radiotherapy (70 cGy) and 4 cycles of chemotherapy, and was followed up for 1 year without recurrence.
Carcinosarcoma, also known as true malignant mixed tumor, is composed of two components, malignant epithelial cells and malignant mesenchymal cells. Only 0.2% of tumors are primary carcinosarcomas, and more than 99% originate from pleomorphic adenomas[12]. We summarize the features of 13 cases published so far, and the clinicopathological features and treatment of the reported cases and the case presented here are detailed in Table 1. According to statistics, carcinosarcoma patients are generally older, 35-83 years old, with an average of about 62.6 years old. The majority of the individuals receiving treatment are male, with the parotid gland being the most frequently affected area, followed by the submandibular gland. A carcinosarcoma usually presents as a rapidly growing mass in the face that may cause pain, and those that occur in the parotid gland may cause facial paralysis. Carcinosarcomas consist of a mixture of distinct carcinomatous and sarcomatous components, any of which is capable of metastasis, most commonly to the lung, bone, and central nervous system with occasional metastases to the abdominal cavity[12].
| Characteristic | Number | % |
| Sex | ||
| Male | 11 | 78.6 |
| Female | 3 | 21.4 |
| Mean age (years) | 62.6 (35-83) | |
| Tumor location | ||
| Parotid | 10 | 71.4 |
| Submandibular | 4 | 28.6 |
| Tumor size (cm) | ||
| Unknown | 3 | 21.4 |
| ≤ 2 | 0 | 0 |
| 2-4 | 2 | 14.3 |
| ≥ 4 | 9 | 64.3 |
| Positive lymph nodes | ||
| None/unknown | 13 | 92.9 |
| ≥ 1 | 1 | 7.1 |
| Distant metastases | ||
| No | 9 | 64.3 |
| Yes | 5 | 35.7 |
| Surgery | ||
| No | 0 | 0 |
| Yes | 14 | 100 |
| Type of surgery | ||
| Partial gland excision | 0 | 0 |
| Total gland excision | 14 | 100 |
| Radiation | ||
| No/unknown | 7 | 50 |
| External beam | 7 | 50 |
| Chemotherapy | ||
| No/unknown | 9 | 64.3 |
| Yes | 5 | 35.7 |
Radiographically, the main imaging findings of this case were a mass of soft tissue shadow in the deep lobe of the right parotid gland, extending to the parapharyngeal area along the widened mastoid sulcus. The local edge of the parapharyngeal area was slightly blurred, and obvious uneven enhancement, local ring enhancement, and low-density liquefactive area and separation were seen inside, which was considered as a malignant tumor. It was basically consistent with previous reports. In the literature, CT often shows a well-demarcated mass in the deep lobe of the parotid gland with an internal cystic necrotic area with irregular borders and septal contrast enhancement[13]. On magnetic resonance imaging (MRI), multilocular cystic mass lesions are often hypointense on T1-weighted images and hyperintense on T2-weighted images[8]. CT of the head and neck can accurately describe the tumor location and detect local invasion, identifying malignant characteristics such as ill-defined borders and calcifications. MRI has better soft tissue resolution and can better assess peripheral soft tissue and nerve involvement[14]. As a result of cystic degeneration or necrosis, carcinosarcoma may be misdiagnosed as an abscess during imaging examinations[15]. The final diagnosis of the tumor requires pathological examination.
Microscopically, the carcinosarcoma is biphasic, with variable proportions of malignant epithelial and malignant mesenchymal components. Adenocarcinoma and undifferentiated carcinoma are the most common types of cancer components, followed by squamous cell carcinoma[16]. The common types of sarcomas are chondrosarcoma, fibrosarcoma, leiomyosarcoma, osteosarcoma, rhabdomyosarcoma, and liposarcoma[16]. All the 13 cases had adenocarcinoma components, of which three also had squamous cell carcinoma components. In addition to osteosarcoma, the sarcomatous components were also mixed with chondrosarcoma, rhabdomyosarcoma, and fibrosarcoma. In this case, there were three components of benign pleomorphic adenoma, salivary duct carcinoma, and osteosarcoma, which were consistent with the histological characteristics of salivary gland carcinosarcoma. Immunohistochemical staining of the present case showed that the glandular epithelial marker CK7 was positive in the benign pleomorphic adenoma and ductal carcinoma components. Myoepithelial markers CK5/6, S-100, SMA, and calponin showed higher expression levels in benign pleomorphic adenoma and lower expression levels in ductal carcinoma. AR and HER2 positive expression is helpful to identify salivary duct carcinoma, especially AR has high specificity and sensitivity[17]. The CK negative and vimentin positive components of sarcomas support the diagnosis of carcinosarcoma, consistent with the results of Jha et al[11] and Woo and Son[12].
Studies on molecular genetics have shown that pleomorphic adenoma and CAxPA both have specific genetic changes, mainly involving the rearrangement of PLAG1 and HMGA2[18]. As far as we know, only one case of carcinosarcoma has been reported by FISH testing to date. According to literature reports, FISH for PLAG1 and HMGA2 was performed separately for the sarcomatoid and carcinomatous components of the tumor and for the small independent PA adjacent to the carcinosarcoma, and PLAG1 was rearranged in both components of the carcinosarcoma, while HMGA2 was translocalized in the PA alone[7]. Therefore, this carcinosarcoma may originate from PA with PLAG1 translocation, rather than from PA with different translocations coexisting nearby. In this case, we performed FISH for PLAG1, HMGA2, and MDM2 for the pleomorphic adenoma component, the carcinomatous component, and the osteosarcoma component of the tumor. PLAG1 gene rearrangement was positive in the pleomorphic adenoma and carcinomatous components, but negative in the sarcomatous component. HMGA2 and MDM2 were negative in all three regions. It was confirmed that the ductal carcinoma in the carcinosarcoma component may originate from the pleomorphic adenoma region. Related studies have shown that the occurrence and development of osteosarcoma are closely related to the presence of MDM2 amplification in patients[19]. However, MDM2 expression was negative in the sarcoma component of this case, and the boundary between the sarcoma and carcinomatous component was not clear, so it is suspected that the sarcoma component may originate from ductal carcinoma. When PLAG1 and HMGA2 gene rearrangements are found, they may be helpful for the diagnosis of carcinosarcoma. However, if not, it may be the differential expression of genes, which is also a potential defect of this testing.
Regarding differential diagnosis, carcinosarcoma of the salivary gland should be differentiated from simple salivary gland sarcoma and myoepithelial carcinoma: (1) Salivary gland sarcoma is a rare malignant tumor, which mainly includes malignant schwannoma, hemangiopericytoma, malignant fibrous histiocytoma, fibrosarcoma, Kaposi's sarcoma, rhabdomyosarcoma, etc[20]. Microscopically, the main component is diffuse spindle tumor cells without epithelial components. Immunohistochemical staining can be used for differentiation, and salivary adenosarcoma does not express epithelial or myoepithelial markers; and (2) Myoepithelial carcinoma is an uncommon malignant tumor consisting mainly of myoepithelium, with complex and diverse morphology and lack of obvious ductal differentiation. Carcinosarcoma of the salivary gland should be differentiated from spindle myoepithelial carcinoma when the sarcomatous component of the salivary gland shows a large number of spindle cells. Myoepithelial carcinoma is diffusely and strongly positive for CK7, P63, S-100, and caplonin. Immunohistochemical examination can identify the cellular components of myoepithelial carcinoma, which is helpful for the differential diagnosis.
In treatment, according to the statistics in Table 1, all cases of carcinosarcoma with an osteosarcoma component were treated by surgery, of which seven patients were treated with postoperative radiotherapy and/or chemotherapy, and five developed distant metastasis. Overall survival was 37% after 5 years, with surgery followed by radiotherapy having a significant survival advantage compared with isolated surgical resection[2]. Consequently, postoperative adjuvant radiotherapy and the extension of the radiotherapy to regional lymph nodes are extremely important, even if the nodes are not metastatic[21].
Due to the poor prognosis of carcinosarcoma and the high rate of recurrence and metastasis, clinicians should diagnose this rare tumor on the basis of its histological appearance, immunohistochemical features, and specific genetic alterations to avoid misdiagnosis and delay in treatment.
| 1. | Tarsitano A, Pizzigallo A, Giorgini F, Marchetti C. Giant pleomorphic adenoma of the parotid gland: an unusual case presentation and literature review. Acta Otorhinolaryngol Ital. 2015;35:293-296. [PubMed] |
| 2. | Gupta A, Koochakzadeh S, Neskey DM, Nguyen SA, Lentsch EJ. Salivary Carcinosarcoma: An Extremely Rare and Highly Aggressive Malignancy. Laryngoscope. 2020;130:E335-E339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Ghaloo SK, Qayyum MU, Shaikh OS, Faisal M, Keerio AA, Hussain RT. Carcinoma ex-pleomorphic adenoma of mandible: A case report and review of literature. Int J Surg Case Rep. 2022;100:107729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 4. | Kirklin JW, McDonald JR, Harrington SW, New GB. Parotid tumors; histopathology, clinical behavior, and end results. Surg Gynecol Obstet. 1951;92:721-733. [PubMed] |
| 5. | Liess BD, Hirschi S, Zitsch RP 3rd, Frazier S, Konrad A. Carcinosarcoma of the parotid gland: report of a case with immunohistochemical findings. Ann Otol Rhinol Laryngol. 2007;116:702-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Taki NH, Laver N, Quinto T, Wein RO. Carcinosarcoma de novo of the parotid gland: case report. Head Neck. 2013;35:E161-E163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Katsakhyan L, LiVolsi VA, Chalian AA, Zhang PJ. Giant Cell Carcinosarcoma of the Parotid Gland With a PLAG 1 Translocation in Association With a Pleomorphic Adenoma With HMGA2 Translocation. Am J Clin Pathol. 2020;154:811-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Endo Y, Ohashi R, Inai S, Yokoshima K, Nakamizo M, Shimizu A, Okubo K, Naito Z. Carcinosarcoma ex Pleomorphic Adenoma of the Submandibular Gland in a 64-Year-Old Man: A Case Report. J Nippon Med Sch. 2018;85:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Rumnong V, Banerjee AK, Joshi K, Kataria RN. Carcinosarcoma of parotid gland having osteosarcoma as sarcomatous component: a case report. Indian J Pathol Microbiol. 1993;36:492-494. [PubMed] |
| 10. | Marcotullio D, de Vincentiis M, Iannella G, Cerbelli B, Magliulo G. Mucoepidermoid Carcinoma Associated with Osteosarcoma in a True Malignant Mixed Tumor of the Submandibular Region. Case Rep Otolaryngol. 2015;2015:694684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Jha V, Kolte S, Goyal S, Mandal AK. Osteosarcoma Arising in Carcinosarcoma De Novo Parotid Gland in a Young Man: An Unusual Case with Review of Literature. J Clin Diagn Res. 2017;11:ED08-ED10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Woo CG, Son SM. Carcinosarcoma of the parotid gland with abdominal metastasis: a case report and review of literature. World J Surg Oncol. 2018;16:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Gozgec E, Ogul H, Ceylan O. Two Extremely Rare Case of Carcinosarcoma Ex Pleomorphic Adenoma. Ear Nose Throat J. 2022;1455613221130888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 14. | Santisi AM, DiMarcangelo MT, Zhang X, Ahmad N, Brody JD. Carcinosarcoma of the parotid gland with mucoepidermoid carcinoma component. Radiol Case Rep. 2020;15:1202-1206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Phan HNM, Hong YT, Hong KH. Primary Carcinosarcoma of the Parotid Gland Mimicking as Parotid Abscess With Deep Neck Infection. J Craniofac Surg. 2017;28:e210-e213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Tanahashi J, Daa T, Kashima K, Kondo Y, Yada N, Kuratomi E, Hirokawa M, Yokoyama S. Carcinosarcoma ex recurrent pleomorphic adenoma of the submandibular gland. APMIS. 2007;115:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Nakaguro M, Tada Y, Faquin WC, Sadow PM, Wirth LJ, Nagao T. Salivary duct carcinoma: Updates in histology, cytology, molecular biology, and treatment. Cancer Cytopathol. 2020;128:693-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 18. | Yousaf A, Sulong S, Abdullah B, Lazim NM. Heterogeneity of Genetic Landscapes in Salivary Gland Tumors and Their Critical Roles in Current Management. Medeni Med J. 2022;37:194-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Limbach AL, Lingen MW, McElherne J, Mashek H, Fitzpatrick C, Hyjek E, Mostofi R, Cipriani NA. The Utility of MDM2 and CDK4 Immunohistochemistry and MDM2 FISH in Craniofacial Osteosarcoma. Head Neck Pathol. 2020;14:889-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Zhao E, Song Y, Tan X, Shi W. Primary spindle-cell sarcoma of the parotid gland. Asian J Surg. 2023;46:1871-1873. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | An HJ, Baek HJ, Kim JP, Kim MH, Song DH. Fine-Needle Aspiration Cytology of Carcinosarcoma in the Salivary Gland: An Extremely Rare Case Report. J Pathol Transl Med. 2018;52:136-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pathology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sridharan G, India S-Editor: Lin C L-Editor: Wang TQ P-Editor: Zhao S