Published online Nov 6, 2023. doi: 10.12998/wjcc.v11.i31.7629
Peer-review started: June 29, 2023
First decision: September 26, 2023
Revised: October 10, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: November 6, 2023
Processing time: 130 Days and 7.8 Hours
Sunitinib, a multi-targeted tyrosine kinase inhibitor (TKI), has been approved for the salvage treatment of gastrointestinal stromal tumors (GIST). Hyperammone
A 66-year-old male with metastatic GIST was admitted because of reduced consciousness. Imatinib was administered as the first-line systemic therapy. He experienced repeated episodes of peritonitis due to tumor perforation, and surgery was performed. Progressive disease was confirmed based on increased liver metastasis, and sunitinib was initiated as a salvage treatment. However, 23 d after the third course of sunitinib, he presented to the emergency room with an episode of altered consciousness and behavioral changes. Based on the patient clinical history and examination findings, sunitinib-induced encephalopathy was suspected. Sunitinib was discontinued, and the patient was treated for hyperammonemia. The patient had a normal level of consciousness four days later, and the serum ammonia level gradually decreased. No further neurological symptoms were reported in subsequent follow-ups.
TKI-induced hyperammonemic encephalopathy is potentially life-threatening. Patients receiving TKIs experiencing adverse reactions should undergo systemic evaluation and prompt treatment.
Core Tip: This case is distinctive because the patient was diagnosed with a rare hyperammonemic encephalopathy caused by sunitinib, which was administered as treatment for gastrointestinal stromal tumors. We emphasize the need for systemic evaluation and prompt action for patients receiving tyrosine kinase inhibitors who present with adverse effects.
- Citation: Hayakawa T, Funakoshi S, Hamamoto Y, Hirata K, Kanai T. Sunitinib-induced hyperammonemic encephalopathy in metastatic gastrointestinal stromal tumors: A case report. World J Clin Cases 2023; 11(31): 7629-7634
- URL: https://www.wjgnet.com/2307-8960/full/v11/i31/7629.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i31.7629
In clinical practice, targeted therapies such as tyrosine kinase inhibitors (TKIs) are widely used to treat several malignancies, including gastrointestinal stromal tumors (GIST). Common adverse effects of multi-targeted TKIs reported in clinical studies include hand-foot syndrome, diarrhea, hypertension, and metabolic and electrolyte imbalances[1].
One rare adverse event is hyperammonemic encephalopathy, which can lead to cerebral edema, seizures, herniation, or even death, if not recognized early[2]. However, the incidence of this side effect has rarely been reported, and its mechanism is unknown. Here we report a 66-year-old man undergoing sunitinib treatment for metastatic GIST, without underlying liver cirrhosis, who presented to the emergency department with disturbance of consciousness and was found to have hyperammonemic encephalopathy. We hope that this case will raise awareness of TKI-related hyperammonemic encephalopathy, as clinical suspicion of this condition will lead to early diagnosis and treatment.
A 66-year-old man was brought to our hospital with an episode of altered consciousness and behavioral changes.
The patient had a metastatic liver tumor of GIST.
The patient had a medical history of type 2 diabetes mellitus and situs inversus.
The patient had a history of smoking for > 40 years. The patient had no history of alcohol consumption. There was no relevant family history.
Physical examination revealed hypertension (blood pressure = 193/118 mmHg) and a Glasgow Coma Scale score of 13 (eye 4, verbal 3, motor 6). The patient exhibited asterixis but was otherwise unremarkable.
Laboratory examination results at the time of admission were as follows: Platelet count, 82000/mm3; total bilirubin level, 2.0 mg/dL; albumin level, 3.7 g/dL; aspartate aminotransferase level, 44 U/L; alanine transaminase level, 25 U/L; creatinine level, 1.25 mg/dL, and PT 97%. In addition, a markedly elevated ammonia level of 122 µmol/L (11-51 µmol/L) was observed. And there were no abnormalities in electrolytes or glucose metabolism.
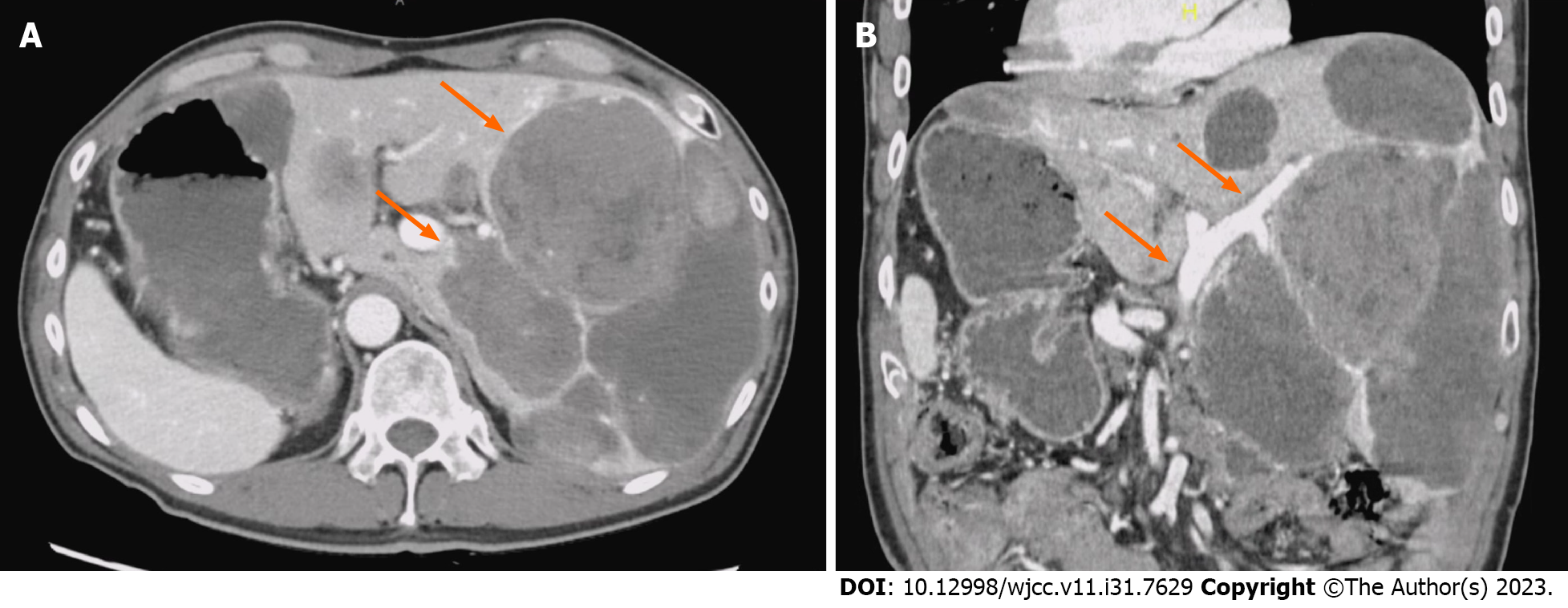
Computed tomography (CT) and magnetic resonance imaging of the brain showed no findings of posterior reversible encephalopathy syndrome (PRES) or metastatic brain tumors. Contrast-enhanced CT imaging of the abdomen and pelvis revealed progressive disease with increased liver metastasis. There was no portal vein obstruction or significant portosystemic shunt, although situs inversus was observed (Figure 1).
The final diagnosis was drug-induced hyperammonemic encephalopathy related to sunitinib.
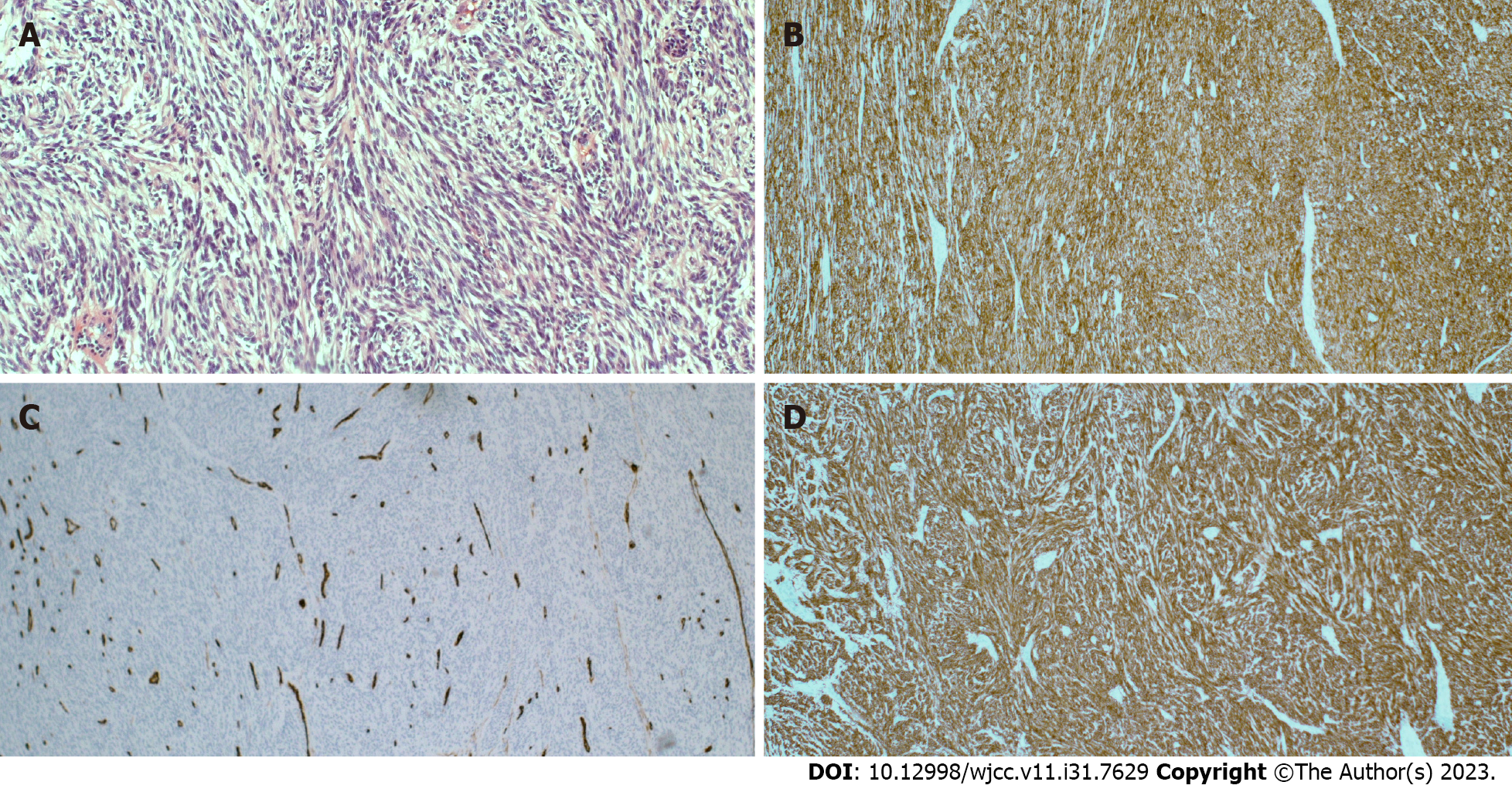
The patient was diagnosed with GIST and liver metastases. Although the patient’s progress was good, he had repeated episodes of peritonitis due to tumor perforation. Surgery was performed, including primary resection, partial resection of the small intestine, and partial resection of the colon. The pathological examination revealed a GIST (Figure 2). Imatinib was then resumed, and his disease was well controlled for 11 mo; however, abdominal CT revealed progressive disease due to increased liver metastasis, and oral sunitinib was initiated as second-line treatment. He had a history of situs inversus but no fundamental liver disease such as liver cirrhosis. However, 23 days after the third course of sunitinib, the patient was brought to the emergency room with an episode of altered consciousness and behavioral changes.
Based on his clinical history and various examinations, sunitinib-induced encephalopathy was suspected after excluding other common causes of encephalopathy, such as hypoglycemia, metastatic brain tumors, PRES, encephalopathy caused by other drugs, and encephalopathy due to shunt or portal vein obstruction. The patient was administered branched-chain amino acids for hyperammonemia. Sunitinib was discontinued based on a previous report of sunitinib-induced hyperammonemia. Four days later, the patient regained a normal level of consciousness. The patients was orally administered lactulose and rifaximin. The serum ammonia level gradually decreased to 70 µmol/L, and the patient was discharged from the hospital on day 14 with no symptom flare-up.
Sunitinib therapy was not resumed. The patient did not develop any neurological symptoms during the subsequent months of follow-up.
Our patient had mild thrombocytopenia, likely due to sunitinib treatment. Sunitinib is a small molecule and an inhibitor of certain receptor tyrosine kinases, including VEGFR types 1 and 2 (FLT-1 and FLK-1/KDR), PDGFR-a and PDGFR-b, stem cell factor receptor (c-KIT), and FLT-3 and RET kinases[3]. TKIs, including sunitinib, have been associated with the onset of PRES; however, few reports of hyperammonemia exist. Hyperammonemia has been frequently reported with other chemotherapeutic agents such as vincristine, doxorubicin, cytarabine, cyclophosphamide, etoposide, and 5-fluorouracil; however, its incidence is less well known and possibly underreported with TKIs[4,5]. The reported cases of drug-induced hyperammonemic encephalopathy associated with TKIs including sunitinib are presented in Table 1. The present case was characterized by a longer period from the start of treatment to the onset of hyperammonemic encephalopathy than those of cases reported in the past; however, the recovery period after sunitinib discontinuation was comparable to those reported in the past.
| Sex | Female/Male, (n) | 5/6 |
| Age | Median (range), yr | 47 (20-74) |
| Diagnosis (n) | GIST | 5 (45) |
| CRC | 2 (18) | |
| RCC | 2 (18) | |
| BC | 1 (9) | |
| pNET | 1 (9) | |
| Drug (n) | Sunitinib | 6 (55) |
| Regorafenib | 4 (36) | |
| Pazopanib | 1 (9) | |
| Ammonia level | Median (range), μmoL/L | 151 (87-278) |
| Time to symptom | Median (range), d | 17 (1-390) |
| Time to recovery | Median (range), d | 3 (1-12) |
The mechanism of encephalopathy remains unclear; however, in light of a similar presentation, it was reasonably suspected to be the result of the class effect of these TKIs[4]. Sunitinib is thought to decrease the ability of the liver to handle NH3 through its anti-angiogenic properties[6]. Sunitinib is primarily metabolized by the liver via cytochrome CYP3A4, with the formation of equally pharmacologically active N-desethyl metabolites, and a decrease in liver reserve may contribute to the development of hyperammonemia[7]. The anti-angiogenetic effect of the VEGFR TKIs could also inhibit new vasculature formation in many organs including the brain, which may interfere with vascular-cerebral permeability or the “blood-brain barrier.” Thus, a higher amount of ammonia may cross the blood-brain barrier and enter the central nervous system[4].
Hyperammonemia did not occur during the initial course of sunitinib treatment in this patient. Increased tumor volume in the liver may have impaired the ability to metabolize ammonia through the urea cycle. In addition, the patient had mild renal impairment and decreased urinary excretion of ammonia, which may have also contributed to hyperammonemia. Situs inversus can be complicated by the congenital absence of the portal vein, and rare cases of portal vein shunts that can cause hyperammonemia have been reported[8]. However, these could be excluded using CT scans.
Hyperammonemic encephalopathy must be urgently managed. The treatment of drug-induced hyperammonemic encephalopathy is often aimed at reducing ammonia production and absorption using an approach similar to that used for hepatic encephalopathy[9]. Oral and/or transrectal lactulose and branched-chain amino acids are recommended, but their benefits are uncertain. Rifaximin is a virtually unabsorbed antibiotic that is thought to reduce ammonia production by eliminating ammonia-producing colonic bacteria in the digestive tract and may also be useful. In addition, because ammonia is not protein-bound and is a low-molecular-weight molecule (17 g/moL) similar to urea, clearance with hemodialysis is extremely effective if there is no improvement with these medical treatments[10].
Based on his clinical history and various examinations, sunitinib-induced encephalopathy was suspected after excluding other common causes of consciousness disturbance. In addition to the cessation of sunitinib, branched-chain amino acids, lactulose, and rifaximin were initiated, effectively reducing the serum ammonia level with a clinical improvement in the patient’s neurological symptoms.
Here, we report a rare case of sunitinib-induced hyperammonemic encephalopathy in a man with metastatic GIST. We suggest checking the ammonia levels in patients on sunitinib who present with altered sensorium, even if there were no liver problems. Further accumulation of reports is desirable because the CNS side effects of TKIs are not well recognized and may be underreported.
| 1. | Blay JY. Pharmacological management of gastrointestinal stromal tumours: an update on the role of sunitinib. Ann Oncol. 2010;21:208-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Neves Briard J, Lezaic N, Keezer MR. Pearls & Oy-sters: Chemotherapy-associated hyperammonemic encephalopathy. Neurology. 2020;94:e874-e877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25:884-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 700] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 4. | Kongsuphon N, Soukavanitch M, Teeraaumpornpunt N, Konmun J, Ativitavas T, Ngamphaiboon N. Multi-Targeted Tyrosine Kinase Inhibitor-Induced Hyperammonemic Encephalopathy: a Report of Two Cases Using Pazopanib, Sunitinib, and Regorafenib. J Gastrointest Cancer. 2019;50:601-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Nott L, Price TJ, Pittman K, Patterson K, Fletcher J. Hyperammonemia encephalopathy: an important cause of neurological deterioration following chemotherapy. Leuk Lymphoma. 2007;48:1702-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Lee NR, Yhim HY, Yim CY, Kwak JY, Song EK. Sunitinib-induced hyperammonemic encephalopathy in gastrointestinal stromal tumors. Ann Pharmacother. 2011;45:e56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, Armand JP, Scigalla P, Raymond E. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 909] [Cited by in RCA: 909] [Article Influence: 43.3] [Reference Citation Analysis (8)] |
| 8. | Kasahara M, Nakagawa A, Sakamoto S, Tanaka H, Shigeta T, Fukuda A, Nosaka S, Matsui A. Living donor liver transplantation for congenital absence of the portal vein with situs inversus. Liver Transpl. 2009;15:1641-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Willson KJ, Nott LM, Broadbridge VT, Price T. Hepatic encephalopathy associated with cancer or anticancer therapy. Gastrointest Cancer Res. 2013;6:11-16. [PubMed] |
| 10. | Haroon S, Ko S, Wong A, Tan PS, Lee E, Lau T. Sunitinib-associated hyperammonemic encephalopathy successfully managed with higher intensity conventional hemodialysis: A case report. Medicine (Baltimore). 2021;100:e24313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gao F, China; Lin Q, China; Zong L, China; Govindarajan KK, India S-Editor: Liu JH L-Editor: A P-Editor: Yuan YY