Published online Oct 26, 2023. doi: 10.12998/wjcc.v11.i30.7440
Peer-review started: August 1, 2023
First decision: September 13, 2023
Revised: September 19, 2023
Accepted: September 27, 2023
Article in press: September 27, 2023
Published online: October 26, 2023
Processing time: 84 Days and 18.2 Hours
Neonatal hypertension is a rare but potentially serious condition that requires careful monitoring and treatment. Pharmacogenomics can help guide individualized drug therapy and improve outcomes.
We report a case of a preterm infant with multiple complications, including bronchopulmonary dysplasia (BPD), sepsis, intracranial hemorrhage, and hyper
This case illustrates the importance of regular blood pressure monitoring and etiological investigation in preterm infants with hypertension. Pharmacogenomics can provide useful information for individualized drug therapy and safety in this population.
Core Tip: This study presents a case report of a premature infant with hypertension and reviews the literature on pharmacogenomics-based individualized treatment. The findings suggest that pharmacogenomics can be used to personalize treatment for hypertension in preterm infants.
- Citation: Tang LF, Xu A, Liu K. Pharmacogenomics-based individualized treatment of hypertension in preterm infants: A case report and review of the literature. World J Clin Cases 2023; 11(30): 7440-7449
- URL: https://www.wjgnet.com/2307-8960/full/v11/i30/7440.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i30.7440
We report a rare and unique case of a preterm infant with multiple complications, including hypertension, bronchopulmonary dysplasia (BPD), sepsis, intracranial hemorrhage, and congenital hypothyroidism. The confluence of these complications in a single infant is noteworthy, and to the best of our knowledge, this is the first report of such a case. Through this report, we aim to provide insights into the challenges faced and the treatment strategies employed, thereby potentially guiding future clinical decisions in similar cases.
The preterm infant, delivered at 29 + 1 wk of gestation, presented with the following chief complaints.
Respiratory distress: Shortly after birth, the infant exhibited signs consistent with respiratory distress syndrome. This was evidenced by difficulty in breathing and the need for immediate respiratory support.
Low birth weight: The infant’s weight at birth was 680 g, classifying him as an ultra-low birth weight infant.
Gastrointestinal issues: The child displayed symptoms of upper gastrointestinal bleeding, which raised concerns regarding the digestive system’s integrity and functionality.
Recurrent infections: The infant was susceptible to multiple severe infections during his hospital stay, including sepsis, pulmonary infections, and fungemia, requiring extended antibiotic treatment.
Hypertension: Starting from the 28th d after birth, the infant showed abnormal blood pressure readings, with a noted increase in both systolic and diastolic pressures.
Apnea episodes: The infant faced recurrent apnea episodes, necessitating medical intervention.
Hypothyroidism: On the 44th d after delivery, the infant was diagnosed with congenital hypothyroidism.
Nutritional challenges: The infant exhibited signs of feeding intolerance, gastrointestinal bleeding post feeding, and subsequent challenges related to his immature gastrointestinal digestion and absorption functions.
The infant’s journey commenced during the second trimester of the mother’s fifth pregnancy. Born prematurely at a gestational age of 29 + 1 wk, his birth was the culmination of a pregnancy marked by complications. The following are prenatal characteristics.
Gestational diabetes mellitus: The mother was diagnosed with gestational diabetes mellitus, a condition that can increase the risk of preterm birth and other complications.
Twin transfusion syndrome: Prenatal ultrasounds depicted the severity of twin transfusion syndrome (grade III). This condition, where blood moves from one twin (the donor) to the other (the recipient), was further complicated by the reversal of umbilical blood flow in the B fetus.
Intrauterine growth restriction: The B fetus showcased intrauterine growth restriction, a condition where a fetus doesn’t grow as expected in the womb.
Amniotic fluid imbalance: The ultrasound revealed an absence of amniotic fluid around the B fetus, contrasted by an excess in the A fetus. Post delivery, the neonate’s health journey was challenging.
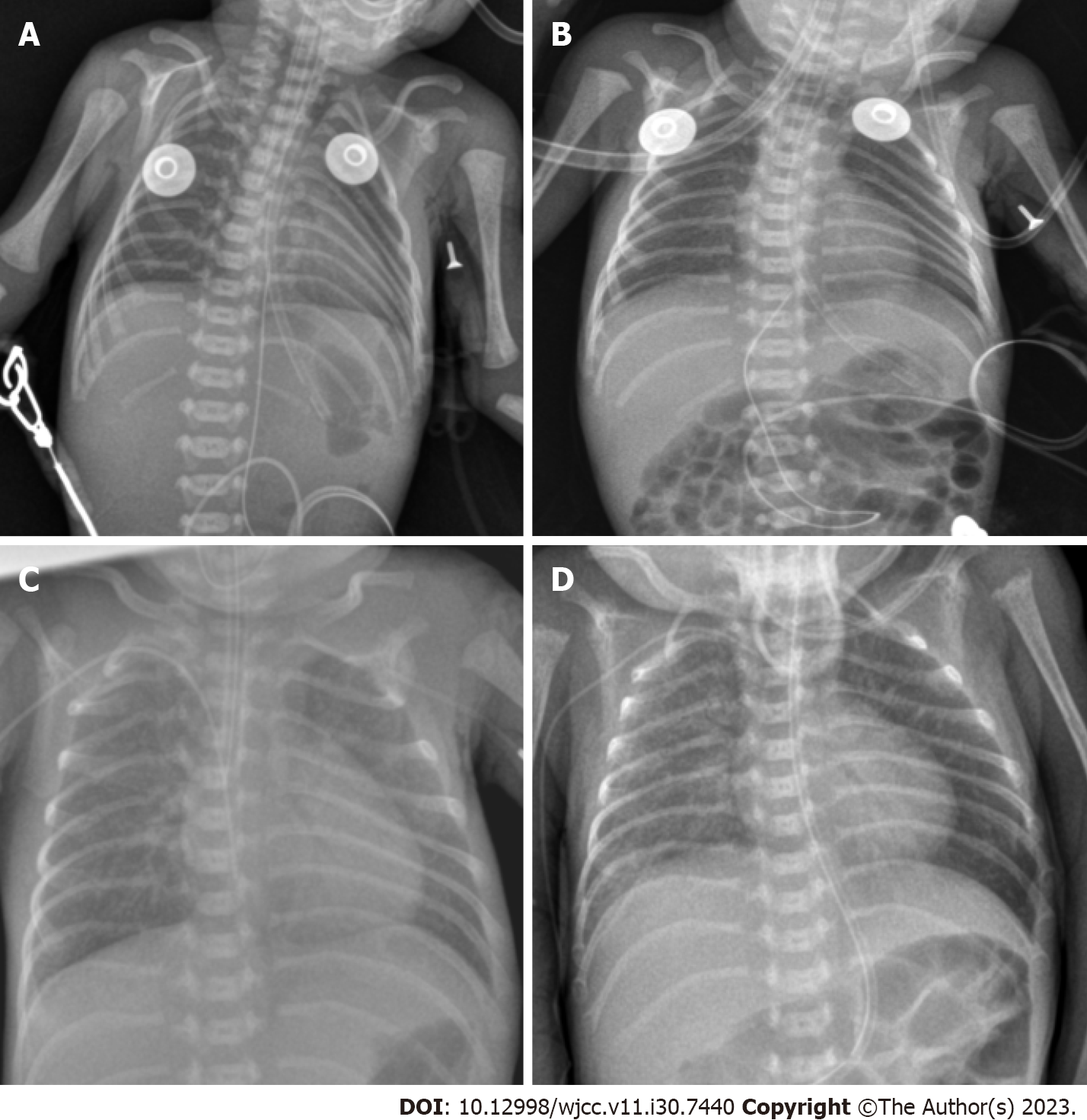
Respiratory complications: Soon after birth, he suffered from respiratory distress syndrome, necessitating immediate intubation and exogenous pulmonary surfactant administration. Despite initial interventions, the infant had to be intubated multiple times due to recurring respiratory failure (Figure 1).
Infections: During his hospital stay, the infant faced severe infections, including sepsis, pulmonary infections, and fungemia. This led to a prolonged course of various antibiotics.
Gastrointestinal and nutritional challenges: Failed umbilical vein catheterization on the day of birth and subsequent gastrointestinal bleeding highlighted the infant’s digestive system vulnerabilities. Feeding posed a significant challenge due to the immaturity of his gastrointestinal system, leading to bleeding post feeding with breast milk and formula.
Cardiac concerns: An echocardiogram on the first day of hospitalization indicated patent ductus arteriosus, patent foramen ovale, tricuspid regurgitation, and raised pulmonary artery pressure. Although some of these conditions resolved, the patent foramen ovale and tricuspid regurgitation persisted.
Endocrine issues: On the 44th d post-delivery, congenital hypothyroidism was identified, requiring therapeutic intervention.
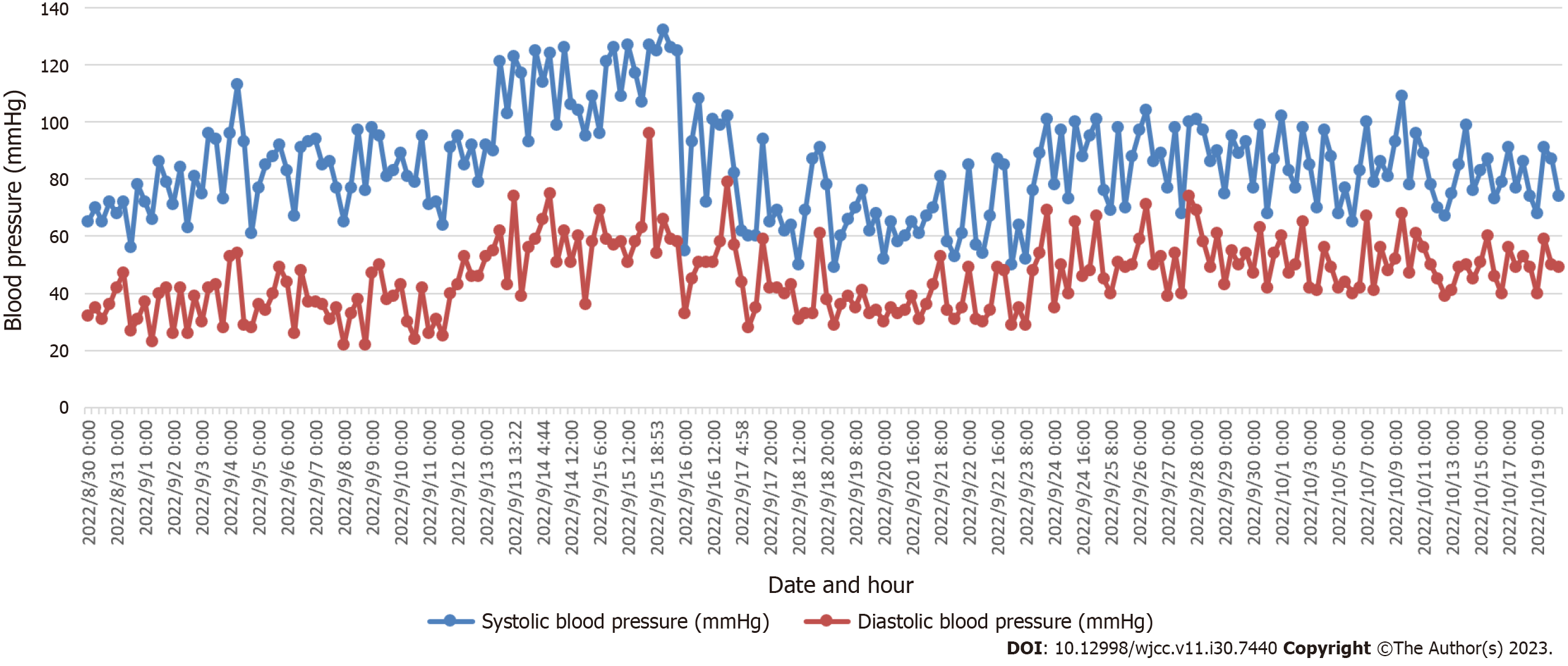
Hypertension: By the end of the first month, the infant exhibited hypertension, particularly after the administration of certain medications. This necessitated pharmacological intervention to manage and stabilize his blood pressure (Figure 2).
Genetic testing: Given the rapid onset of hypertension post-medication and the varying response to treatment, genetic testing was conducted. This revealed specific genotypes in the ABCB1 gene for both dexamethasone and amlodipine, as well as a particular genotype in the CYP3A5 gene.
The pregnancy was marked with complications, including the mother’s gestational diabetes and indications of twin transfusion syndrome on prenatal ultrasounds.
This being the mother’s fifth pregnancy, the infant was a twin and labeled as a donor in twin transfusion syndrome due to the complications identified in prenatal screenings.
Upon delivery, the infant weighed 680 g. No anomalies were noted in the placenta or umbilical cord. Initial Apgar scores were relatively low but showed improvement within ten minutes.
The infant underwent extensive treatments, including a range of antibiotics such as cefoperazone sulbactam sodium, flucloxacillin, meropenem, and others. The need for multiple medications indicated recurrent severe infections. Additionally, due to complications, the infant was provided with omeprazole, etamsylate, and vitamin K1.
Echocardiography on the first day post-birth revealed patent ductus arteriosus, patent foramen ovale, tricuspid regurgitation, and elevated pulmonary artery pressure. Follow-up echocardiographies showed closure of the ductus arteriosus but persistent patent foramen ovale and tricuspid regurgitation.
The final diagnosis encompassed BPD, sepsis, intracranial hemorrhage, congenital hypothyroidism, and hypertension.
Upon being diagnosed with respiratory distress syndrome and respiratory failure immediately after birth, the infant underwent the following treatments.
Intubation: Due to the severity of the respiratory distress syndrome, the infant was intubated to facilitate breathing.
Exogenous pulmonary surface active substance: Administered Gursul 120 mg as an initial treatment.
High-frequency oscillatory ventilation: Used for one day, followed by mechanical ventilation in constant-frequency mode for five subsequent days.
Constant-frequency mechanized ventilation: The infant faced recurrent respiratory challenges, leading to two more episodes of intubation, each lasting five days.
Nasal Bi-level positive airway pressure ventilation and continuous positive airway pressure ventilation: Used between the intubation sessions.
High-flow nasal cannula: Implemented on the 52nd d after birth for 4 d, followed by nasal cannula oxygen inhalation (Figure 1).
Infection management: The infant was diagnosed with multiple severe infections, including sepsis, pulmonary infections, and fungemia.
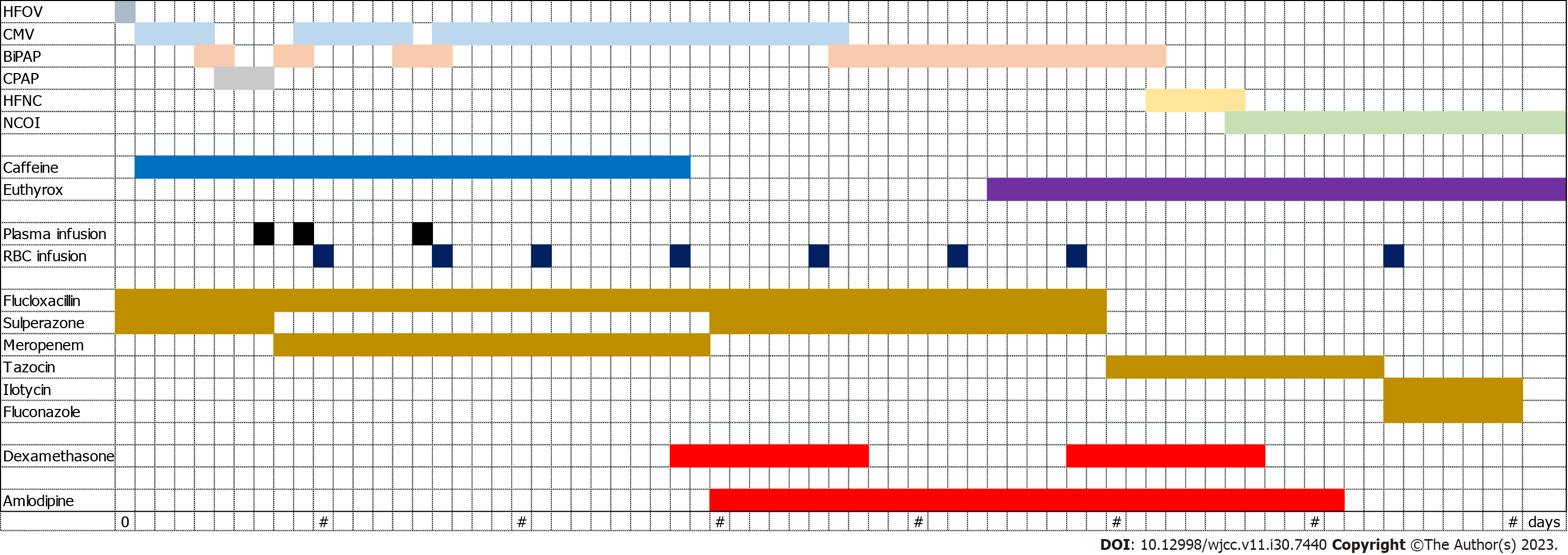
Antibiotic treatment: Administered a cocktail of antibiotics over a span of 70 d (Figure 3), including: Cefoperazone sulbactam sodium, flucloxacillin, meropenem, piperacillin tazobactam sodium, erythromycin, fluconazole.
Vascular access: After a failed attempt at umbilical vein catheterization on the day of delivery, a central venous catheter was successfully implanted on the 16th d post-birth and was in place for 32 d.
Hemorrhage and anemia management: Administered omeprazole, etamsylate, and vitamin K1 to manage gastrointestinal bleeding and brain hemorrhage. Transfused with frozen plasma three times and suspended red blood cells ten times (Figure 3).
Hypoalbuminemia, edema, and oliguria: Managed by infusing human serum albumin.
Recurrent apnea: Managed by supplying caffeine intravenously through drip for 27 d (Figure 3).
Congenital hypothyroidism: Diagnosed on the 44th d post-delivery and treated with oral levothyroxine pills until discharge (Figure 3).
Nutritional support: Enteral nutrition was initiated on the second day post-birth. Due to the immature state of his gastrointestinal system, complications like gastrointestinal bleeding and feeding intolerance arose. Thus, the infant was given enteral nutrition support for 28 d.
Total parenteral nutrition (TPN)-related cholestasis and liver function impairment: Addressed with adenosine butadisulfonic acid, ursodeoxycholic acid capsules, and compound glycyrrhizin.
Cardiovascular management: Based on echocardiography findings, the patient was treated with appropriate fluid restriction, refraining from the use of ibuprofen or indomethacin.
Hypertension management: The infant exhibited elevated blood pressure levels post the administration of dexamethasone, hydrochlorothiazide, and spironolactone (Figure 2). Initiated treatment with amlodipine (0.1 mg, once per day). After observing a significant drop in blood pressure, the dosage was adjusted to 0.075 mg (Figure 3). The medication was finally discontinued on the 61st d post-birth after stabilization of blood pressure.
Pharmacogenomic analysis: On the 34th d of life, due to the unusual response to medications, a pharmacogenomic gene test was conducted by drawing 2 mL of venous blood from the infant. The sample, anticoagulated with ethylene diamine tetraacetic acid, was sent for high-throughput sequencing. The results showed: The ABCB1 gene, associated with the glucocorticoid dexamethasone, had the genotype GG (rs1045642), indicating minimal effect on its toxicity, dose, and efficacy, and thus was recommended for regular use. The ABCB1 gene of the antihypertensive drug amlodipine also had the genotype GG (rs1045642), suggesting a reduced-function phenotype. The CYP3A5 gene presented a *3/*3 genotype, which is linked to a slow metabolic phenotype with an increased potency, suggesting the need for dosage adjustments.
The infant’s hospitalization journey was fraught with a series of medical challenges. However, the comprehensive and timely interventions ensured his steady progress.
Respiratory recovery: By the 52nd d after birth, there was a notable improvement in the infant’s respiratory condition. He transitioned from mechanical ventilatory support to high-flow nasal cannula, eventually only requiring nasal cannula oxygen inhalation.
Stabilization of infections: Post administration of a diverse antibiotic regimen, the infant showed no signs of recurrent infections, indicating successful treatment of sepsis, pulmonary infections, and fungemia.
Cardiac improvements: Follow-up echocardiograms showed the closure of the ductus arteriosus, but the persistence of patent foramen ovale and tricuspid regurgitation, both of which would require continued monitoring.
Endocrine management: With the diagnosis of congenital hypothyroidism, oral levothyroxine was administered, ensuring stabilization of thyroid function.
Hypertension management: The introduction of amlodipine led to the regulation of the infant’s blood pressure, with subsequent modifications in dosage ensuring stabilization (Figure 2).
Genetic insights: Pharmacogenomic gene testing provided invaluable insights into the infant’s unique metabolic responses to certain medications, guiding precise dosing and management.
The infant was discharged on the 100th d after birth, weighing 2730 g. A robust follow-up plan was put in place.
Routine pediatric visits: Scheduled visits to the pediatrician ensured continuous monitoring of the infant’s growth, development, and overall health.
Cardiac monitoring: Regular echocardiograms were recommended to monitor the patent foramen ovale and tricuspid regurgitation.
Endocrine follow-up: Periodic thyroid function tests were scheduled to monitor the efficacy of the levothyroxine therapy and adjust dosages if needed.
Hypertension monitoring: Blood pressure checks at regular intervals ensured that the infant remained normotensive post-discharge.
Nutritional guidance: Given the initial challenges with feeding, a nutritionist’s guidance was sought to ensure appropriate dietary intake and monitor growth milestones.
Neonatal hypertension is uncommon in the neonatal intensive care unit (NICU), with incidence inconsistently reported between 0.2% and 3.2%[1,2], and is predominantly seen in preterm infants. In contrast, severe full-term infants present earlier with a higher incidence of intractable hypertension[3]. A 2018 multicenter study done in Australia demonstrated a correlation between neonatal hypertension and acute kidney injury[4], which should be further researched. There also remains a lack of uniform diagnostic criteria. The definition of neonatal blood pressure proposed by the American academy of pediatrics in 1987 is generally accepted. After correcting for gestational age, borderline hypertension is defined as three different time points of systolic blood pressure/diastolic blood pressure between the 90th and 95th percentiles for a given age and sex, and hypertension is defined as higher than the 95th percentile. A multicenter study done in the Philadelphia region revealed a linear relationship between gestational age, daytime age, and body weight; and a progressive increase in blood pressure after birth[5]. In our case, the blood pressure of the infant increased on the 28th d after birth (postmenstrual age 33 + 1 wk), and the systolic blood pressure (132 mmHg) and diastolic blood pressure (96 mmHg) were higher than those of the 95th percentile. Therefore, the diagnosis of neonatal hypertension was confirmed.
Most signs of newborn hypertension are unusual and identified during unrelated cardiac monitoring[6]. Mild cases are mostly asymptomatic or have nonspecific manifestations such as feeding difficulties, apnea, or tachycardia. Infants with severely elevated blood pressure may present with life-threatening conditions such as convulsions, heart failure, shock[7], and intracranial hemorrhage. Even in early childhood, a minority of preterm newborns may manifest with hypertension only after hospital discharge[8]. During such infants’ hospitalization, feeding problems and frequent apnea may be signs of hypertension, which must be identified. According to the relevant literature[9], there are various causes of elevated blood pressure in neonates, which can be divided into primary and secondary factors. Primary hypertension is primarily associated with familial genetic factors, maternal hypertension during pregnancy, and smoking. According to a 2019 meta-analysis study, hypertension during pregnancy increases the risk of cardiovascular disease in infants and children, and may be associated with DNA methylation[10]. In this case, the boy had no apparent family history of hypertension, his mother’s blood pressure was monitored within the normal range during pregnancy, and she only had high fasting glucose, which was diagnosed as gestational diabetes mellitus. As the mother had reasonable glycemic control after dietary modification and did not smoke during pregnancy, the association was deemed unlikely. There are many kinds of secondary hypertension involving multiple organs of the body, the most important of which are renal vascular disease and renal parenchymal disease[11]; these include aortic or renal artery thrombosis related to umbilical artery catheterization, renal artery or aortic stenosis or dysplasia, and renal parenchymal lesions. There are also congenital adrenal hyperplasia, hyperthyroidism, intracranial hypertension, BPD, and related genetic diseases, and some drugs. According to the literature and pharmacological understanding, dexamethasone, indomethacin, adrenergic medications, aminophylline, caffeine, erythropoietin, and amphotericin B are frequent pharmaceuticals that might elevate blood pressure. Animal investigations show that dexamethasone may be associated with tyrosine hydroxylase[12], vascular endothelial glucocorticoid receptors[13], human angiotensinogen gene polymorphisms[14], and other variables.
In this case, hypertension began four weeks after delivery and progressively worsened. Relevant ancillary investigations were performed sequentially to further clarify the predisposing factors further. Urinary routine and renal function were regular and did not support substantial renal disease: 17 hydroxyprogesterone, aldosterone, and cortisol were normal, FT4 and thyroid stimulating hormone were controlled within the normal range with oral levothyroxine tablets, and endocrine system disorders causing elevated blood pressure were not considered. No aortic stenosis, polycystic kidney, renal tumor, renal agenesis, nor thrombosis was found by cardiac, renal, and vascular ultrasound. Cranial ultrasound showed grade III-IV intracranial hemorrhage on the next day of life, and no enlargement was observed subsequently. In addition, in this case, we used a variety of medications associated with elevated blood pressure, including dexamethasone, caffeine, levothyroxine tablets, vitamin D, and long-term TPN. It is noteworthy that this case required multiple and prolonged tracheal intubations and mechanical ventilation, nasal bi-level positive airway pressure, continuous positive airway pressure, high-flow oxygen therapy, and oxygen therapy for more than two months. BPD is a definite diagnosis, and the fact that this patient acquired hypertension after several days of intravenous dexamethasone infusions made the possibility of BPD and dexamethasone more significant.
The treatment of neonatal hypertension is controversial. In the case of asymptomatic persons, there is no pressing need to treat them[15]. We can remove the trigger first, and vasodilators, calcium channel blockers, beta-blockers, angiotensin-converting enzyme inhibitors, and diuretics can be used if blood pressure is persistently over the 99th percentile, or if organ involvement is evident[16]. At present, there is still a lack of large-scale, multi-center clinical trial data. Furthermore, different experiences have been reported in our country and abroad, including the type, dose, and course of treatment. This infant’s hypertension was well controlled with oral amlodipine, and no significant side effects were found. In addition, a study[17] revealed that prenatal administration of calcium channel blockers to pregnant women with hypertension might reduce the development of neonatal hypertension, which is a phenomenon that requires further investigation.
The distribution, metabolism, and excretion processes of drug therapy in neonates are significantly different compared to older children, particularly in preterm infants, who are more sensitive. Pharmacogenomics refers to gene polymorphisms related to individual differences in drug response, including drug metabolism enzyme genes, drug transporter genes, and drug target genes[18]. Gene polymorphisms may cause differences in pharmacokinetics, efficacy, and side effects. It has been used in the treatment of epilepsy[19], schizophrenia[20], malignant tumors[21], bronchial asthma[22], and other diseases, but there are few pharmacogenomics studies on children. We sent the infant for genetic testing to determine medication safety; the medications of interest were dexamethasone and amlodipine, and the genes implicated were ABCB1 and CYP2A5. According to the literature, the ABCB1 gene is located on the long arm of chromosome 7 and contains 29 exons[23], the most widely studied of which are exon 21, G2677T/A (rs2032582), exon 26, C3435T (rs1065642), exon 12, and C1236T (rs1128503). In 2000, a study[24] reported for the first time that ABCB1 gene polymorphism was associated with the expression of P-gp, a drug transporter. P-gp transports the drug to the outside of the cell under the energy of adenosine triphosphate and is a protective protein of the body[25]. It can affect the metabolism of a variety of drugs, especially antitumor drugs[26] and immunosuppressants[27]. Dexamethasone is a long-acting glucocorticoid that may induce an increase in blood pressure and blood glucose, as well as osteoporosis and femoral head necrosis. This child had the wild-type (GG) ABCB1 C3435T genotype, which does not affect the pharmacokinetics of dexamethasone. Cytochrome P450 is the most important enzyme system for drug metabolism in the body, and the CYP3A enzyme family is its members, mainly including CYP3A4 and CYP3A5[28], which metabolize amlodipine into inactive products in the liver. The CYP3A5 (rs776746) gene is located on chromosome 7[29], and the wild type is *1. The infant was homozygous for the mutation *3/*3, which cannot express the CYP3A5*1 protein, resulting in the decrease or disappearance of CYP3A5 enzyme activity and the increase of blood drug concentration. According to a study done in 2016[30], individuals with the CYP3A5*3/*3 genotype responded better to amlodipine than those with the CYP3A5*1 genotype (*1/*1 + *1/*3) when amlodipine was administered for hypertension after renal transplantation, with larger decreases in diastolic blood pressure. Furthermore, Kim et al[31] discovered that in healthy individuals, those with the ABCB1 GG (rs1045642) genotype had a lower amlodipine clearance rate than those with the AA (rs1045642) genotype. Therefore, the infant’s blood pressure returned to normal following a dose decrease of amlodipine.
Several physical and chemical factors may contribute to the development of hypertension in high-risk and preterm infants in the NICU. Therefore, regular blood pressure monitoring before discharge and an aggressive search for causative factors are essential for the clinical management of critically ill neonates. After the exclusion of causative factors, most neonates may not require pharmacologic intervention, but a few may require antihypertensive medications to control blood pressure. In recent years, genetic polymorphism has become a hot topic to guide the individualized treatment of adult clinical medication. Newborns, especially premature infants, are not the epitome of adults, and their metabolism of drugs is more complex. This infant’s condition was complex and complicated with severe hypertension. Through the guidance of pharmacogenomics, precision medication has been effective and safe, which is worthy of promotion. Although studies on neonatal pharmacogenomics are available, large-sample, multicenter, high-quality studies are still needed so that pharmacogenomics can be thoroughly applied to infants, allowing for the potential for significant improvements in clinical care.
We owe our thanks to Kai Liu for his work on revising in this manuscript.
| 1. | Flynn JT. Neonatal hypertension: diagnosis and management. Pediatr Nephrol. 2000;14:332-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 103] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Hjorten R, Flynn JT. Neonatal Hypertension. Clin Perinatol. 2022;49:27-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Sahu R, Pannu H, Yu R, Shete S, Bricker JT, Gupta-Malhotra M. Systemic hypertension requiring treatment in the neonatal intensive care unit. J Pediatr. 2013;163:84-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Kraut EJ, Boohaker LJ, Askenazi DJ, Fletcher J, Kent AL; Neonatal Kidney Collaborative (NKC). Correction: Incidence of neonatal hypertension from a large multicentre study [Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates-AWAKEN]. Pediatr Res. 2018;84:314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Shah S, Kaul A, Khandare J, Dhalait S. Comparison of Invasive Arterial Blood Pressure Monitoring vs. Non-Invasive Blood Pressure Monitoring in Preterm Infants < 37 Weeks in the Neonatal Intensive Care Unit- A Prospective Observational Study. J Trop Pediatr. 2021;67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | Lüscher TF. Unanswered questions in hypertension: prematurity and long-term trajectories, masked and white coat hypertension. Eur Heart J. 2020;41:1527-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Xiao N, Tandon A, Goldstein S, Lorts A. Cardiogenic shock as the initial presentation of neonatal systemic hypertension. J Neonatal Perinatal Med. 2013;6:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Edstedt Bonamy AK, Mohlkert LA, Hallberg J, Liuba P, Fellman V, Domellöf M, Norman M. Blood Pressure in 6-Year-Old Children Born Extremely Preterm. J Am Heart Assoc. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Harer MW, Kent AL. Neonatal hypertension: an educational review. Pediatr Nephrol. 2019;34:1009-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Kazmi N, Sharp GC, Reese SE, Vehmeijer FO, Lahti J, Page CM, Zhang W, Rifas-Shiman SL, Rezwan FI, Simpkin AJ, Burrows K, Richardson TG, Santos Ferreira DL, Fraser A, Harmon QE, Zhao S, Jaddoe VWV, Czamara D, Binder EB, Magnus MC, Håberg SE, Nystad W, Nohr EA, Starling AP, Kechris KJ, Yang IV, DeMeo DL, Litonjua AA, Baccarelli A, Oken E, Holloway JW, Karmaus W, Arshad SH, Dabelea D, Sørensen TIA, Laivuori H, Raikkonen K, Felix JF, London SJ, Hivert MF, Gaunt TR, Lawlor DA, Relton CL. Hypertensive Disorders of Pregnancy and DNA Methylation in Newborns. Hypertension. 2019;74:375-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 11. | Adelman RD. Long-term follow-up of neonatal renovascular hypertension. Pediatr Nephrol. 1987;1:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Kumai T, Asoh K, Tateishi T, Tanaka M, Watanabe M, Shimizu H, Kobayashi S. Involvement of tyrosine hydroxylase up regulation in dexamethasone-induced hypertension of rats. Life Sci. 2000;67:1993-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Goodwin JE, Zhang J, Gonzalez D, Albinsson S, Geller DS. Knockout of the vascular endothelial glucocorticoid receptor abrogates dexamethasone-induced hypertension. J Hypertens. 2011;29:1347-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Pandey VG, Jain S, Rana A, Puri N, Arudra SK, Mopidevi B, Kaw M, Nasjletti A, Kumar A. Dexamethasone promotes hypertension by allele-specific regulation of the human angiotensinogen gene. J Biol Chem. 2015;290:5749-5758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Starr MC, Flynn JT. Correction to: Neonatal hypertension: cases, causes, and clinical approach. Pediatr Nephrol. 2019;34:1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Dionne JM, Flynn JT. Management of severe hypertension in the newborn. Arch Dis Child. 2017;102:1176-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Habli M, Clifford CC, Brady TM, Rodriguez Z, Eschenbacher M, Wu M, DeFranco E, Gresh J, Kamath-Rayne BD. Antenatal exposure to nonsteroidal anti-inflammatory drugs and risk of neonatal hypertension. J Clin Hypertens (Greenwich). 2018;20:1334-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Altman RB, Benowitz N, Gurwitz D, Lunshof J, Relling M, Lamba J, Wieben E, Mooney S, Giacomini K, Weiss S, Johnson JA, McLeod H, Flockhart D, Weinshilboum R, Shuldiner AR, Roden D, Krauss RM, Ratain M. Genetic nondiscrimination legislation: a critical prerequisite for pharmacogenomics data sharing. Pharmacogenomics. 2007;8:519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Balestrini S, Sisodiya SM. Pharmacogenomics in epilepsy. Neurosci Lett. 2018;667:27-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 20. | Kawanishi Y, Tachikawa H, Suzuki T. Pharmacogenomics and schizophrenia. Eur J Pharmacol. 2000;410:227-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Saulsberry L, Olopade OI. Precision oncology: Directing genomics and pharmacogenomics toward reducing cancer inequities. Cancer Cell. 2021;39:730-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Daya M, Ortega VE. Asthma genomics and pharmacogenomics. Curr Opin Immunol. 2020;66:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Hodges LM, Markova SM, Chinn LW, Gow JM, Kroetz DL, Klein TE, Altman RB. Very important pharmacogene summary: ABCB1 (MDR1, P-glycoprotein). Pharmacogenet Genomics. 2011;21:152-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 347] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 24. | Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmöller J, Johne A, Cascorbi I, Gerloff T, Roots I, Eichelbaum M, Brinkmann U. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A. 2000;97:3473-3478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1340] [Cited by in RCA: 1273] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 25. | Borst P, Schinkel AH. P-glycoprotein ABCB1: a major player in drug handling by mammals. J Clin Invest. 2013;123:4131-4133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 26. | Coyle B, Kessler M, Sabnis DH, Kerr ID. ABCB1 in children’s brain tumours. Biochem Soc Trans. 2015;43:1018-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Vafadari R, Bouamar R, Hesselink DA, Kraaijeveld R, van Schaik RH, Weimar W, Baan CC, van Gelder T. Genetic polymorphisms in ABCB1 influence the pharmacodynamics of tacrolimus. Ther Drug Monit. 2013;35:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Niwa T, Murayama N, Emoto C, Yamazaki H. Comparison of kinetic parameters for drug oxidation rates and substrate inhibition potential mediated by cytochrome P450 3A4 and 3A5. Curr Drug Metab. 2008;9:20-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Sangkuhl K, Claudio-Campos K, Cavallari LH, Agundez JAG, Whirl-Carrillo M, Duconge J, Del Tredici AL, Wadelius M, Rodrigues Botton M, Woodahl EL, Scott SA, Klein TE, Pratt VM, Daly AK, Gaedigk A. PharmVar GeneFocus: CYP2C9. Clin Pharmacol Ther. 2021;110:662-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 30. | Huang Y, Wen G, Lu Y, Wen J, Ji Y, Xing X, Li Y, Yuan H. CYP3A4*1G and CYP3A5*3 genetic polymorphisms alter the antihypertensive efficacy of amlodipine in patients with hypertension following renal transplantation. Int J Clin Pharmacol Ther. 2017;55:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Kim KA, Park PW, Park JY. Effect of ABCB1 (MDR1) haplotypes derived from G2677T/C3435T on the pharmacokinetics of amlodipine in healthy subjects. Br J Clin Pharmacol. 2007;63:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Canelli RJ, United States; Nawab M, India S-Editor: Qu XL L-Editor: A P-Editor: Yuan YY