Published online Nov 6, 2023. doi: 10.12998/wjcc.v11.i31.7562
Peer-review started: August 31, 2023
First decision: September 19, 2023
Revised: September 23, 2023
Accepted: October 26, 2023
Article in press: October 26, 2023
Published online: November 6, 2023
Processing time: 66 Days and 20 Hours
Various reconstruction options have been introduced to treat decubitus ulcers. A combined flap that takes advantage of the fasciocutaneous and muscle flaps has been proven to be effective in reconstructing decubitus ulcers in previous studies. However, no studies have measured combined flap thickness. This is the first study to demonstrate the superiority of the combined flap by measuring its thickness using enhanced abdominopelvic computed tomography (APCT).
To evaluate combined flap modality as a useful reconstruction option for decubitus ulcers using measurements obtained through APCT.
Fifteen patients with paraplegia who underwent combined flap surgery for reconstruction of decubitus ulcers between March 2020 and December 2021 were included. The defects in the skin and muscle components were reconstructed separately. The inner gluteus muscle flap was split and manipulated to obliterate dead space. The outer fasciocutaneous flap was transposed to cover the muscle flap and opening of the decubitus ulcer. Subsequently, we performed enhanced APCT at 3 wk and 6 mo postoperatively to measure the flap thickness.
The mean flap thickness was 32.85 ± 8.89 mm at 3 wk postoperatively and 29.27 ± 8.22 mm at 6 mo postoperatively. The flap thickness was maintained without any major complications such as contour deformities or recurrence.
Although there was a significant decrease in flap thickness as measured by APCT, the combined flap provided sufficient padding and maintained its thickness even at 6 mo after reconstruction, suggesting that the combined flap modality may be a useful reconstruction option for patients with paraplegic decubitus ulcers.
Core Tip: In this study, we examined the use of combined fasciocutaneous and gluteus maximus muscle flaps to reconstruct grade IV decubitus ulcers in patients with paraplegia. Radiological evaluation was employed to measure flap thickness, and the combined flap approach was found to provide successful reconstruction, with only minor complications. The study revealed a slight reduction in flap thickness over time; however, adequate coverage was maintained. This combined flap method offers a valuable treatment option for challenging decubitus ulcers in patients with paraplegia, and addresses the issues of flap thickness and recurrence.
- Citation: Kim EC, Park JD, Wee SY, Kim SY. Measurement of combined flap thickness for reconstruction of decubitus ulcer using computed tomography. World J Clin Cases 2023; 11(31): 7562-7569
- URL: https://www.wjgnet.com/2307-8960/full/v11/i31/7562.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i31.7562
Reconstruction of decubitus ulcers is challenging owing to the high rates of recurrence and complications. Surgical reconstruction is rarely required for grade I or II decubitus ulcers, which are typically managed conservatively with proper nutrition, adequate positional changes, and dressing. However, surgical reconstruction is recommended in patients with grade III and IV decubitus ulcers. The selection of an appropriate reconstruction option is a critical factor contributing to its success[1]. Various reconstruction methods have been used, including musculocutaneous, fasciocutaneous, and perforator flaps. Recently, fasciocutaneous and perforator flaps have gained popularity owing to their low donor site morbidity[2,3]. Nevertheless, their limited volume and bulk compared with muscle flaps may lead to recurrence owing to inadequate padding. In cases of deep and wide decubitus ulcers, muscle flaps can provide sufficient padding to effectively obliterate dead space.
However, to date, there is no universally accepted gold standard for the reconstruction of decubitus ulcers, leading many surgeons to explore new methods. One such approach combines the advantages of a perforator-based fasciocutaneous flap and a muscle flap, and has shown promise in decubitus ulcer reconstruction[4-6]. However, to our knowledge, no study has investigated whether flap thickness is maintained or whether adequate padding is provided in patients who undergo reconstruction using this combined flap. Therefore, the primary objective of our study was to measure the thickness of combined flap coverage in patients with paraplegia with decubitus ulcers using radiological evaluation.
This retrospective clinical study was conducted between March 2020 and December 2021 at Soonchunhyang University Gumi Hospital. Patients who underwent combined flap coverage for reconstruction of grade IV decubitus ulcers in the ischial or trochanteric regions were included. Owing to severity of the ulcer and problems of recurrence, all patients with ischial or trochanteric ulcers underwent the same surgical procedure regardless of defect size. This study was approved by the Institutional Review Board of the Soonchunhyang Medical Center Office of the Human Research Protection Program (IRB No. 2021-14) and was conducted in accordance with the principles of the Declaration of Helsinki. All patients provided written informed consent for the use and publication of their images. This study included only patients who were in a paraplegic state, and ambulatory patients were excluded. Patients with a history of gluteus maximus flap reconstruction were also excluded. After admission, all patients underwent thorough irrigation and surgical debridement of the necrotic tissue. Appropriate antibiotics were administered based on the bone biopsy and wound culture results. Negative-pressure wound therapy was administered for bed preparation. Preoperative enhanced abdominopelvic computed tomography (APCT) was used to evaluate each patient's wound, and computed tomography was used to identify the perforator of the gluteal region prior to surgery.
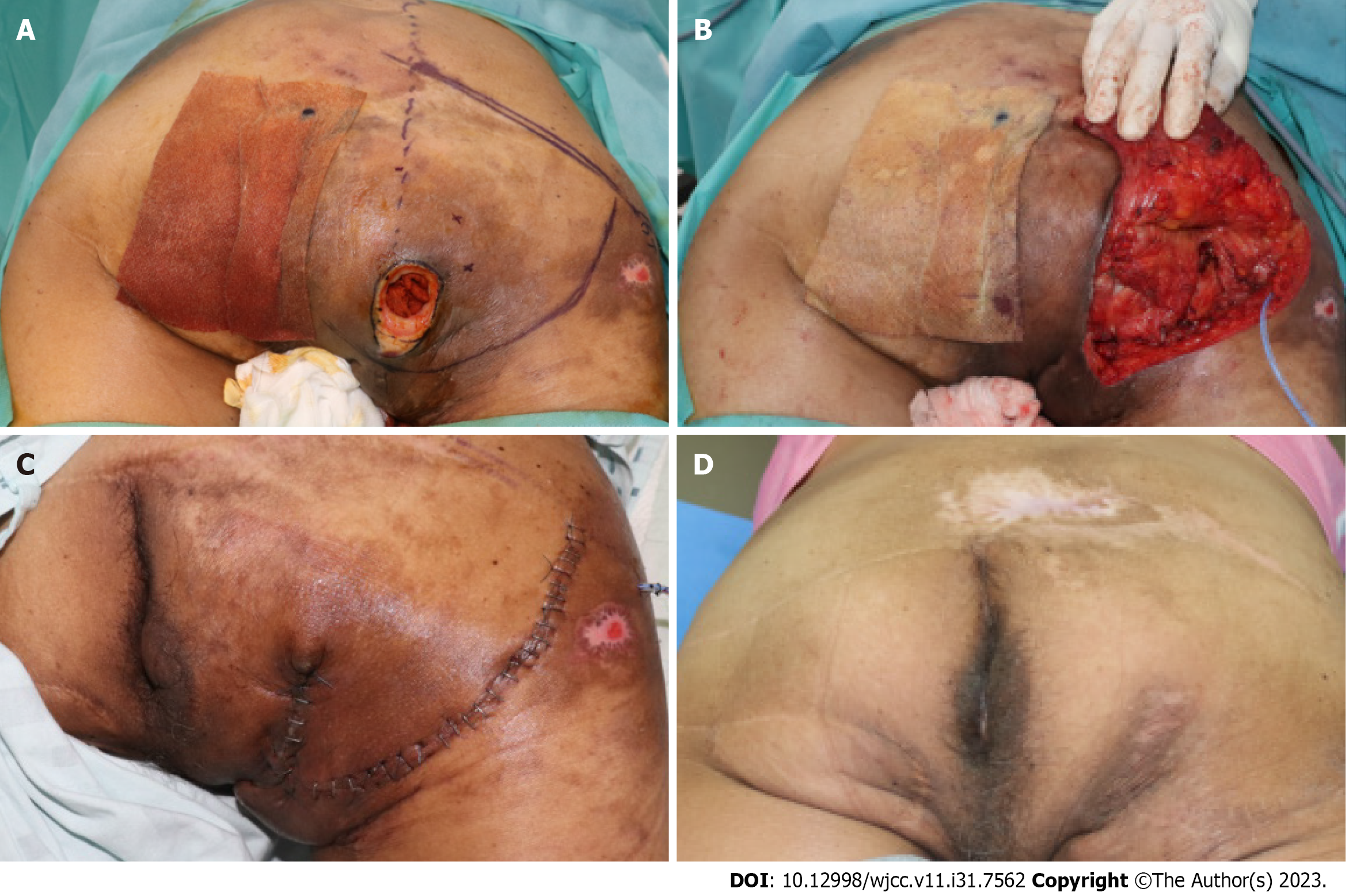
All reconstruction procedures were performed under general anesthesia, with each patient being in the prone position. The ulcer and bursa were stained with gentian violet ink and completely removed using Versajet (Smith-Nephew, Hull, United Kingdom) until the healthy soft tissue was exposed. The protruding bone was removed using a rongeur and a burr. After debridement, a perforator-based fasciocutaneous flap was designed, considering the defect size, location, and donor site closure. The fasciocutaneous flap was elevated with careful and meticulous perforator dissection and separated from the underlying gluteus maximus muscle. The gluteus maximus muscle was identified, and an incision line was made on the muscle depending on the size and location of the exposed bone defect. The gluteus maximus was dissected parallel to the muscle fiber and split from its origin or insertion. After transposing, the muscle flap was anchored to the surrounding fascia or periosteum. To prevent seroma and hematoma formation, drainage tubes were inserted under the muscle and fasciocutaneous flaps. Subsequently, a perforator-based fasciocutaneous flap was transposed, rotated, or advanced to cover the muscle flap (Figure 1).
During the postoperative period, drainage tubes were removed after 10 d, and sutures were removed at 3 wk postoperatively. The patient was maintained in the prone position for 3-4 wk, and wheelchair mobility was encouraged 4 wk after reconstruction.
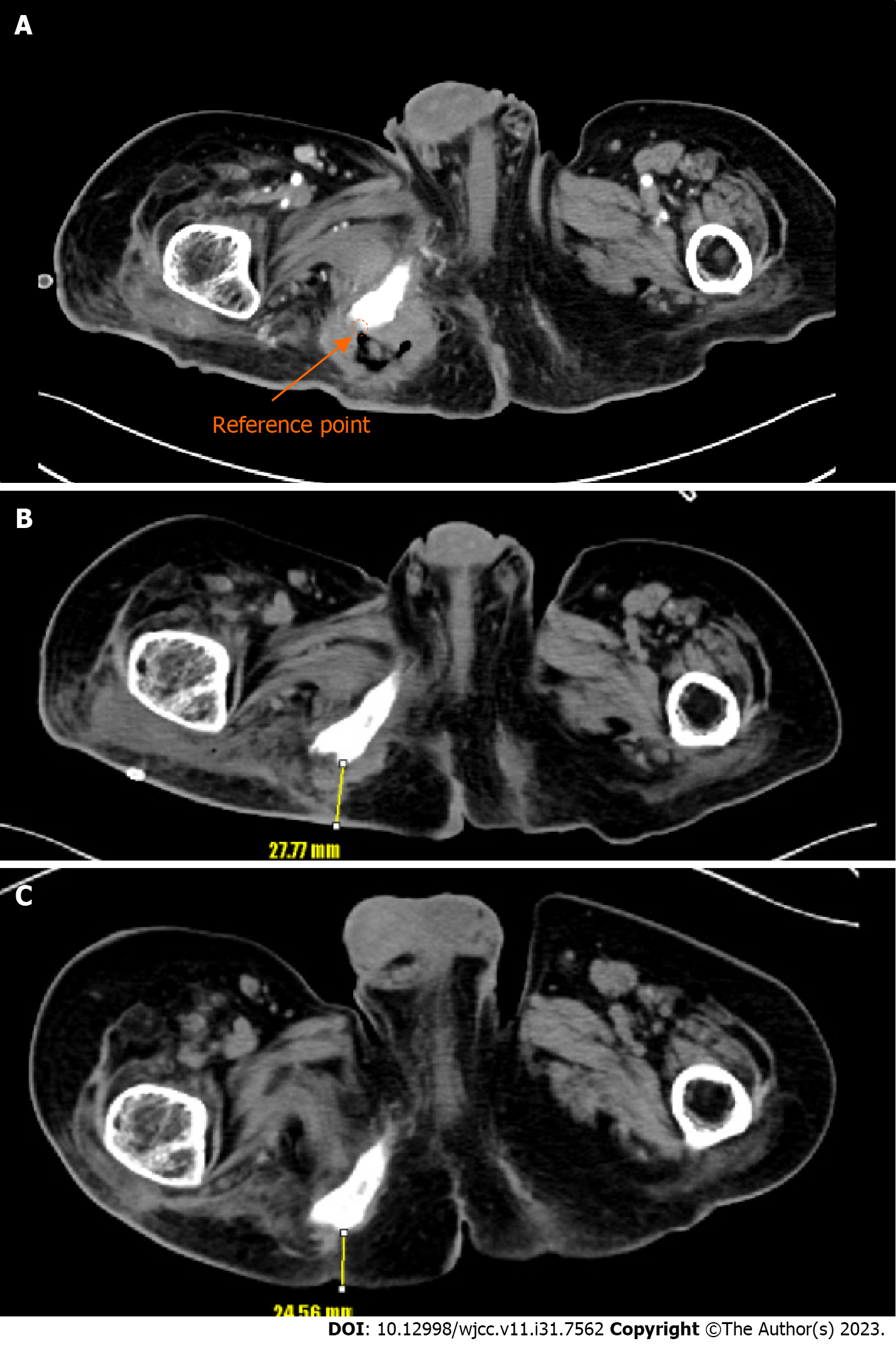
Enhanced APCT was used to measure the thickness of combined flap coverage. Preoperative computed tomography (CT) examinations were performed on the day after admission, and the first postoperative enhanced CT examination was conducted at 3 wk after reconstruction when the wound was stabilized. A second postoperative CT examination was performed in an outpatient clinic at 6 mo after reconstruction, with a slice thickness of 5 mm. On the axial image slice of the preoperative CT scan, the deepest point where the bone was exposed served as a reference point. The flap thickness, defined as the vertical distance from the reference point to the skin, was measured on an axial image slice of the postoperative CT image at the same level as the reference point (Figure 2). Flap thicknesses were compared at 3 wk and 6 mo after reconstruction using digital calipers in the picture archiving and communication system image review software. All measurements were performed by the first author using the aforementioned protocol.
Statistical analyses were performed using SPSS software version 26 (IBM SPSS Statistics for Windows, version 26; IBM Corp., Armonk, NY, United States). A paired t-test was used to analyze the difference between flap thicknesses at 3 wk and 6 mo after reconstruction.
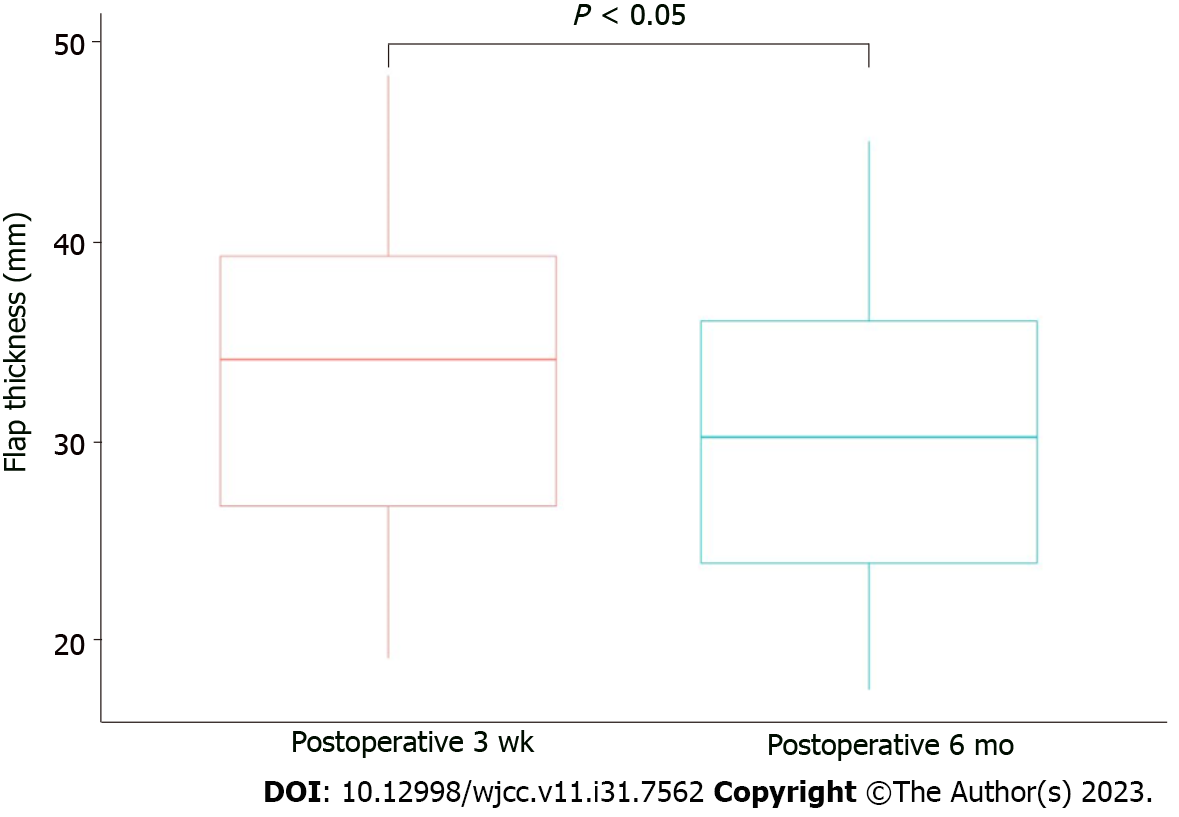
Fifteen patients (11 men and 4 women) underwent reconstruction using a gluteus maximus muscle flap and a perforator-based fasciocutaneous combined flap. The mean patients’ age was 60.6 (range, 42-72) years. The mean follow-up period was 10.5 (range, 6-18) mo. The data for each patient, including age, sex, defect location, defect size, and flap type, are presented in Table 1. The defect size of the decubitus ulcer, the size of the muscle flaps, and the size of the fasciocutaneous flap ranged from 16 cm2 to 156 cm2 (mean, 36 cm2), from 40 to 150 cm2 (mean, 59 cm2), and from 40 cm2 to 90 cm2 (mean, 59 cm2) respectively. Flap thickness measurements at 3 wk and 6 mo postoperatively using CT are shown in Table 2 and Figure 3. The mean flap thickness at 3 wk postoperatively was 32.85 ± 8.89 mm, and at 6 mo postoperatively, it was 29.27 ± 8.22 mm. A significant reduction in flap thickness was noted when comparing the postoperative CT images obtained at 3 wk and 6 mo. Notably, there were no cases of partial or total flap loss, and only two minor complications were observed. One case of wound dehiscence occurred after the stitch-out process but resolved with wound revision, and another case of seroma was resolved with aspiration at the outpatient clinic.
| Patient No. | Sex | Age | BMI (kg/ | Location | Site | Onset (mo) | Status | Past history | Defect size (cm2) | Muscle flap size (cm2) | FC flap size (cm2) | Complication | Follow-up time (mo) |
| 1 | M | 50 | 20 | Ischial sore | Lt. | 3 | Paraplegia | None | 6 × 4 | 5 × 8 | 5 × 12 | None | 8 |
| 2 | M | 42 | 25.9 | Trochanteric sore | Rt. | Unknown | Paraplegia | C1VD | 12 × 13 | 15 × 10 | 6 × 15 | Wound dehiscence | 12.5 |
| 3 | M | 48 | 27.6 | Ischial sore | Rt. | 1 | Paraplegia | DM | 4 × 4 | 12 × 8 | 8 × 10 | Seroma | 15 |
| 4 | M | 52 | 18.8 | Ischial sore | Rt. | 2 | Paraplegia | None | 4 × 5 | 12 × 5 | 4 × 12 | None | 9 |
| 5 | M | 58 | 22.5 | Ischial sore | Rt. | 84 | Paraplegia | None | 7 × 3 | 10 × 8 | 5 × 12 | None | 10 |
| 6 | M | 64 | 19.5 | Ischial sore | Lt. | 1 | Paraplegia | None | 6 × 8 | 5 × 6 | 4 × 10 | None | 8 |
| 7 | M | 72 | 23.8 | Ischial sore | Lt. | 2 | Paraplegia | HTN, DM | 5 × 6 | 6 × 8 | 5 × 10 | None | 9 |
| 8 | M | 68 | 21.2 | Trochanteric sore | Lt. | 12 | Paraplegia | HTNDM | 4 × 5 | 6 × 7 | 5 × 12 | None | 18 |
| 9 | F | 65 | 23.6 | Ischial sore | Rt. | 1 | Paraplegia | HTN | 3 × 5 | 8 × 10 | 6 × 10 | None | 10 |
| 10 | F | 70 | 27.3 | Ischial sore | Rt. | 24 | Quadriplegia | None | 6 × 7 | 6 × 8 | 5 × 13 | None | 8 |
| 11 | M | 63 | 22.4 | Ischial sore | Lt. | 3 | Paraplegia | HTNDM | 7 × 8 | 5 × 8 | 5 × 12 | None | 10 |
| 12 | F | 71 | 25.7 | Ischial sore | Lt. | 2 | Paraplegia | HTNDM | 5 × 5 | 6 × 8 | 4 × 10 | None | 12 |
| 13 | M | 60 | 27.0 | Trochanteric sore | Rt. | 3 | Quadriplegia | None | 4 × 4 | 5 × 8 | 5 × 10 | None | 9 |
| 14 | M | 62 | 23.0 | Ischial sore | Rt. | 2 | Paraplegia | DM | 4 × 5 | 7 × 10 | 5 × 13 | None | 10 |
| 15 | M | 64 | 25.5 | Ischial sore | Lt. | 1 | Quadriplegia | HTNDM | 5 × 6 | 6 × 8 | 5 × 12 | None | 9 |
| Patient No. | 3 wk follow-up postoperative flap thickness (mm) | 6 mo follow-up postoperative flap thickness (mm, %) | Difference (mm, %) |
| 1 | 34.05 | 31.01 (91.7) | 3.04 (-8.3) |
| 2 | 35.37 | 30.17 (85.3) | 5.20 (-14.7) |
| 3 | 48.37 | 45.08 (93.2) | 3.29 (-6.8) |
| 4 | 19.02 | 17.58 (92.4) | 1.44 (-7.6) |
| 5 | 41.98 | 37.40 (89.1) | 4.58 (-10.9) |
| 6 | 27.77 | 24.56 (88.4) | 3.21 (-11.6) |
| 7 | 37.29 | 35.58 (95.4) | 1.71 (-4.6) |
| 8 | 24.03 | 20.06 (83.4) | 3.97 (-16.6) |
| 9 | 28.95 | 25.53 (88.1) | 3.42 (-11.9) |
| 10 | 40.05 | 36.43 (90.9) | 4.62 (-9.1) |
| 11 | 43.72 | 37.33 (85.3) | 6.39 (-14.7) |
| 12 | 38.55 | 33.01 (85.6) | 5.54 (-14.4) |
| 13 | 19.87 | 17.46 (87.8) | 2.41 (-12.2) |
| 14 | 25.64 | 23.12 (90.1) | 2.52 (-9.9) |
| 15 | 28.13 | 24.77 (88.0) | 3.36 (-12.0) |
| Average | 32.85 ± 8.89 | 29.27 ± 8.22 (88.98) | 3.58 (-11.02) |
Successful reconstruction of decubitus ulcers depends on various factors, including the patient's nutritional status, medical history, and postoperative care[7]. Among these factors, selection of an appropriate flap type is crucial for procedure success[1]. Recently, fasciocutaneous and perforator flaps have gained popularity for decubitus ulcer reconstruction because of their advantages in preserving muscle function and minimizing donor-site morbidity. However, their relatively thin nature may not be sufficient for obliterating large dead spaces, making muscle flaps an ideal option for such cases[8-11]. The gluteus maximus is the preferred reconstructive option for decubitus ulcers because it provides adequate padding to cover the exposed bone[12]. It can provide sufficient muscle bulking for a large dead space and can also be re-advanced or re-rotated if the decubitus ulcer recurs[13].
In this study, we addressed the challenge of a small opening relative to a large inner pocket, resulting in a discrepancy between the sizes of the skin and muscle components that require reconstruction. To overcome this issue, we employed a combined flap approach to separately cover defects of different sizes in the skin and muscle components while harnessing the advantages of both fasciocutaneous and muscle flaps. The inner gluteus muscle flap was manipulated to provide the desired shape and volume to effectively obliterate dead space. The outer fasciocutaneous flap was rotated or transposed to cover the muscle flap and open the decubitus ulcer. This combination of fasciocutaneous and gluteus maximus muscle flaps enabled us to minimize the wide dead space and provide a double-layer thickness.
Several studies have explored the treatment of ischial ulcers using a combination of fasciocutaneous and gluteus maximus muscle flaps. For example, Ku et al[4] treated ischial ulcers by combining the gluteus maximus muscle flap with an inferior gluteal artery perforator fasciocutaneous flap, reporting a lower recurrence rate (8%-64%) compared to ischial ulcers treated with other muscle flaps or fasciocutaneous flaps. Similarly, Borgognone et al[14] treated ischial ulcers using a combination of the gluteus maximus muscle flap and a rhomboid-shaped fasciocutaneous flap, highlighting the advantage of preserving the tissue from hypoxic damage by independently supplying blood to each flap. However, previous reports have assessed the utility of the combined flap primarily based on recurrence during the postreconstruction follow-up period and have not provided an adequate evaluation of flap thickness. In contrast, our study measured flap thickness using CT.
In our cohort, we observed no major complications after combined flap coverage, and patients underwent successful reconstruction without recurrence during the follow-up period. To ascertain whether the flap thickness was maintained, we compared CT examinations conducted at 3 wk postoperatively with those conducted 6 mo postoperatively. On average, 88.98% of flap thickness was retained after 6 mo, with a slight decrease observed in the statistical analysis.
Our study had some limitations. First, the sample size was relatively small. Second, flap thickness was indirectly assessed using CT rather than intraoperative measurements, which may have introduced measurement errors. Finally, the 6-month follow-up period may be insufficient considering the typical recurrence period of decubitus ulcers, which is approximately 1 year[15]. Larger studies with longer follow-up periods are warranted to further explore the effectiveness of combined flaps.
Therefore, it is essential to address the reasons for changes in flap thickness in our cohort. First, over time, the subsiding soft tissue swelling may have contributed to the reduction in flap thickness. Second, the supine position during the APCT scan may have affected the flap thickness. Third, atrophic changes owing to interruption of the reflex arc or ischemia cannot be ruled out. Nevertheless, we did not observe any clinical findings, such as concavity of the flap surface.
In conclusion, our study demonstrated the successful reconstruction of grade IV decubitus ulcers in patients with paraplegia using a combination of fasciocutaneous and gluteus maximus muscle flaps. We used enhanced APCT to measure flap thickness and observed a slight but statistically significant reduction. Importantly, the patient achieved successful reconstruction without major complications. Therefore, the combined flap method is a valuable treatment option for decubitus ulcers in patients with paraplegia.
Decubitus ulcers, especially grades III and IV, pose significant challenges to reconstruction owing to their high recurrence rates. Surgical options including muscle and fasciocutaneous flaps have been explored; however, there is no universally accepted gold standard.
This study aimed to assess the thickness of combined fasciocutaneous and gluteus maximus muscle flaps used in patients with paraplegia for grade IV decubitus ulcer reconstruction to address the need for effective treatments with minimal complications.
The primary objective of this study was to measure flap thickness using radiological evaluation in patients with paraplegia who underwent combined flap reconstruction for severe decubitus ulcers.
This retrospective clinical study included patients with paraplegia who underwent combined flap coverage for grade IV decubitus ulcers. Flap thickness was measured using enhanced abdominopelvic computed tomography at 3 wk and 6 mo postoperatively.
This study demonstrated a successful reconstruction without major complications. Flap thickness decreased slightly but significantly between 3 wk and 6 mo postoperatively, with an average retention of 88.98%.
Combined fasciocutaneous and gluteus maximus muscle flaps offer an effective option for grade IV decubitus ulcer reconstruction in patients with paraplegia with the potential to maintain flap thickness over time.
Larger studies with longer follow-up periods are needed to further assess the effectiveness of combined flaps. Additionally, exploring the factors influencing flap thickness changes can enhance our understanding of the long-term outcomes of decubitus ulcer reconstruction.
| 1. | Foster RD, Anthony JP, Mathes SJ, Hoffman WY, Young D, Eshima I. Flap selection as a determinant of success in pressure sore coverage. Arch Surg. 1997;132:868-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Coşkunfirat OK, Ozgentaş HE. Gluteal perforator flaps for coverage of pressure sores at various locations. Plast Reconstr Surg. 2004;113:2012-7; discussion 2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Higgins JP, Orlando GS, Blondeel PN. Ischial pressure sore reconstruction using an inferior gluteal artery perforator (IGAP) flap. Br J Plast Surg. 2002;55:83-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Ku I, Lee GK, Yoon S, Jeong E. A dual padding method for ischial pressure sore reconstruction with an inferior gluteal artery perforator fasciocutaneous flap and a split inferior gluteus maximus muscle flap. Arch Plast Surg. 2019;46:455-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Ramirez OM, Swartz WM, Futrell JW. The gluteus maximus muscle: experimental and clinical considerations relevant to reconstruction in ambulatory patients. Br J Plast Surg. 1987;40:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Han HH, Choi EJ, Moon SH, Lee YJ, Oh DY. Combined V-Y Fasciocutaneous Advancement and Gluteus Maximus Muscle Rotational Flaps for Treating Sacral Sores. Biomed Res Int. 2016;2016:8714713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Keys KA, Daniali LN, Warner KJ, Mathes DW. Multivariate predictors of failure after flap coverage of pressure ulcers. Plast Reconstr Surg. 2010;125:1725-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Demirseren ME, Ceran C, Aksam B, Demiralp CO. Clinical Experience With the Combination of a Biceps Femoris Muscle Turnover Flap and a Posterior Thigh Fasciocutaneous Hatchet Flap for the Reconstruction of Ischial Pressure Ulcers. Ann Plast Surg. 2016;77:93-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Burm JS, Hwang J, Lee YK. A New Option for the Reconstruction of Primary or Recurrent Ischial Pressure Sores: Hamstring-Adductor Magnus Muscle Advancement Flap and Direct Closure. Ann Plast Surg. 2018;80:400-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Lee SS, Huang SH, Chen MC, Chang KP, Lai CS, Lin SD. Management of recurrent ischial pressure sore with gracilis muscle flap and V-Y profunda femoris artery perforator-based flap. J Plast Reconstr Aesthet Surg. 2009;62:1339-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | McGregor JC, Buchan AC. The tensor fasciae latae flap and its use in the closure of trochanteric and ischial pressure sores. Paraplegia. 1980;18:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Hwang K, Nam YS, Han SH, Hwang SW. The intramuscular course of the inferior gluteal nerve in the gluteus maximus muscle and augmentation gluteoplasty. Ann Plast Surg. 2009;63:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Bialowas C, Nguyen B, Patel A. Best Solutions for Perineal and Pressure Sore Reconstruction. Plast Reconstr Surg. 2021;148:1026e-1039e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Borgognone A, Anniboletti T, De Vita F, Schirosi M, Palombo P. Ischiatic pressure sores: our experience in coupling a split-muscle flap and a fasciocutaneous flap in a 'criss-cross' way. Spinal Cord. 2010;48:770-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Bamba R, Madden JJ, Hoffman AN, Kim JS, Thayer WP, Nanney LB, Spear ME. Flap Reconstruction for Pressure Ulcers: An Outcomes Analysis. Plast Reconstr Surg Glob Open. 2017;5:e1187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Frittoli B S-Editor: Qu XL L-Editor: A P-Editor: Qu XL