Published online Nov 6, 2023. doi: 10.12998/wjcc.v11.i31.7508
Peer-review started: August 25, 2023
First decision: September 19, 2023
Revised: September 19, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: November 6, 2023
Processing time: 73 Days and 0.7 Hours
Type 2 diabetes mellitus (T2DM), which is distinguished by increased glucose levels in the bloodstream, is a metabolic disease with a rapidly increasing incidence worldwide. Nevertheless, the etiology and characteristics of the mechanism of T2DM remain unclear. Recently, abundant evidence has indicated that the intestinal microbiota is crucially involved in the initiation and progression of T2DM. The gut microbiome, the largest microecosystem, engages in material and energy metabolism in the human body. In this review, we concentrated on the correlation between the gut flora and T2DM. Meanwhile, we summarized the pathogenesis involving the intestinal flora in T2DM, as well as therapeutic approaches aimed at modulating the gut microbiota for the management of T2DM. Through the analysis presented here, we draw attention to further exploration of these research directions.
Core Tip: In this review, we summarized the pathogenesis that intestinal flora is involved in type 2 diabetes mellitus (T2DM), as well as therapeutic approaches aimed at modulating the gut microbial for the management of T2DM.
- Citation: Li SX, Guo Y. Gut microbiome: New perspectives for type 2 diabetes prevention and treatment. World J Clin Cases 2023; 11(31): 7508-7520
- URL: https://www.wjgnet.com/2307-8960/full/v11/i31/7508.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i31.7508
Diabetes, a metabolic disease with a rapidly increasing incidence, is characterized by deficiencies in insulin secretion, diminished sensitivity of target organs to insulin and metabolic disorders. According to the statistics supplied by the International Diabetes Federation, 463 million individuals suffer from this condition worldwide, and a conservative estimate of the global population of adult patients with diabetes indicates that it may reach 700 million by 2045[1]. Diabetes is broadly divided into three major types: Gestational diabetes, type 1 diabetes, and type 2 diabetes mellitus (T2DM), which represent over 90% of all diagnosed cases[1,2]. Therefore, the acquisition of knowledge of the unclear etiology and effective treatment strategies is crucial. Extensive discussions have revealed that the primary contributing factors include genetics, excessive caloric intake, and a lack of effective exercise[3]. Meanwhile, developing T2DM is closely related to obesity, which is recognized as the principal pathogenetic factor[4]. Furthermore, mounting evidence has proven that T2DM is linked to inadequate insulin production or insulin resistance (IR)[5].
Recently, increasing evidence has indicated that the intestinal microbiota is the essential element for maintaining health and the main pathogenic factor for various diseases that significantly influences the normal development of physiological systems[6,7]. Certainly, intensive discussions have identified the intestinal flora and the factors contributing to and mechanisms of T2DM as key factors in the advancement of metabolic diseases and the progression of T2DM[8]. According to many studies, T2DM patients have compositional differences in the characteristics of the intestinal microbiota compared to healthy people. Hence, for the purpose of preventing and improving T2DM and complicating diseases, as well as to provide literature references for the participation of the intestinal flora in the treatment of T2DM, this review summarizes the relevance and focuses on the systems in which the gut flora are engaged in the pathogenesis of this condition. These findings have allowed us to identify the significance of the intestinal flora as a candidate target for managing T2DM and related metabolic diseases.
The intestinal flora in the digestive system is currently considered an emerging ‘organ’ with immunologic, endocrinological, and energy-metabolic-like functions[9], and it consists of a vast quantity of bacteria, fungi and other parasitic organisms. The mature microbial ecosystem in the gut is originally formed during the introduction of solid food, which generally materializes between the ages of one and three years[10]. Once established, the overall structure and function of the intestinal flora remain relatively unchanged until old age. The adult intestine is a place of residence for a wide variety of bacterial species, has a range of approximately 500-1000 of bacterial classes, a large quantity of approximately 1012-1014, and a total scale of approximately 1-2 kg. Additionally, researchers have estimated that the human body comprises close to 30 to 40 trillion cells. In contrast, research shows that the number of bacterial cells in the intestinal microbiome exceeds 10 times the total number of cells composing the human body[7]. Meanwhile, compared to the human genomic sequence, the human microbiome encompasses a significantly higher number of genes: more than 150 times that of the human genome[9]. These numbers indicate the abundance and complexity of the intestinal flora. According to research, the majority of the normal gut microbiota is composed of anaerobic bacteria. These bacterial species are well adapted to flourish in the oxygen-depleted environment of the gut tract. The majority of the total intestinal microbiota, accounting for nearly 98% of the microbial population, is subjugated by the following four key bacterial phyla: Proteobacteria, Firmicutes, Actinobacteria and Bacteroidetes[11]. Within these four phyla, Firmicutes constitutes the highest proportion, reaching up to 64%, and mainly includes Lactobacillus, Enterococcus, and Clostridium, which are involved in numerous metabolic processes. Proteobacteria comprises Escherichia, Salmonella, and Helicobacter. Bifidobacterium, Corynebacterium, and Mycobacterium are considered the core constituents of Actinobacteria. Bacteroidetes includes bacterial genera such as Bacteroides, Prevotella, and Parabacteroides. Certain studies indicate that Verrucomicrobia might be involved in the augmentation of glucose regulation and insulin responsiveness.
The gut microbiome, which is broadly identified as a critical factor for human health, is intimately correlated with various aspects of human physiological function, the immune response and metabolic nutrition. These aspects encompass but are not exclusively constrained by the following functions: the metabolism of nondigestible dietary residues, which subsequently supply energy for gut motility, likewise organisms through short-chain fatty acids; adjustments of epithelial cellular growth and differentiation[9], as these cells form an intestinal barrier to safeguard against pathogens and harmful substances[6]; involvement in various metabolic processes, such as fermentation of carbohydrates and dietary components, and vitamin synthesis; and modulation of the immune system and inflammatory responses[12,13]. Microbial flora have the potential to impact the absorption and transportation of saccharides across intestinal epithelial cells. Certain microorganisms can modulate the expression of glucose transporters on these cells, thus influencing the uptake and utilization of glucose[14,15]. Akkermansia muciniphila effectively reverses the metabolic disturbances induced by a high-fat diet, which include an increase in fat accumulation, metabolic endotoxemia, inflammation in adipose tissue, and IR[16].
Recently, the development of 16S rRNA sequencing has transformed scholars' comprehension of taxonomic studies of the gut microbiome. The initial exploration of the intestinal microbiota in individuals with T2DM was documented in 2010. T2DM is linked to alterations in the gut flora[17].
Emerging evidence suggests that the formation and structure of intestinal microbes have the potential to modulate the occurrence of diabetes, particularly T2DM[18]. Furthermore, a study analyzing bacterial gene functions reported a discrepancy in the gut flora composition at the molecular level among people with diabetes and those without the illness[19]. Many studies have noted the interconnection between T2DM and fluctuations in the microbial composition of intestinal microorganisms, predominantly at the class and phylum levels. Compared with the normal population, an interesting finding is that one of the main characteristics of the microbiota during the initial and progressing periods of T2DM is that the proportional representation of Bacteroides and β-proteus increases significantly with dietary alterations[20], and the remarkably higher levels of Bacilli, including the Lactobacillus group[17], specifically in the advanced stages. Furthermore, the levels of Clostridium, Bifidobacteria, and Firmicutes in the gut microbiota of T2DM patients exhibit meaningful decreases[17,18,21], and the number of Verrucomicrobia decreases significantly[15]. However, importantly, one of the main alterations related to T2DM includes a notable decrease in the frequency of specific butyrate-synthesizing bacteria, such as the species Faecalibacterium prausnitzii and Roseburia intestinalis[22]. According to the latest reports, the enrichment of the Betaproteobacteria family[17] and Proteobacteria[23] is associated with low-grade inflammation in individuals with T2DM. Alterations in the levels and variety of bacterial groups, along with other modifications in the gut flora, may cause metabolic dysregulation and IR through various mechanisms, thus generating T2DM.
The principal determinant of the risk for T2DM is obesity, which is particularly characterized by excess visceral adipose accumulation[24]. Fat accumulation could cause increased IR and decreased insulin secretion, leading to decreased glucose absorption by cells and higher blood glucose levels, which eventually trigger the occurrence of T2DM. Recently, the decrease in the diversity and proportional distribution of microbes have also been implicated in obesity[25,26]. Numerous mechanisms have been postulated to elucidate how the intestinal colony contributes to obesity and visceral adiposity[10,27], including assimilating energy from dietary consumption, regulating metabolism and triggering low-grade inflammation. In obese individuals, several factors, such as increased intestinal permeability and dysfunction of the gut barrier and immune system characterized by a chronic low-grade immune response, might further facilitate the advancement of IR and T2DM[28,29] (Table 1).
| Classification | Species of bacteria | Content |
| Fimicutes | Eubacterium rectale, Lactobacillus gasseri, Roseburia spp., Faecalibacterium | Down |
| Streptococcus mutans, Clostridium ramosum | Up | |
| Bacteroidales | Bacteroides spp., Parabacteroides | Up |
| Verrucomicrobia | Akkermansia muciniphila | Down |
| Proteobacteria | Escherichia coli | Up |
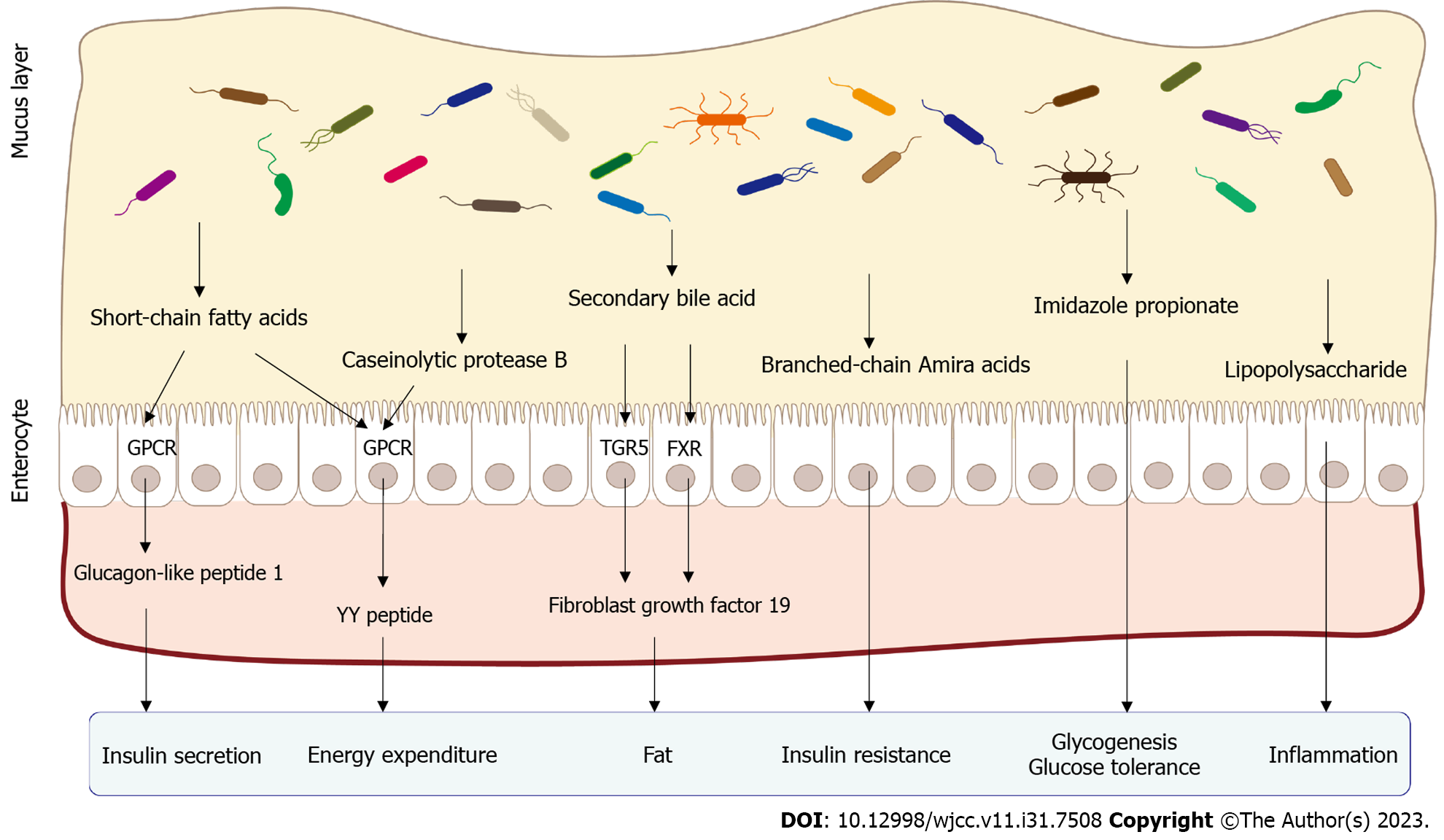
Bile acid plays a crucial role as an integral component of bile, which is manufactured by the liver and stockpiled within the gallbladder, together with the cholesterol end metabolite. Bile acids enter the gastrointestinal tract. Initially, bile acids, a component of bile, are exuded into the intestine. The sophisticated processes of primary bile acid metabolism are predominantly regulated by the gut microbiota and then enzymatically transformed into secondary bile acids[30,31]. Secondary bile acids may affect amylaceum metabolism and insulin susceptibility through diverse signaling pathways[32]. Some studies have suggested that certain secondary bile acids activate a continuum of specific nuclear receptors, namely, farnesoid X receptor (FXR) and Takeda G protein-coupled bile acid receptor 1 (GPBAR1)[33], which participate in the regulation of glucose and lipid metabolism. FXR activation by secondary bile acids has been linked to increased glycemic tolerance and insulin susceptibility. By activating FXR, hepatic glucose production and insulin signaling are potentially affected in peripheral tissues, ultimately leading to an improvement in glycemic control. Fibroblast growth factor 19/15 (FGF19/15) is secreted by intestinal cells and is induced by FXR activation. FGF19/15 has been proven to optimize insulin sensitivity by facilitating dextrose uptake into cells, stimulating glycogen synthesis in the liver, and diminishing hepatic glucose production[33,34]. Secondary bile acids also activate Takeda G protein-coupled receptor 5 (TGR5). TGR5 was initially discovered in 2002[35] and subsequently characterized in 2003[36]. Through the establishment of TGR5 mice[37,38], researchers discovered that their overall reservoir of bile acids was diminished. In skeletal muscle, TGR-5 activation has been validated to increase the utilization of glucose and aliphatic acids, thereby promoting energy consumption. In intestinal L cells, TGR-5 activation stimulates the production of glucagon-like peptide 1 (GLP-1), an incretin hormone responsible for regulating glucose homeostasis. GLP-1 shows promise for therapeutic applications due to its ability to stimulate insulin secretion from pancreatic beta cells and inhibit glucagon release. Moreover, it has shown promising potential in reversing IR and correcting irregular glucose metabolism[33,39]. Both FXR and TGR5 are expressed in pancreatic β-cells and serve a purpose in accelerating glucagon synthesis and insulin discharge in reaction to glucose. Therefore, the proper functioning of the intestinal flora is vital for the synthesis, alteration, and transmission of bile acids. Recent research has indicated that an imbalance in the intestinal flora interferes with the ability of bile acids to regulate glucose metabolism[40,41], which further disturbs carbohydrate metabolism and results in the onset of T2DM.
Short-chain fatty acids (SCFAs), one of the metabolites that has been researched extensively, are synthesized by specific bacteria in the intestine through fermentation using a variety of substrates. Several common bacteria, such as Bacteroides, Streptococcus, Clostridium, Eubacterium, and Bifidobacterium, breakdown a variety of dietary fibers and cumbersome carbohydrates through zymolysis, eventually resulting in the generation of SCFAs. SCFAs belong to the group of organic carboxylic acids, including monoprop, lactic acid, acetic acid, isobutyric acid, isovaleric acid, isohexanoic acid, and butyrate, among others.
Gut hormones: As ligands for FFAR2 and FFAR3, the receptors that bind to free fatty acids, SCFAs can transmit signals related to the energy status and metabolic processes, including the regulation of energy balance and metabolism. Free fatty acids (FFAs) are always produced by colonic enteroendocrine L cells. In addition to their roles in inhibiting glucagon secretion and glucose-dependent insulin secretion, FFAs have also been validated to stimulate the production of specific hormones in the intestinal tract, such as GLP-1 and peptide YY (PYY)[42,43]. GLP-1 functions as an insulinotropic hormone to stimulate insulin secretion while also serving as an anorectic hormone to promote sensations of satiety. Through the utilization of C57BL6 mice and free fatty acid receptor 2 knockout mice, researchers have observed that propionate promotes the liberation of GLP-1 and PYY, while the absence of FFA2 negatively impacts the secretion of intestinal hormones induced by short-chain fatty acids[42]. Mice deficient in FFAR2 and FFAR3 exhibit diminished secretion of GLP-1 in response to SCFAs both in vitro and in vivo, resulting in impaired glucose tolerance[44]. Colonic infusions of SCFA mixtures (including acetate, propionate, and butyrate) lead to elevated energy expenditure and PYY levels while reducing lipolysis[45].
Energy supply: SCFAs, particularly ethanoate and propionate, can enter the bloodstream from the colon and are subsequently transported to the liver. The metabolic pathway of hepatic gluconeogenesis is directly stimulated by SCFAs, a process that entails the synthesis of glucose from noncarbohydrate sources. It contributes approximately 30% of the energy required for liver metabolism[46]. Furthermore, studies have estimated that the zymolysis of SCFAs by microbes in the colon supplies a substantial amount of the energy required for the metabolism of colonocytes, which comprise approximately one-tenth of the entire energy expenditure in the normal state[47]. Butyrate, a type of SCFA, has shown potential in improving insulin sensitivity in response to the diet, glucose metabolism and the burning of calories, potentially through several mechanisms that are still being investigated[48]. SCFAs exert various effects on fat accumulation within the human body[49,50]. In particular, SCFAs inhibit fatty acid production by suppressing the activity of key enzymes involved in fatty acid synthesis and increasing thermogenesis, which results in the burning of more calories and potentially leads to a reduction in fat accumulation.
Intestinal glucose metabolism: Butyrate and propionate, two of the major SCFAs, exert considerable effects on the process of intestinal gluconeogenesis, which involves glucose production, thereby promoting improvements in body weight and glycemic regulation. Studies have indicated that butyrate increases glucose synthesis in the intestine by promoting the activation of genes related to intestinal gluconeogenesis. Additionally, propionate and butyrate have been identified as crucial catalysts required for the activation of intestinal gluconeogenesis, which plays a major role in fostering metabolic wellness[51].
Moreover, SCFAs potentially stimulate β-cells to deliver insulin and activate G-protein coupled receptors, thereby aiding in increasing glucose metabolism or insulin sensitivity by engaging diverse pathways[44].
Intestinal barrier: SCFAs contribute to the maintenance of a healthy gut environment through multiple mechanisms, including enhancing the acidic pH in the colon to restrain the proliferation of harmful bacteria. An additional mechanism is the ability of SCFAs to prevent gastrointestinal dysfunction and regulate fluid and electrolyte equilibrium. Multiple research findings have presented supporting evidence for the beneficial effects of SCFAs on inflammatory responses, including their anti-inflammatory ability to suppress the production of proinflammatory factors[47,52]. The development of T2DM is strongly influenced by the presence of chronic inflammation[53,54]. Impaired intestinal barrier function may cause persistent inflammation, triggering the development of IR in T2DM patients[55,56]. Given these implications, the utilization of SCFAs and their associated molecular targets represents a potential avenue for therapeutic intervention in the management and treatment of these disorders.
Some bacteria in the gut microbiota produce endotoxins, particularly lipopolysaccharide (LPS), which are constituents located in the outer membrane that are present in gram-negative bacteria. In individuals diagnosed with T2DM, endotoxins penetrate more easily due to the impaired intestinal barrier function, such as the elevated permeability of the intestinal barrier, culminating in an increased concentration of LPS in the bloodstream. After the endotoxin enters the bloodstream, it engages with the immune system, inciting the onset of inflammation. Endotoxins bind to Toll-like receptors (TLRs) and other receptors, activating immune cells to produce inflammatory mediators. Meanwhile, endotoxins may cause disorders in the host immune system. Increased systemic levels of LPS may be able to induce a chronic low-grade inflammatory reaction, ultimately contributing to impaired glucose metabolism and insulin signaling in individuals with T2DM[57,58]. In addition, the disruption of the intestinal flora may increase the abundance of gram-negative bacteria, which in turn increases LPS production and release. LPS in the circulation forms a complex with CD14 and is subsequently identified by TLR-4. Afterward, the activation of mitogen-activated protein kinase and TLR signaling pathways triggers a cascade of nonspecific inflammatory responses that ultimately result in disturbances in insulin secretion and insulin transport[59]. Simultaneously, disturbances in the intestinal flora contribute to the impairment of gut epithelial barrier function, resulting in metabolic endotoxin-related pathogenesis. The presence of mild inflammation caused by endotoxemia implies that intestinal microbiota-derived metabolites may exert effects on initiating metabolic disturbances and the development of T2DM. Interestingly, previous research has shown that the concentration of endotoxins in individuals may serve as a prognostic biomarker for identifying humans with a higher risk of T2DM[60] (Figure 1).
The modulation of the intestinal microbiota has become a substantial focus as a promising area of research for preventing and treating T2DM, as numerous studies published in recent years have recognized the notable role of gut flora in the physiopathology of T2DM.
Balanced dietary choices, particularly the types of foods and nutrient contents, represent appropriate changes in shaping the health of the intestinal microbiome and metabolites derived from gut microorganisms[61], and they might prevent and lower the predisposition to obesity and T2DM[62] and promote glycemic control[63]. Over the last several decades, scientific evidence has highlighted the significant effects of dietary factors on both the prevention and healing of T2DM[64]. Altering the structure and composition of diet is regarded as a basic and essential adjunct approach for treating diabetes effectively.
Dietary fiber has received considerable attention among the various dietary factors due to its significant effect on improving glycemic control in T2DM patients[65,66]. Consuming a diet rich in dietary fiber, including whole grains and nuts, perhaps promotes positive effects on glucose regulation and insulin sensitivity, thereby decreasing the probability of developing T2DM[67,68]. By increasing the intake of fiber-rich foods, insulin susceptibility may be increased by promoting dietary fiber fermentation by the gut microbiome and the creation of prominent end products, such as short-chain fatty acids, butanoate, and propanoic acid ester[69].
Making dietary choices, such as replacing saturated and trans fats with healthier fats, selecting carbohydrates with a low glycemic index (GI) and consuming lean proteins, can enhance insulin sensitivity and lower the likelihood of developing T2DM. Closer adherence to the diets that are primarily based on plants, such as the DASH, Portfolio, and Mediterranean diets, is linked to a lower incidence of developing T2DM in the future[70,71].
Regarding traditional calorie-restricted diets, intermittent fasting (IF) by individuals affected by T2DM has shown potential advantages in glucose control, weight management and IR[72,73]. Increasing evidence suggests that intermittent fasting has the capacity to alter the composition and diversity of the gut microflora and modulate microbial metabolite products. This diet may be responsible for the maintenance of gut, glucose and lipid metabolic health[74].
Therefore, these changes in the intestinal microbial community resulting from dietary modifications might have a major impact on enhancing glycemic control in T2DM patients[75,76].
Probiotics are living microscopic organisms that provide health advantages to humans by synthesizing vitamins, assisting in the digestion of food, and suppressing the proliferation of hazardous bacteria[77]. The increasing popularity of probiotics as functional foods or dietary supplements is due to their perceived effects on the intestinal microbial community[78-80]. Changes in metabolic disorders related to T2DM are usually observed after the utilization of probiotics[81], which encompass increased insulin sensitivity[82,83], the amelioration of impaired glucose tolerance[84], improved intestinal integrity[85], modulation of the gut flora and a reduction in systemic levels of LPSs[86,87]. The mechanisms of T2DM that are affected by individuals using probiotics as a supplemental treatment have been suggested[88].
Based on accumulating evidence indicates, studies not only showcase the positive effects of various probiotic strains but also provide a valuable understanding of the effects of a few particular strains of probiotics on T2DM. The administration of Bifidobacterium lactis, Streptococcus thermophilus, Lactobacillus bulgaricus, and Lactobacillus acidophilus as supplements has shown promising results in enhancing glycemic control in adults diagnosed with T2DM[89]. Clinical and experimental research has revealed that Lactobacillus gasseri, Bifidobacterium bifidum, Lactobacillus casei, and Lactobacillus helveticus effectively decrease fasting blood glucose levels and HbA1c levels in individuals diagnosed with T2DM[90]. Lactobacillus casei, a probiotic strain that is considered beneficial for individuals with T2DM, has been shown to improve SIRT1 and fetuin-A levels, which are correlated with various metabolic processes[91]. Consequently, Lactobacillus casei supplementation may have the potential to effectively manage diabetes[92] and change the gut microbiota ecosystem in patients suffering from T2DM, specifically by increasing the copiousness of beneficial microflora[93]. Lactobacillus reuteri DSM 17938 enhances insulin sensitivity and increases the diversity of gut microorganisms[94]. Numerous studies have examined the effects of Lactobacillus rhamnosus GG on glycemic parameters, reporting its potential advantages in safeguarding against alterations such as decreased blood glucose levels in the fasting state, enhancing the insulin hormone sensitivity index, and improving glucose control, specifically in individuals with impaired glucose tolerance[95]. Lactobacillus casei CCFM419 shows promising potential in promoting metabolic health, exerting anti-inflammatory effects, reducing IR and improving the composition of beneficial gut microbes, possibly helping to improve the amelioration of hyperglycemia in individuals with T2DM[96]. Supplementation with Akkermansia probiotics improves insulin sensitivity and is associated with improving metabolic health indicators[97].
Prebiotics, which are indigestible food components, exert a beneficial effect by preferentially promoting the proliferation and function of a single or a restricted group of specific bacteria that already exist in the colon[98]. Inulin, one of the extensively researched prebiotics, tends to enhance glucose by provoking the production of the hormone GLP-1. It can help alleviate T2DM by suppressing inflammation and modulating the composition of the gut microbiota[99,100].
Importantly, the combination of prebiotics and probiotic substances may exert a greater positive effect on decreasing fasting glycemia levels and HbA1C levels in T2DM patients compared to the administration of prebiotics or probiotics individually[101,102]. The amalgamation of multiple beneficial probiotic strains promotes more encyclopedic and substantial synergistic effects on T2DM[103,104] because of their distinct mechanisms and complementary effects.
In the future, supplementation with beverages containing probiotics and/or prebiotics has the potential to emerge as a complementary approach in conjunction with medication and lifestyle modifications to effectively manage T2DM[105,106].
Regular physical activity, a budget-friendly lifestyle modification for the prevention and intervention of obesity and T2DM by increasing energy consumption and the metabolic rate[107], might improve glycemic homeostasis and insulin receptor sensitivity[108]. A controlling effect of exercise on the extent and nature of the intestinal microbiota has been proposed, particularly in terms of the underlying mechanisms. The microflora of individuals who exercise exhibits higher biodiversity and metabolic aptitude, and different types and intensities of exercise promote noticeable shifts in the constitution and capacity of the intestinal bacterial population[109,110] because exercise stimulates gut motility, dietary changes and the activity of immune cells. Meanwhile, according to some references, a strong correlation exists between exercise interventions that induce transformations in gut microorganisms and improvements in metabolic improvements, including glucose homeostasis and insulin sensitivity[111]. On the other hand, a better understanding of the role of the gut bacterial population in the response to physical activity is needed and may provide valuable insights into personalized approaches and therapeutic interventions for enhancing glucose metabolism and insulin sensitivity[112].
Fecal microbiota transplantation (FMT) is a technique used to restore the equilibrium of gut microbiota in clinical trials and is recognized as an effective method to rectify dysbiosis. Essentially, FMT involves transferring beneficial microbes from the healthy gut tract of donors into patients with gut microbiota dysbiosis, thereby reestablishing a harmonious microbial ecosystem in the intestinal tract to treat diseases[113].
Studies have indicated that FMT from individuals with a lean weight who were diagnosed with metabolic syndrome might result in improved insulin sensitivity, glucose levels, and the alleviation of metabolic syndrome. This positive outcome may be at least partially attributed to the increased abundance of butyrate-producing bacteria[114-116].
Similarly, research has shown that FMT from healthy Chinese individuals into mice effectively decreases fasting plasma glucose levels[117]. Similar studies have shown that reverse IR is effectively reversed by restoring the microbiota in mice with T2DM using FMT[118].
Based on the ongoing exploration of the “brain-gut-microbiota axis”, the mood of patients with diabetes, particularly those with diabetic neuropathy, may be affected by their intestinal flora. Interestingly, promising research findings have shown that patients with stable glycemic levels and well-controlled conditions who underwent fecal bacteria transplantation as a therapy for T2DM experienced positive outcomes[119]. Based on the aforementioned studies, we propose the hypothesis that fecal bacteria transplantation holds promise in ameliorating the symptoms of T2DM.
Metformin, a widely used medication for treating T2DM, was discovered in the study of Galega officinalis, a plant commonly known as Goat’s rue, in 1922. Since then, metformin has been widely used due to its ability to lower glucose levels through its interaction with the gastrointestinal system. Moreover, numerous studies have provided supporting evidence that the modulation of gut microbiota participates in the mechanism through which metformin improves glycemic regulation[120]. Notably, metformin has been found to alter the abundance and composition of various microbial taxa. Specifically, it decreases the levels of Clostridium spp. and Intestinibacter spp. while increasing the abundance of Bifidobacterium bifidum, Lactobacillus, Bilophila wadsworthia, Escherichia and Shigella spp.[121,122]. Propionate helps decrease hepatic blood dextrose levels, while butyrate improves insulin sensitivity. Metformin has been observed to regulate blood glucose levels in individuals with conditions such as T2DM due to its effects on propionate and butyrate production[123,124]. Furthermore, an expanding repertoire of research has indicated that metformin exerts its effects on endocrine cell activity through diverse pathways, encompassing the regulation of bile acid conversion, intestinal barrier integrity and permeability, reductions in endotoxin levels, and increasing the production of the hormones released by endocrine cells, such as GLP-1 and PYY peptides, in the gut[125]. Furthermore, metformin has the potential to increase the population of Akkermansia muciniphila, a type of bacteria known for its beneficial effects on insulin sensitivity and glucose regulation[126]. Akkermansia protects the intestinal barrier in individuals with T2DM, aiding in the preservation of the wholeness of the intestinal mucosa and thereby reducing inflammatory reactions.
Acarbose, the other antidiabetic medication with a connection to the microorganism community, is an α-glucosidase inhibitor used to manage the condition of individuals with T2DM. Acarbose treatment represses the enzymatic transformation of complex oligosaccharides to monosaccharides and disaccharides and lowers blood glucose levels in the small intestine by modifying the diversity and abundance of designated microbial families in patients with T2DM[127].
According to recent studies, the composition of the gut microbial community changes in diabetic rats following treatment with pioglitazone, vildagliptin, DPP-4 inhibitors and sitagliptin[128-130]. Recent studies have revealed that SGLT2 inhibitors may also exert their hypoglycemic and therapeutic effects on T2DM by influencing on the intestinal microbiota[131].
Bacteriophages, which are viruses that specifically target bacteria, are highly prevalent microorganisms in the gut. They have a prominent role in shaping the structure of the gut flora, ultimately impacting health[132]. An increased quantity of bacteriophages has been observed in the digestive tract of individuals with diabetes[133]. This finding highlights the significance of the presence of bacteriophages in individuals with diabetes.
The fecal virome transplant operation, which is the transfer of a healthy virome from the donor to the receiver, may induce positive changes in altering the gut microbiome and host metabolome in patients with T2DM[134].
The active artificial manipulation of gut microbial genes has the potential to control the structure and function of the intestinal ecosystem. Through a targeted manipulation, promoting the expression of beneficial metabolic genes may be feasible. For example, administering engineered Lactobacillus gasseri or Escherichia coli Nissle 1917, which produce GLP-1, may render intestinal cells responsive to glucose, making them sensitive to glucose and inducing insulin production when glucose levels increase[135,136], potentially improving glucose regulation in individuals with T2DM. The production of high-fat diet-fed mice transplanted with engineered Escherichia coli Nissle 1917 that generate N-acylphosphatidylethanolamines lead to a decrease in glycemia and IR.
A large number of emerging papers has increasingly recognized the involvement of intestinal flora in the onset and progression of T2DM. The gut microbial community plays a role in regulating T2DM through various mechanisms and multiple targets, including the regulation and production of bacterial metabolites and bile acid metabolism and inflammation mediated by LPS, which may contribute to the occurrence and extension of T2DM. By conditioning the intestinal flora as a therapeutic approach, scientists may not only be able to regulate IR and the blood glucose status but also to improve T2DM outcomes.
Our understanding of the complex field of the intestinal ecosystem is still in its nascent stages, and numerous questions remain unanswered and details remain unknown. First, currently, the majority of studies still primarily focus on bacteria while disregarding the contributions of nonbacterial microorganisms, such as viruses and fungi. Crucially, we should delve deeper into the complete microbial ecosystem to gain a comprehensive grasp of their implications for health and diseases such as T2DM. Second, specific related microbial taxa or metabolic derivatives that fulfill a role in the pathogenesis and escalation of T2DM must be identified to provide valuable targeted interventions. Third, the establishment of standardized reference databases is expected to analyze the comparisons of microbiome data and sustain our exploration of the involvement of intestinal microbes in metabolic disorders such as T2DM. Fourth, an imperative goal is to not only concentrate on the gut microbiome at certain time points but also maintain a continuous determination of the fluctuating variations in the gut flora and metabolic products. These alterations occur throughout various stages of diabetes and its complications, which might enable us to identify potential biomarkers for improved prevention and treatment measures. Fifth, the creation of a noninvasive diagnostic technique for early detection, such as examining biomarkers in blood and feces, is formidable and challenging. In addition, maintaining the sustained presence of exogenous microbes in the gut is another challenge. Last but not least, deeper research based on the gut microbiome to predict T2DM holds the potential to formulate novel and personalized approaches to prevent and treat IR and T2DM.
Continued investigation is essential to obtain a more comprehensive understanding of the mutual influences between the intestinal ecosystem and T2DM and to provide a crucial foundation for predicting and treating diabetes in the future. Absolutely, we believe that the treatment of T2DM based on the value of the gut flora has promise as a safer and more reliable approach, which shows promise in minimizing side effects and improving long-term effectiveness.
| 1. | Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3709] [Cited by in RCA: 4587] [Article Influence: 573.4] [Reference Citation Analysis (7)] |
| 2. | Holman N, Young B, Gadsby R. Current prevalence of Type 1 and Type 2 diabetes in adults and children in the UK. Diabet Med. 2015;32:1119-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 226] [Article Influence: 20.5] [Reference Citation Analysis (1)] |
| 3. | Lyssenko V, Jonsson A, Almgren P, Pulizzi N, Isomaa B, Tuomi T, Berglund G, Altshuler D, Nilsson P, Groop L. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med. 2008;359:2220-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 628] [Article Influence: 34.9] [Reference Citation Analysis (1)] |
| 4. | Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2756] [Cited by in RCA: 2906] [Article Influence: 132.1] [Reference Citation Analysis (1)] |
| 5. | Asemi Z, Zare Z, Shakeri H, Sabihi SS, Esmaillzadeh A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab. 2013;63:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 285] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 6. | Bull MJ, Plummer NT. Part 1: The Human Gut Microbiome in Health and Disease. Integr Med (Encinitas). 2014;13:17-22. [PubMed] |
| 7. | Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat Med. 2018;24:392-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 991] [Cited by in RCA: 1683] [Article Influence: 240.4] [Reference Citation Analysis (0)] |
| 8. | Weinstein N, Garten B, Vainer J, Minaya D, Czaja K. Managing the Microbiome: How the Gut Influences Development and Disease. Nutrients. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Jian M, Zhou Y, Li Y, Zhang X, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J; MetaHIT Consortium, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9101] [Cited by in RCA: 8114] [Article Influence: 507.1] [Reference Citation Analysis (4)] |
| 10. | Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2779] [Cited by in RCA: 3175] [Article Influence: 226.8] [Reference Citation Analysis (0)] |
| 11. | Gallo A, Passaro G, Gasbarrini A, Landolfi R, Montalto M. Modulation of microbiota as treatment for intestinal inflammatory disorders: An uptodate. World J Gastroenterol. 2016;22:7186-7202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Scheperjans F. Gut microbiota, 1013 new pieces in the Parkinson's disease puzzle. Curr Opin Neurol. 2016;29:773-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1783] [Cited by in RCA: 2142] [Article Influence: 133.9] [Reference Citation Analysis (0)] |
| 14. | Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, Khan MT, Zhang J, Li J, Xiao L, Al-Aama J, Zhang D, Lee YS, Kotowska D, Colding C, Tremaroli V, Yin Y, Bergman S, Xu X, Madsen L, Kristiansen K, Dahlgren J, Wang J. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe. 2015;17:852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 458] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 15. | Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529:212-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 965] [Cited by in RCA: 1232] [Article Influence: 123.2] [Reference Citation Analysis (0)] |
| 16. | Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066-9071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2639] [Cited by in RCA: 3484] [Article Influence: 268.0] [Reference Citation Analysis (1)] |
| 17. | Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, Chen Y, Ji L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS One. 2013;8:e71108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 481] [Cited by in RCA: 655] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 18. | Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3971] [Cited by in RCA: 5016] [Article Influence: 358.3] [Reference Citation Analysis (1)] |
| 19. | Qiu J, Zhou H, Jing Y, Dong C. Association between blood microbiome and type 2 diabetes mellitus: A nested case-control study. J Clin Lab Anal. 2019;33:e22842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 20. | Sato J, Kanazawa A, Ikeda F, Yoshihara T, Goto H, Abe H, Komiya K, Kawaguchi M, Shimizu T, Ogihara T, Tamura Y, Sakurai Y, Yamamoto R, Mita T, Fujitani Y, Fukuda H, Nomoto K, Takahashi T, Asahara T, Hirose T, Nagata S, Yamashiro Y, Watada H. Gut dysbiosis and detection of "live gut bacteria" in blood of Japanese patients with type 2 diabetes. Diabetes Care. 2014;37:2343-2350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 324] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 21. | Ríos-Covián D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilán CG, Salazar N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front Microbiol. 2016;7:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1002] [Cited by in RCA: 1440] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 22. | Roager HM, Vogt JK, Kristensen M, Hansen LBS, Ibrügger S, Mærkedahl RB, Bahl MI, Lind MV, Nielsen RL, Frøkiær H, Gøbel RJ, Landberg R, Ross AB, Brix S, Holck J, Meyer AS, Sparholt MH, Christensen AF, Carvalho V, Hartmann B, Holst JJ, Rumessen JJ, Linneberg A, Sicheritz-Pontén T, Dalgaard MD, Blennow A, Frandsen HL, Villas-Bôas S, Kristiansen K, Vestergaard H, Hansen T, Ekstrøm CT, Ritz C, Nielsen HB, Pedersen OB, Gupta R, Lauritzen L, Licht TR. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: a randomised cross-over trial. Gut. 2019;68:83-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 325] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 23. | Masoud Abd El Gayed E, Kamal El Din Zewain S, Ragheb A, ElNaidany SS. Fat mass and obesity-associated gene expression and disease severity in type 2 diabetes mellitus. Steroids. 2021;174:108897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Frank J, Gupta A, Osadchiy V, Mayer EA. Brain-Gut-Microbiome Interactions and Intermittent Fasting in Obesity. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 25. | Peters BA, Shapiro JA, Church TR, Miller G, Trinh-Shevrin C, Yuen E, Friedlander C, Hayes RB, Ahn J. A taxonomic signature of obesity in a large study of American adults. Sci Rep. 2018;8:9749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 214] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 26. | Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9796] [Cited by in RCA: 9026] [Article Influence: 451.3] [Reference Citation Analysis (1)] |
| 27. | Dugas LR, Lie L, Plange-Rhule J, Bedu-Addo K, Bovet P, Lambert EV, Forrester TE, Luke A, Gilbert JA, Layden BT. Gut microbiota, short chain fatty acids, and obesity across the epidemiologic transition: the METS-Microbiome study protocol. BMC Public Health. 2018;18:978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1620] [Cited by in RCA: 1937] [Article Influence: 138.4] [Reference Citation Analysis (0)] |
| 29. | Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2249] [Cited by in RCA: 3697] [Article Influence: 462.1] [Reference Citation Analysis (0)] |
| 30. | Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1644] [Cited by in RCA: 2142] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 31. | Fiorucci S, Distrutti E. Bile Acid-Activated Receptors, Intestinal Microbiota, and the Treatment of Metabolic Disorders. Trends Mol Med. 2015;21:702-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 390] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 32. | Zheng X, Chen T, Jiang R, Zhao A, Wu Q, Kuang J, Sun D, Ren Z, Li M, Zhao M, Wang S, Bao Y, Li H, Hu C, Dong B, Li D, Wu J, Xia J, Wang X, Lan K, Rajani C, Xie G, Lu A, Jia W, Jiang C. Hyocholic acid species improve glucose homeostasis through a distinct TGR5 and FXR signaling mechanism. Cell Metab. 2021;33:791-803.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 317] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 33. | Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, Atkins AR, Khvat A, Schnabl B, Yu RT, Brenner DA, Coulter S, Liddle C, Schoonjans K, Olefsky JM, Saltiel AR, Downes M, Evans RM. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 611] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 34. | Shapiro H, Kolodziejczyk AA, Halstuch D, Elinav E. Bile acids in glucose metabolism in health and disease. J Exp Med. 2018;215:383-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 353] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 35. | Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Itadani H, Tanaka K. Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun. 2002;298:714-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 786] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 36. | Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435-9440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1060] [Cited by in RCA: 1269] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 37. | Vassileva G, Golovko A, Markowitz L, Abbondanzo SJ, Zeng M, Yang S, Hoos L, Tetzloff G, Levitan D, Murgolo NJ, Keane K, Davis HR Jr, Hedrick J, Gustafson EL. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem J. 2006;398:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 223] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 38. | Maruyama T, Tanaka K, Suzuki J, Miyoshi H, Harada N, Nakamura T, Miyamoto Y, Kanatani A, Tamai Y. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J Endocrinol. 2006;191:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 228] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 39. | Swann JR, Want EJ, Geier FM, Spagou K, Wilson ID, Sidaway JE, Nicholson JK, Holmes E. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4523-4530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 607] [Cited by in RCA: 565] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 40. | Martin AM, Yabut JM, Choo JM, Page AJ, Sun EW, Jessup CF, Wesselingh SL, Khan WI, Rogers GB, Steinberg GR, Keating DJ. The gut microbiome regulates host glucose homeostasis via peripheral serotonin. Proc Natl Acad Sci U S A. 2019;116:19802-19804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 41. | Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1566] [Cited by in RCA: 2608] [Article Influence: 260.8] [Reference Citation Analysis (1)] |
| 42. | Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, Ghatei MA, Bloom SR, Frost G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond). 2015;39:424-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 395] [Cited by in RCA: 614] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 43. | Brüssow H, Parkinson SJ. You are what you eat. Nat Biotechnol. 2014;32:243-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 44. | Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1524] [Cited by in RCA: 1741] [Article Influence: 124.4] [Reference Citation Analysis (0)] |
| 45. | Canfora EE, van der Beek CM, Jocken JWE, Goossens GH, Holst JJ, Olde Damink SWM, Lenaerts K, Dejong CHC, Blaak EE. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Sci Rep. 2017;7:2360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 256] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 46. | Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165:1332-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2533] [Cited by in RCA: 4654] [Article Influence: 465.4] [Reference Citation Analysis (0)] |
| 47. | Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509-1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1337] [Cited by in RCA: 1656] [Article Influence: 97.4] [Reference Citation Analysis (0)] |
| 48. | De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1214] [Cited by in RCA: 1674] [Article Influence: 139.5] [Reference Citation Analysis (0)] |
| 49. | Lin HV, Frassetto A, Kowalik EJ Jr, Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G, Marsh DJ. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7:e35240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 760] [Cited by in RCA: 957] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 50. | Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, Takahashi T, Miyauchi S, Shioi G, Inoue H, Tsujimoto G. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 833] [Cited by in RCA: 1127] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 51. | Sawicki CM, Livingston KA, Obin M, Roberts SB, Chung M, McKeown NM. Dietary Fiber and the Human Gut Microbiota: Application of Evidence Mapping Methodology. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 52. | de Las Heras Gala T, Herder C, Rutters F, Carstensen-Kirberg M, Huth C, Stehouwer CDA, Nijpels G, Schalkwijk C, Flyvbjerg A, Franks PW, Dekker J, Meisinger C, Koenig W, Roden M, Rathmann W, Peters A, Thorand B; IMI DIRECT Consortium. Association of changes in inflammation with variation in glycaemia, insulin resistance and secretion based on the KORA study. Diabetes Metab Res Rev. 2018;34:e3063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012;3:279-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 616] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 54. | Sharma S, Tripathi P. Gut microbiome and type 2 diabetes: where we are and where to go? J Nutr Biochem. 2019;63:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 298] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 55. | Horton F, Wright J, Smith L, Hinton PJ, Robertson MD. Increased intestinal permeability to oral chromium (51 Cr) -EDTA in human Type 2 diabetes. Diabet Med. 2014;31:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 56. | Cox AJ, Zhang P, Bowden DW, Devereaux B, Davoren PM, Cripps AW, West NP. Increased intestinal permeability as a risk factor for type 2 diabetes. Diabetes Metab. 2017;43:163-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 57. | Camargo A, Jimenez-Lucena R, Alcala-Diaz JF, Rangel-Zuñiga OA, Garcia-Carpintero S, Lopez-Moreno J, Blanco-Rojo R, Delgado-Lista J, Perez-Martinez P, van Ommen B, Malagon MM, Ordovas JM, Perez-Jimenez F, Lopez-Miranda J. Postprandial endotoxemia may influence the development of type 2 diabetes mellitus: From the CORDIOPREV study. Clin Nutr. 2019;38:529-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 58. | Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4095] [Cited by in RCA: 4742] [Article Influence: 249.6] [Reference Citation Analysis (1)] |
| 59. | Masi S, Gkranias N, Li K, Salpea KD, Parkar M, Orlandi M, Suvan JE, Eng HL, Taddei S, Patel K, Darbar U, Donos N, Deanfield JE, Hurel S, Humphries SE, D'Aiuto F. Association between short leukocyte telomere length, endotoxemia, and severe periodontitis in people with diabetes: a cross-sectional survey. Diabetes Care. 2014;37:1140-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 60. | Shu L, Shen XM, Li C, Zhang XY, Zheng PF. Dietary patterns are associated with type 2 diabetes mellitus among middle-aged adults in Zhejiang Province, China. Nutr J. 2017;16:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 61. | Glenn AJ, Li J, Lo K, Jenkins DJA, Boucher BA, Hanley AJ, Kendall CWC, Shadyab AH, Tinker LF, Chessler SD, Howard BV, Liu S, Sievenpiper JL. The Portfolio Diet and Incident Type 2 Diabetes: Findings From the Women's Health Initiative Prospective Cohort Study. Diabetes Care. 2023;46:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 62. | Magkos F, Hjorth MF, Astrup A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2020;16:545-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 299] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 63. | Khudhair Z, Alhallaf R, Eichenberger RM, Field M, Krause L, Sotillo J, Loukas A. Administration of Hookworm Excretory/Secretory Proteins Improves Glucose Tolerance in a Mouse Model of Type 2 Diabetes. Biomolecules. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Yao B, Fang H, Xu W, Yan Y, Xu H, Liu Y, Mo M, Zhang H, Zhao Y. Dietary fiber intake and risk of type 2 diabetes: a dose-response analysis of prospective studies. Eur J Epidemiol. 2014;29:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 185] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 65. | McRae MP. Dietary Fiber Intake and Type 2 Diabetes Mellitus: An Umbrella Review of Meta-analyses. J Chiropr Med. 2018;17:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 66. | Kyrø C, Tjønneland A, Overvad K, Olsen A, Landberg R. Higher Whole-Grain Intake Is Associated with Lower Risk of Type 2 Diabetes among Middle-Aged Men and Women: The Danish Diet, Cancer, and Health Cohort. J Nutr. 2018;148:1434-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 67. | Hu Y, Ding M, Sampson L, Willett WC, Manson JE, Wang M, Rosner B, Hu FB, Sun Q. Intake of whole grain foods and risk of type 2 diabetes: results from three prospective cohort studies. BMJ. 2020;370:m2206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 68. | Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3:289-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1231] [Cited by in RCA: 1562] [Article Influence: 111.6] [Reference Citation Analysis (1)] |
| 69. | Robertson MD, Bickerton AS, Dennis AL, Vidal H, Frayn KN. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr. 2005;82:559-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 70. | Tison SE, Shikany JM, Long DL, Carson AP, Cofield SS, Pearson KE, Howard G, Judd SE. Differences in the Association of Select Dietary Measures With Risk of Incident Type 2 Diabetes. Diabetes Care. 2022;45:2602-2610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 71. | Wang X, Li Q, Liu Y, Jiang H, Chen W. Intermittent fasting versus continuous energy-restricted diet for patients with type 2 diabetes mellitus and metabolic syndrome for glycemic control: A systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2021;179:109003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 72. | Borgundvaag E, Mak J, Kramer CK. Metabolic Impact of Intermittent Fasting in Patients With Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis of Interventional Studies. J Clin Endocrinol Metab. 2021;106:902-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 73. | Liu Z, Dai X, Zhang H, Shi R, Hui Y, Jin X, Zhang W, Wang L, Wang Q, Wang D, Wang J, Tan X, Ren B, Liu X, Zhao T, Pan J, Yuan T, Chu C, Lan L, Yin F, Cadenas E, Shi L, Zhao S. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat Commun. 2020;11:855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 383] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 74. | Fijan S. Microorganisms with claimed probiotic properties: an overview of recent literature. Int J Environ Res Public Health. 2014;11:4745-4767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 583] [Cited by in RCA: 597] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 75. | Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J, Yu L, Xu C, Ren Z, Xu Y, Xu S, Shen H, Zhu X, Shi Y, Shen Q, Dong W, Liu R, Ling Y, Zeng Y, Zhang Q, Wang J, Wang L, Wu Y, Zeng B, Wei H, Zhang M, Peng Y, Zhang C. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359:1151-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1659] [Article Influence: 207.4] [Reference Citation Analysis (68)] |
| 76. | Li D, Li Y, Yang S, Lu J, Jin X, Wu M. Diet-gut microbiota-epigenetics in metabolic diseases: From mechanisms to therapeutics. Biomed Pharmacother. 2022;153:113290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 77. | Neef A, Sanz Y. Future for probiotic science in functional food and dietary supplement development. Curr Opin Clin Nutr Metab Care. 2013;16:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 78. | Mayorga Reyes L, González Vázquez R, Cruz Arroyo SM, Melendez Avalos A, Reyes Castillo PA, Chavaro Pérez DA, Ramos Terrones I, Ramos Ibáñez N, Rodríguez Magallanes MM, Langella P, Bermúdez Humarán L, Azaola Espinosa A. Correlation between diet and gut bacteria in a population of young adults. Int J Food Sci Nutr. 2016;67:470-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 79. | Martin FP, Wang Y, Sprenger N, Yap IK, Lundstedt T, Lek P, Rezzi S, Ramadan Z, van Bladeren P, Fay LB, Kochhar S, Lindon JC, Holmes E, Nicholson JK. Probiotic modulation of symbiotic gut microbial-host metabolic interactions in a humanized microbiome mouse model. Mol Syst Biol. 2008;4:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 309] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 80. | He X, Slupsky CM, Dekker JW, Haggarty NW, Lönnerdal B. Integrated Role of Bifidobacterium animalis subsp. lactis Supplementation in Gut Microbiota, Immunity, and Metabolism of Infant Rhesus Monkeys. mSystems. 2016;1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 81. | Panwar H, Rashmi HM, Batish VK, Grover S. Probiotics as potential biotherapeutics in the management of type 2 diabetes - prospects and perspectives. Diabetes Metab Res Rev. 2013;29:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 82. | Hsieh MC, Tsai WH, Jheng YP, Su SL, Wang SY, Lin CC, Chen YH, Chang WW. The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: a randomized, double-blinded, placebo-controlled trial. Sci Rep. 2018;8:16791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 83. | Asemi Z, Samimi M, Tabassi Z, Naghibi Rad M, Rahimi Foroushani A, Khorammian H, Esmaillzadeh A. Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: a randomized controlled trial. Eur J Clin Nutr. 2013;67:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 84. | Cani PD, Delzenne NM. Interplay between obesity and associated metabolic disorders: new insights into the gut microbiota. Curr Opin Pharmacol. 2009;9:737-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 241] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 85. | Monnerie S, Comte B, Ziegler D, Morais JA, Pujos-Guillot E, Gaudreau P. Metabolomic and Lipidomic Signatures of Metabolic Syndrome and its Physiological Components in Adults: A Systematic Review. Sci Rep. 2020;10:669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 86. | Jayashree B, Bibin YS, Prabhu D, Shanthirani CS, Gokulakrishnan K, Lakshmi BS, Mohan V, Balasubramanyam M. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol Cell Biochem. 2014;388:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 251] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 87. | Tiderencel KA, Hutcheon DA, Ziegler J. Probiotics for the treatment of type 2 diabetes: A review of randomized controlled trials. Diabetes Metab Res Rev. 2020;36:e3213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 88. | Balakumar M, Prabhu D, Sathishkumar C, Prabu P, Rokana N, Kumar R, Raghavan S, Soundarajan A, Grover S, Batish VK, Mohan V, Balasubramanyam M. Improvement in glucose tolerance and insulin sensitivity by probiotic strains of Indian gut origin in high-fat diet-fed C57BL/6J mice. Eur J Nutr. 2018;57:279-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 89. | Sharma P, Bhardwaj P, Singh R. Administration of Lactobacillus casei and Bifidobacterium bifidum Ameliorated Hyperglycemia, Dyslipidemia, and Oxidative Stress in Diabetic Rats. Int J Prev Med. 2016;7:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 90. | Hove KD, Brøns C, Færch K, Lund SS, Rossing P, Vaag A. Effects of 12 weeks of treatment with fermented milk on blood pressure, glucose metabolism and markers of cardiovascular risk in patients with type 2 diabetes: a randomised double-blind placebo-controlled study. Eur J Endocrinol. 2015;172:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 91. | Khalili L, Alipour B, Asghari Jafar-Abadi M, Faraji I, Hassanalilou T, Mesgari Abbasi M, Vaghef-Mehrabany E, Alizadeh Sani M. The Effects of Lactobacillus casei on Glycemic Response, Serum Sirtuin1 and Fetuin-A Levels in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Iran Biomed J. 2019;23:68-77. [PubMed] |
| 92. | Sato J, Kanazawa A, Azuma K, Ikeda F, Goto H, Komiya K, Kanno R, Tamura Y, Asahara T, Takahashi T, Nomoto K, Yamashiro Y, Watada H. Probiotic reduces bacterial translocation in type 2 diabetes mellitus: A randomised controlled study. Sci Rep. 2017;7:12115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 93. | Mobini R, Tremaroli V, Ståhlman M, Karlsson F, Levin M, Ljungberg M, Sohlin M, Bertéus Forslund H, Perkins R, Bäckhed F, Jansson PA. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: A randomized controlled trial. Diabetes Obes Metab. 2017;19:579-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 207] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 94. | Sanborn VE, Azcarate-Peril MA, Gunstad J. Lactobacillus rhamnosus GG and HbA1c in middle age and older adults without type 2 diabetes mellitus: A preliminary randomized study. Diabetes Metab Syndr. 2020;14:907-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 95. | Li X, Wang E, Yin B, Fang D, Chen P, Wang G, Zhao J, Zhang H, Chen W. Effects of Lactobacillus casei CCFM419 on insulin resistance and gut microbiota in type 2 diabetic mice. Benef Microbes. 2017;8:421-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 96. | Kobyliak N, Falalyeyeva T, Mykhalchyshyn G, Kyriienko D, Komissarenko I. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: Randomized clinical trial. Diabetes Metab Syndr. 2018;12:617-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 97. | Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, de Barsy M, Loumaye A, Hermans MP, Thissen JP, de Vos WM, Cani PD. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25:1096-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 774] [Cited by in RCA: 1583] [Article Influence: 226.1] [Reference Citation Analysis (0)] |
| 98. | Cani PD, Dewever C, Delzenne NM. Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. Br J Nutr. 2004;92:521-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 313] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 99. | Li K, Zhang L, Xue J, Yang X, Dong X, Sha L, Lei H, Zhang X, Zhu L, Wang Z, Li X, Wang H, Liu P, Dong Y, He L. Dietary inulin alleviates diverse stages of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in db/db mice. Food Funct. 2019;10:1915-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 160] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 100. | Morshedi M, Saghafi-Asl M, Hosseinifard ES. The potential therapeutic effects of the gut microbiome manipulation by synbiotic containing-Lactobacillus plantarum on neuropsychological performance of diabetic rats. J Transl Med. 2020;18:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 101. | Mahboobi S, Rahimi F, Jafarnejad S. Effects of Prebiotic and Synbiotic Supplementation on Glycaemia and Lipid Profile in Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Adv Pharm Bull. 2018;8:565-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 102. | Rossen NG, MacDonald JK, de Vries EM, D'Haens GR, de Vos WM, Zoetendal EG, Ponsioen CY. Fecal microbiota transplantation as novel therapy in gastroenterology: A systematic review. World J Gastroenterol. 2015;21:5359-5371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 160] [Cited by in RCA: 164] [Article Influence: 14.9] [Reference Citation Analysis (1)] |
| 103. | Ziegler MC, Garbim Junior EE, Jahnke VS, Lisbôa Moura JG, Brasil CS, Schimitt da Cunha PH, Lora PS, Gemelli T. Impact of probiotic supplementation in a patient with type 2 diabetes on glycemic and lipid profile. Clin Nutr ESPEN. 2022;49:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 104. | Wang L, Lee YK, Bundman D, Han Y, Thevananther S, Kim CS, Chua SS, Wei P, Heyman RA, Karin M, Moore DD. Redundant pathways for negative feedback regulation of bile acid production. Dev Cell. 2002;2:721-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 385] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 105. | Kasińska MA, Drzewoski J. Effectiveness of probiotics in type 2 diabetes: a meta-analysis. Pol Arch Med Wewn. 2015;125:803-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 106. | Wang Y, Dilidaxi D, Wu Y, Sailike J, Sun X, Nabi XH. Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice. Biomed Pharmacother. 2020;125:109914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 107. | Balducci S, Sacchetti M, Haxhi J, Orlando G, D'Errico V, Fallucca S, Menini S, Pugliese G. Physical exercise as therapy for type 2 diabetes mellitus. Diabetes Metab Res Rev. 2014;30 Suppl 1:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 108. | Barton W, Penney NC, Cronin O, Garcia-Perez I, Molloy MG, Holmes E, Shanahan F, Cotter PD, O'Sullivan O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. 2018;67:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 271] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 109. | Campbell SC, Wisniewski PJ, Noji M, McGuinness LR, Häggblom MM, Lightfoot SA, Joseph LB, Kerkhof LJ. The Effect of Diet and Exercise on Intestinal Integrity and Microbial Diversity in Mice. PLoS One. 2016;11:e0150502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 196] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 110. | Motiani KK, Collado MC, Eskelinen JJ, Virtanen KA, Löyttyniemi E, Salminen S, Nuutila P, Kalliokoski KK, Hannukainen JC. Exercise Training Modulates Gut Microbiota Profile and Improves Endotoxemia. Med Sci Sports Exerc. 2020;52:94-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 221] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 111. | Liu Y, Wang Y, Ni Y, Cheung CKY, Lam KSL, Xia Z, Ye D, Guo J, Tse MA, Panagiotou G, Xu A. Gut Microbiome Fermentation Determines the Efficacy of Exercise for Diabetes Prevention. Cell Metab. 2020;31:77-91.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 292] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 112. | Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, Mele MC. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. 2019;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 969] [Cited by in RCA: 2288] [Article Influence: 326.9] [Reference Citation Analysis (4)] |
| 113. | Khoruts A, Sadowsky MJ. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol. 2016;13:508-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 414] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 114. | Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913-6.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1881] [Cited by in RCA: 2078] [Article Influence: 148.4] [Reference Citation Analysis (0)] |
| 115. | Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV, Udayappan SD, Hermes G, Bouter KE, Koopen AM, Holst JJ, Knop FK, Blaak EE, Zhao J, Smidt H, Harms AC, Hankemeijer T, Bergman JJGHM, Romijn HA, Schaap FG, Olde Damink SWM, Ackermans MT, Dallinga-Thie GM, Zoetendal E, de Vos WM, Serlie MJ, Stroes ESG, Groen AK, Nieuwdorp M. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017;26:611-619.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 735] [Article Influence: 81.7] [Reference Citation Analysis (0)] |
| 116. | Zhang PP, Li LL, Han X, Li QW, Zhang XH, Liu JJ, Wang Y. Fecal microbiota transplantation improves metabolism and gut microbiome composition in db/db mice. Acta Pharmacol Sin. 2020;41:678-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 117. | Wang H, Lu Y, Yan Y, Tian S, Zheng D, Leng D, Wang C, Jiao J, Wang Z, Bai Y. Promising Treatment for Type 2 Diabetes: Fecal Microbiota Transplantation Reverses Insulin Resistance and Impaired Islets. Front Cell Infect Microbiol. 2019;9:455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 118. | Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2084] [Cited by in RCA: 2440] [Article Influence: 187.7] [Reference Citation Analysis (0)] |
| 119. | Cai TT, Ye XL, Yong HJ, Song B, Zheng XL, Cui BT, Zhang FM, Lu YB, Miao H, Ding DF. Fecal microbiota transplantation relieve painful diabetic neuropathy: A case report. Medicine (Baltimore). 2018;97:e13543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 120. | Ejtahed HS, Tito RY, Siadat SD, Hasani-Ranjbar S, Hoseini-Tavassol Z, Rymenans L, Verbeke K, Soroush AR, Raes J, Larijani B. Metformin induces weight loss associated with gut microbiota alteration in non-diabetic obese women: a randomized double-blind clinical trial. Eur J Endocrinol. 2019;180:165-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 121. | Malik F, Mehdi SF, Ali H, Patel P, Basharat A, Kumar A, Ashok F, Stein J, Brima W, Malhotra P, Roth J. Is metformin poised for a second career as an antimicrobial? Diabetes Metab Res Rev. 2018;34:e2975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 122. | de la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, Velásquez-Mejía EP, Carmona JA, Abad JM, Escobar JS. Metformin Is Associated With Higher Relative Abundance of Mucin-Degrading Akkermansia muciniphila and Several Short-Chain Fatty Acid-Producing Microbiota in the Gut. Diabetes Care. 2017;40:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 556] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 123. | Lee H, Ko G. Effect of metformin on metabolic improvement and gut microbiota. Appl Environ Microbiol. 2014;80:5935-5943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 306] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 124. | Rodriguez J, Hiel S, Delzenne NM. Metformin: old friend, new ways of action-implication of the gut microbiome? Curr Opin Clin Nutr Metab Care. 2018;21:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 125. | Zhang X, Fang Z, Zhang C, Xia H, Jie Z, Han X, Chen Y, Ji L. Effects of Acarbose on the Gut Microbiota of Prediabetic Patients: A Randomized, Double-blind, Controlled Crossover Trial. Diabetes Ther. 2017;8:293-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 126. | Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, Prifti E, Vieira-Silva S, Gudmundsdottir V, Pedersen HK, Arumugam M, Kristiansen K, Voigt AY, Vestergaard H, Hercog R, Costea PI, Kultima JR, Li J, Jørgensen T, Levenez F, Dore J; MetaHIT consortium, Nielsen HB, Brunak S, Raes J, Hansen T, Wang J, Ehrlich SD, Bork P, Pedersen O. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1208] [Cited by in RCA: 1597] [Article Influence: 145.2] [Reference Citation Analysis (0)] |
| 127. | Su B, Liu H, Li J, Sunli Y, Liu B, Liu D, Zhang P, Meng X. Acarbose treatment affects the serum levels of inflammatory cytokines and the gut content of bifidobacteria in Chinese patients with type 2 diabetes mellitus. J Diabetes. 2015;7:729-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 128. | Yan X, Feng B, Li P, Tang Z, Wang L. Microflora Disturbance during Progression of Glucose Intolerance and Effect of Sitagliptin: An Animal Study. J Diabetes Res. 2016;2016:2093171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 129. | Zhang Q, Xiao X, Li M, Yu M, Ping F, Zheng J, Wang T, Wang X. Vildagliptin increases butyrate-producing bacteria in the gut of diabetic rats. PLoS One. 2017;12:e0184735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 130. | Rankin LC, Artis D. Beyond Host Defense: Emerging Functions of the Immune System in Regulating Complex Tissue Physiology. Cell. 2018;173:554-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 189] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 131. | Ahmed-Sarwar N, Nagel AK, Leistman S, Heacock K. SGLT-2 Inhibitors: Is There a Role in Type 1 Diabetes Mellitus Management? Ann Pharmacother. 2017;51:791-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 132. | Ma Y, You X, Mai G, Tokuyasu T, Liu C. A human gut phage catalog correlates the gut phageome with type 2 diabetes. Microbiome. 2018;6:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 133. | Rasmussen TS, Mentzel CMJ, Kot W, Castro-Mejía JL, Zuffa S, Swann JR, Hansen LH, Vogensen FK, Hansen AK, Nielsen DS. Faecal virome transplantation decreases symptoms of type 2 diabetes and obesity in a murine model. Gut. 2020;69:2122-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 175] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 134. | Duan FF, Liu JH, March JC. Engineered commensal bacteria reprogram intestinal cells into glucose-responsive insulin-secreting cells for the treatment of diabetes. Diabetes. 2015;64:1794-1803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 135. | Duan F, Curtis KL, March JC. Secretion of insulinotropic proteins by commensal bacteria: rewiring the gut to treat diabetes. Appl Environ Microbiol. 2008;74:7437-7438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 136. | Chen Z, Guo L, Zhang Y, Walzem RL, Pendergast JS, Printz RL, Morris LC, Matafonova E, Stien X, Kang L, Coulon D, McGuinness OP, Niswender KD, Davies SS. Incorporation of therapeutically modified bacteria into gut microbiota inhibits obesity. J Clin Invest. 2014;124:3391-3406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology & metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wu K, United States S-Editor: Yan JP L-Editor: A P-Editor: Yan JP