Published online Jan 26, 2023. doi: 10.12998/wjcc.v11.i3.700
Peer-review started: November 23, 2022
First decision: December 13, 2022
Revised: December 23, 2022
Accepted: January 5, 2023
Article in press: January 5, 2023
Published online: January 26, 2023
Processing time: 64 Days and 3.4 Hours
Osteopetrosis is a rare genetic disorder characterized by increased bone density due to defective bone resorption of osteoclasts. Approximately, 80% of autosomal dominant osteopetrosis type II (ADO-II) patients were usually affected by heterozygous dominant mutations in the chloride voltage-gated channel 7 (ClCN7) gene and present early-onset osteoarthritis or recurrent fractures. In this study, we report a case of persistent joint pain without bone injury or underlying history.
We report a 53-year-old female with joint pain who was accidentally diagnosed with ADO-II. The clinical diagnosis was based on increased bone density and typical radiographic features. Two heterozygous mutations in the ClCN7 and T-cell immune regulator 1 (TCIRG1) genes by whole exome sequencing were identified in the patient and her daughter. The missense mutation (c.857G>A) occurred in the CLCN7 gene p. R286Q, which is highly conserved across species. The TCIRG1 gene point mutation (c.714-20G>A) in intron 7 (near the splicing site of exon 7) had no effect on subsequent transcription.
This ADO-II case had a pathogenic CLCN7 mutation and late onset without the usual clinical symptoms. For the diagnosis and assessment of the prognosis for osteopetrosis, genetic analysis is advised.
Core Tip: Autosomal dominant osteopetrosis (ADO-II) is an autosomal dominant form of osteopetrosis. In ADO-II patients, the clinical spectrum ranges from nonsymptomatic to recurrent fractures, anemia, and a favorable prognosis. We reported a 53-year-old female patient with persistent joint pain, who was accidentally diagnosed with ADO-II at a later age. Her asymptomatic daughter was also diagnosed with ADO-II, as confirmed by whole exome sequencing.
- Citation: Gong HP, Ren Y, Zha PP, Zhang WY, Zhang J, Zhang ZW, Wang C. Clinical and genetic diagnosis of autosomal dominant osteopetrosis type II in a Chinese family: A case report. World J Clin Cases 2023; 11(3): 700-708
- URL: https://www.wjgnet.com/2307-8960/full/v11/i3/700.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i3.700
Osteopetrosis, also known as "marble bone disease," is a rare genetic disease characterized by increased bone mass and density due to bone resorption failure[1]. Dr. Albers-Schonberg, a radiologist in Germany, described it for the first time in 1904[2]. It has a broad clinical spectrum, ranging from asymptomatic to life-threatening bone marrow failure and cranial nerve dysfunction. Based on clinical severity and inheritance patterns, osteopetrosis is classified into three types: a "malignant" autosomal recessive infantile form (ARO), a "benign" autosomal dominant form (ADO type II), and an intermediate recessive form[1,3,4]. To date, mutations in at least ten genes have been identified to cause failure of osteoclast differentiation or function in humans, including the T-cell immune regulator gene (TCIRG1), chloride voltage-gated channel 7 (ClCN7), tumor necrosis factor (TNF) superfamily member 11, TNF receptor superfamily member 11a, osteopetrosis-associated transmembrane protein, sorting nexin 10 (SNX10), pleckstrin homology and RUN domain containing M1, and NF-κB essential modulator genes[3,5].
The most prominent characteristic of ADO-II is its dense yet fragile bones. We present a rare case of limb joint pain that was accidentally diagnosed as ADO-II based on clinical findings and genetic analysis.
A 53-year-old woman was admitted to the hospital with a complaint of limb joint pain for 11 mo.
The patient presented with pain in her shoulder, elbow, wrist, and metacarpophalangeal joints with swelling, tenderness and numbness for 1 mo before pain began in her bilateral knee, ankle, toe and finger joints for approximately 10 mo.
The patient had no history of fractures and bone injury.
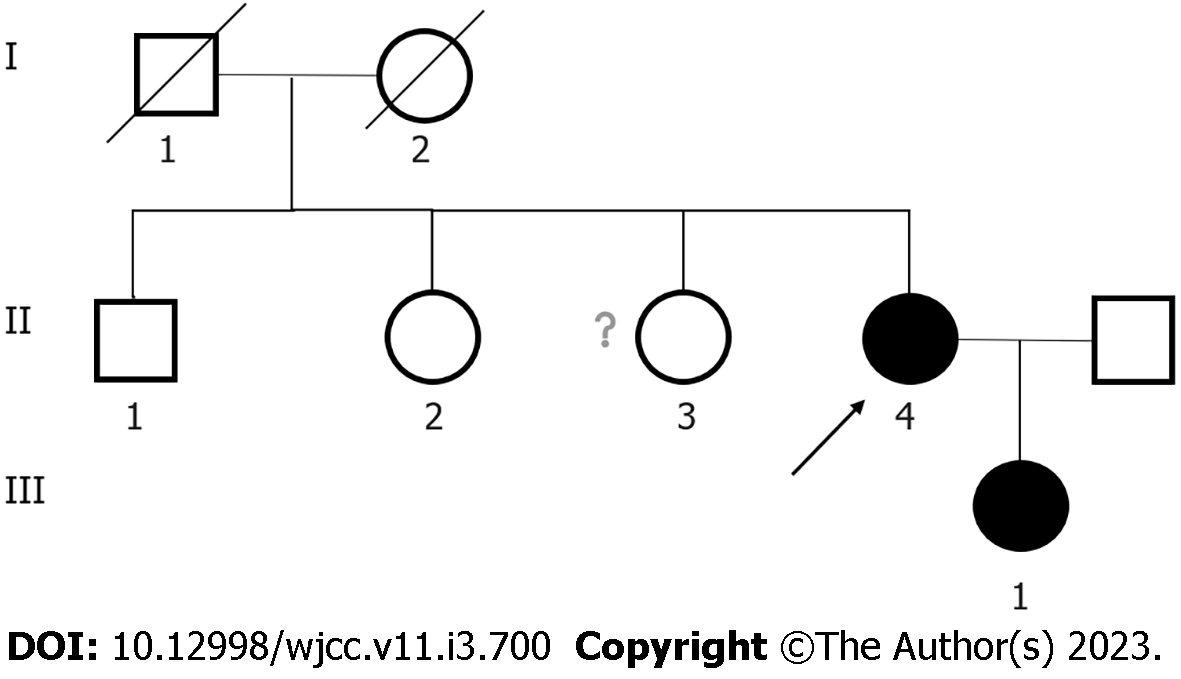
Her parents were not consanguineous. Her father and mother died of bone cancer and esophageal cancer, respectively. She has a daughter, a brother and two sisters who are asymptomatic. The pedigree of her family is shown in Figure 1.
On admission, her blood pressure was 120/70 mmHg, pulse rate 98/min, and respiratory rate 20/min. Her height and body weight were 155 cm and 63 kg, respectively. Physical examinations showed slight swelling of her wrists, hands, knees and ankles, accompanied by tenderness, limited retral swing of the upper limbs and slightly restricted movement of the lower limbs.
The patient had mild anemia; decreased levels of serum bone alkaline phosphatase (BALP), 25-dihydroxy vitamin D3, urinary calcium and phosphorus; and an elevated level of serum phosphorus. The levels of serum calcium, urinary fluoride, lactate dehydrogenase, creatine kinase, C-telopeptide of type I collagen, N-terminal mid-fragment of osteocalcin, and parathyroid hormone (PTH) were within the normal reference ranges. Her spectrum of antinuclear antibodies, anti-cyclic citrullinated peptide, anti-streptolysin O and rheumatoid factor were negative.
The biochemical measurements of her daughter, brother and sister were normal except that her daughter had mild anemia (partial data not shown). All laboratory findings are summarized in Table 1.
| The proband | The proband’s daughter | Reference | |
| Sex | Female | Female | |
| RBC ( 1012/L) | 3.64 | 3.55 | 3.8-5.1 |
| Hb (g/L) | 99 | 105 | 115-150 |
| PLT ( 109/L) | 235 | 110 | 100-300 |
| ALT (IU/mL) | 19 | 30 | < 40 |
| AST (IU/mL) | 29 | 30 | < 35 |
| ALP (IU/mL) | 89 | 55 | 50-135 |
| CK (IU/L) | 80 | NA | 20-140 |
| CK-MB (ng/mL) | 0.35 | NA | < 2.88 |
| LDH (IU/mL) | 232 | NA | 120-250 |
| Serum uric acid (μmol/L) | 313 | 311 | 160-380 |
| Cr (μmol/L) | 49 | 58 | 41-73 |
| eGFR (ml/min/1.73 m2) | 107.16 | 121 | 56-122 |
| Ca (mmol/L) | 2.22 | 2.26 | 2.11-2.52 |
| P (mmol/L) | 1.65 | 1.11 | 0.85-1.51 |
| 25-OH-VD (nmol/L) | 34.9 | NA | 47.7-144 |
| PTH (pmol/L) | 2.96 | NA | 1.6-6.9 |
| B-ALP (μg/L) | 10.57 | NA | 11.4-24.6 |
| CTX (ng/mL) | 0.813 | NA | 0.556-1.008 |
| N-MID OC (ng/mL) | 22.7 | NA | 15-46 |
| Growth hormone (ng/mL) | 0.53 | NA | 0.126-9.88 |
| IGF-1 (ng/mL) | 81.78 | NA | 102-212 |
| ACTH (ng/L) | 19.11 | NA | 5-78 |
| Cortisol (8:00 A.M.) (nmol/L) | 260.8 | NA | 147.3-609.3 |
| Cortisol (12:00 P.M.) (nmol/L) | 68.07 | NA | / |
| 24 h urinary Ca (mmol/L) | 2.45 | NA | 2.5-7.5 |
| 24 h urinary P (mmol/L) | 11.84 | NA | 22-48 |
| 24 h urinary Mg (mmol/L) | 1.62 | NA | 3-5 |
The bone mineral density (BMD) was measured by dual-energy X-ray absorptiometry (iDXA, GE Lunar, United States). The results showed that the hips and lumbar spines of the patient and her daughter significantly increased. The T scores of the patient were higher than those of Chinese female youth, and the Z scores of her daughter were also higher than those of the age-matched Chinese women (Table 2). The results of the BMD tests for her brother and sister revealed low bone mass (osteopenia) compared with the Chinese sex-matched adolescents (data not shown).
| The BMD values of the proband (g/cm2) | T/Z score of the proband | The BMD values of the proband’s daughter (g/cm2) | T/Z score of the proband’sdaughter | |
| L1 | 2.013 | 8.2/8.8 | 1.777 | 5.2/5.4 |
| L2 | 2.146 | 8.7/9.4 | 1.811 | 5.0/5.1 |
| L3 | 2.256 | 9.2/9.9 | 1.785 | 4.5/4.7 |
| L4 | 2.335 | 9.9/10.4 | 1.900 | 5.3/5.5 |
| L1-L4 | 2.203 | 9.1/9.7 | 1.820 | 5.1/5.2 |
| Femoral neck | 1.838 | 7.6/8.2 | 1.391 | 2.5/2.9 |
| Total hip | 1.851 | 6.7/7.2 | 1.344 | 2.7/2.9 |
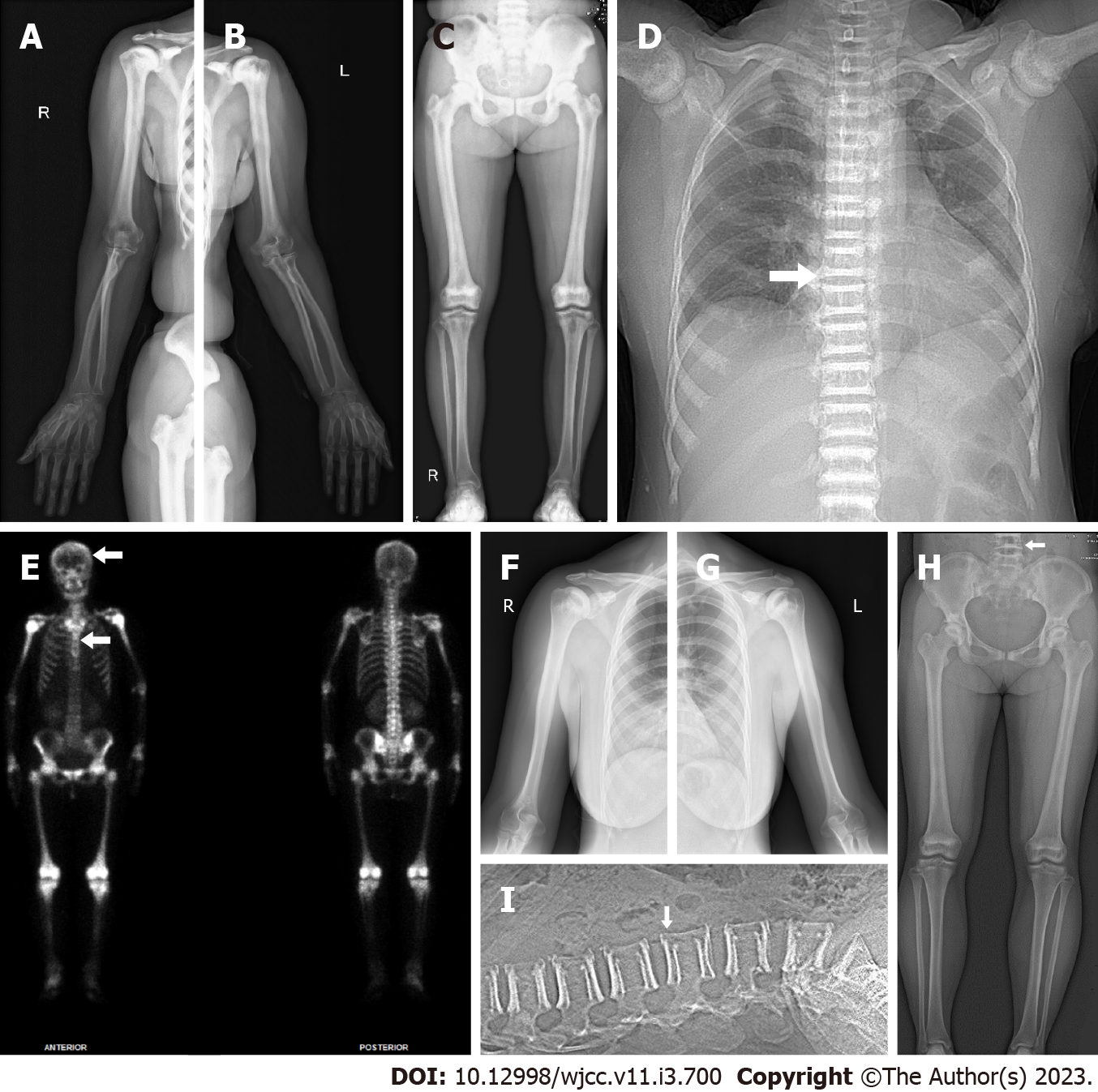
X-ray images of the limbs, including the wrist and ankle joints of the patient, showed increased bone density in the pelvis, femurs, humerus, knees and shoulder joints; mild degeneration in the hips, knees, ankles, shoulders, elbows and wrist joints; and slight soft tissue swelling around the wrist joints (Figure 2A-C). The chest computerized tomography (CT) scan of the patient showed that bone mineral density increased in the bilateral humeral head, sternum, scapula, ribs and multiple thoracic vertebrae (Figure 2D). Whole-body bone single-photon emission computed tomography of the patient revealed extremely high uptake in the long bone, ribs, and spine with no renal or bladder radioactivity visualization. The features from imaging showed a “bone super scan” (Figure 2E). The results of the ultrasound examination of the patient showed synovitis in both the first metatarsophalangeal joints and the wrist joint, tenosynovitis of the fourth compartment of the left wrist, and joint effusion on the right ankle (data not shown).
X-ray images of the limbs of the patient’s daughter showed that the density of the bone tip and flat bone increased, and the marrow cavity became narrow (Figure 2F-H). The CT scan of the lumbar spine of the patient’s daughter showed that bone mineral density increased at the upper and lower edges from the twelfth thoracic vertebra to the first sacral vertebra and slightly decreased in their center (Figure 2I).
A bone biopsy of the patient on the right posterior iliac crest showed cortical bone sclerosis, some thickened bone trabeculae, and active proliferation of bone marrow hematopoietic cells (Supple
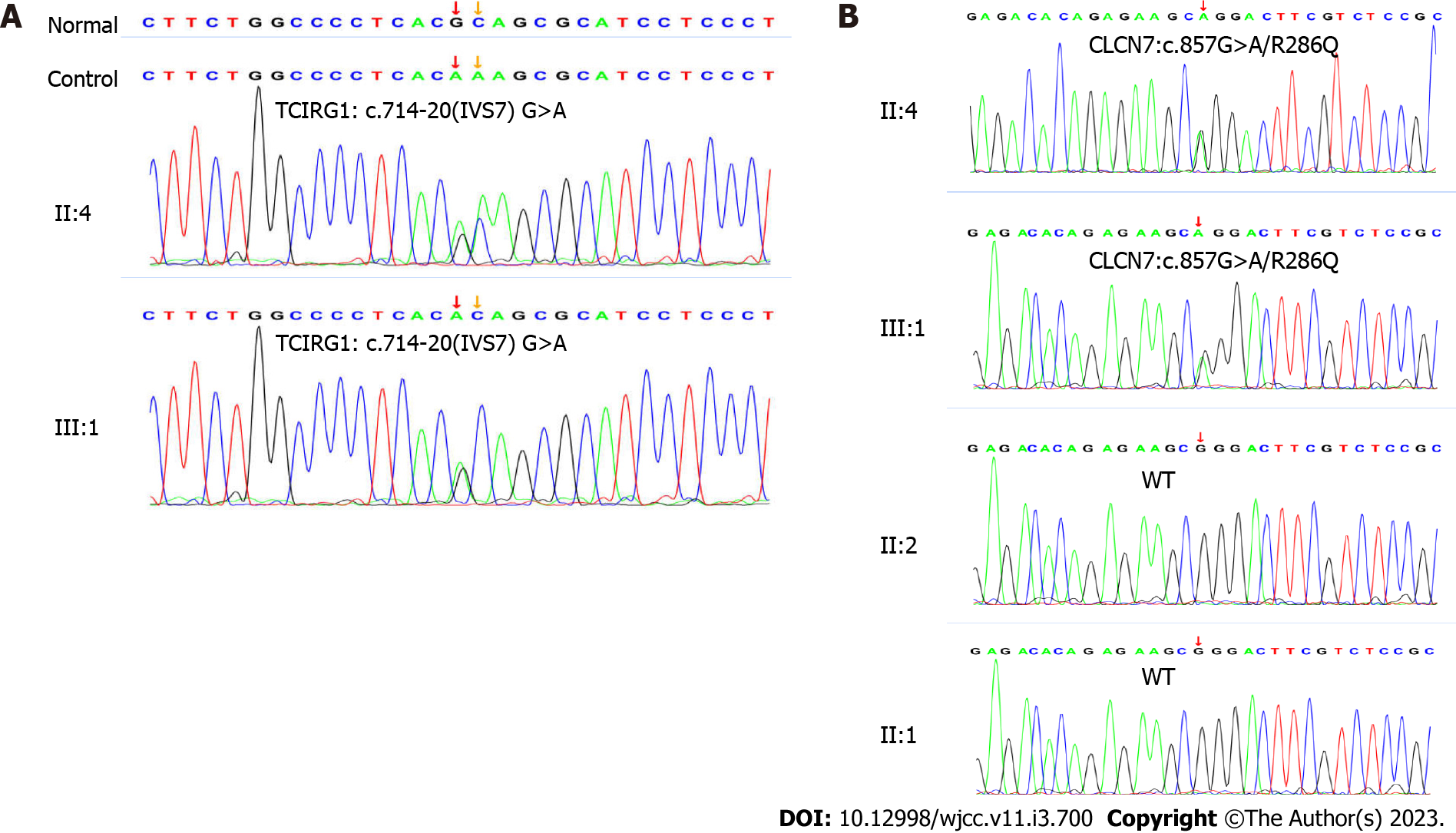
Whole exome sequencing (whole-exome library construction by xGen Exome Research Panel v2.0 (IDT, Iowa, United States), high-throughput sequencing by a DNBSEQ-T7 sequencer (MGI, Beijing, CHN), and not less than 99% of target sequence were sequenced) identified two heterozygous mutations, including c.857G>A (p. Arg286Gln, rs760956030) in exon 10 of the CLCN7 gene (NCBI reference sequence: NM_001287) and c.714-20G>A (-, rs200087340) in intron 7 of the TCIRG1 gene (NCBI reference sequence: NM_006019.4). Her daughter carried the same heterozygous mutation in the CLCN7 and TCIRG1 genes by genetic analysis (Figure 3).
The R286 position was highly conserved among various species in the CLCN7 gene (Supplementary Figure 2). Moreover, Polymorphism Phenotyping v2 (http://genetics.bwh.harvard.edu/pph) prediction results of p. R286Q in the CLCN7 gene was probably damaging, with a score of 1.000 (Supple
Combined with the patient’s medical history and radiological examination results, the final diagnosis was osteopetrosis. In light of genetic typing, the case belonged to ADO II.
During hospitalization, celecoxib capsules, wet packing of Liuhedan (a traditional Chinese medicine recipe), ketoprofen gel, and flurbiprofen paste were all given. Her hemoglobin level increased from 99 g/L to 109 g/L with normal serum phosphorus after 16 days of treatment. Her regular activities were unaffected, and the discomfort and swelling in her joints subsided after she was discharged.
After 2 years of follow-up, her joints were no longer painful, and no complications developed. However, her daughter experienced mild lumbar spine discomfort and received analgesic treatment.
Osteopetrosis is a rare inherited metabolic bone disorder characterized by a generalized increase in bone density due to osteoclastic insufficiency, impaired bone absorption and poor bone remodeling[4]. Once osteoclasts have defective proton pumps, chloride channels, or carbonic anhydrase II proteins, the mineral matrix is unable to be resorbed effectively[6]. Published studies have shown that most defects in genes result in impaired acidification of bone[7].
With a prevalence of approximately 1:20,000 Live births, ADO is far more common and less severe than ARO[8,9]. The clinical phenotype of osteopetrosis is highly variable, making diagnosis difficult for clinicians. Patients with osteopetrosis may present with no symptoms, fractures following minor trauma, osteomyelitis, early arthritis, anemia, hearing and vision problems due to cranial nerve compression, or all of the above[4]. Some differential diagnoses should be ruled out, such as congenital diseases (e.g., hypoparathyroidism, pseudohypoparathyroidism), chemical poisoning (e.g., with fluoride, lead, or beryllium), and malignancies (leukemias and myeloproliferative diseases). This patient had normal urinary fluoride and PTH serum levels without arthritis, and she was eventually diagnosed with ADO-II. Her daughter was asymptomatic, and ADO-II was confirmed by increased BMD, radiological examination, and the p. R286Q mutation. Their anemia was mild due to relatively sufficient marrow cavity retention for normal hematopoiesis[10]. It is worth noting that, in contrast to ARO, this patient had a mildly decreased BALP concentration. The majority of ADO-II patients have an imbalance in osteoblast serum markers, with low BALP and high osteocalcin levels[11]. As a result, bone biomarkers may be useful in disease classification.
Increased BMD and radiographic findings are most commonly used to diagnose osteopetrosis. The classic radiographic features of osteopetrosis are the bare minimum for diagnosis[7]. Radiographic features of osteopetrosis include diffuse bone sclerosis with "bone-within-bone" in the pelvis, long bones, phalanges, and vertebrae. A bone marrow biopsy may be required to confirm the diagnosis and differentiate between osteoclast-poor and osteoclast-rich subtypes of osteopetrosis. Bone marrow biopsy can also distinguish hematological disorders such as myelofibrosis, sickle cell disease, leukemia, and osteoblastic bony metastases. It is, however, more invasive to the patient and carries some risks. Whole exome sequencing, on the other hand, is becoming less expensive and faster to obtain a diagnosis and is not invasive. Therefore, therapeutic approaches must be tailored to each patient.
Approximately 80% of ADO-II patients are affected by heterozygous dominant negative mutations of the CLCN7 gene, while 17% of ARO patients have recessive mutations in the CLCN7 gene[5,12]. CLCN7 encodes the chloride channel involved in osteoclast HCl secretion, which is critical in osteoclast dissolution of bone mineral and organic bone matrix[13,14]. A single nucleotide change (c.857G>A, p. R286Q) in exon 10 of CLCN7 results in a protein with the amino acid glutamine instead of arginine. This variant appears to be located at one of the “hot spots” as the most common CLCN7 mutations causing ADO and three known disease-related CLCN7 mutations at the R286 position (p. R286P, p. R286W and p. R286Q) have previously been reported among Caucasians and Asians[15-17]. The mutations (p. R286Q) in the CLCN7 gene are located in the intramembrane α-helices, creating a positive electrical potential to prevent the fast flux of chloride at the binding site[18]. Approximately 80% of CLCN7-dependent ADO-II patients discovered the disease after fractures, implying that osteoblast malfunction likely results in low-quality bone tissue[5,11]. However, no fractures have occurred in this patient thus far. As a result, even if the mutations are identical, the clinical phenotypes may differ.
Osteopetrosis may also be caused by an intronic nucleotide change in the TCIRG1 gene[19]. TCIRG1 encodes the a3 subunit of H+ ATPase, and V-ATPase with d2/a3 is a major proton pump of osteoclasts[20]. Mutations in TCIRG1 account for approximately 50% of ARO cases[5]. The TCIRG1 variant (c.714-20 G>A) is a point mutation, but there is insufficient evidence to conclude that it is pathogenic. Furthermore, published reports of digenic inheritance suggested that TCIRG1 and CLCN7 interact in the two mutations[15,21].
The majority of benign ADO treatments are symptomatic and supportive. Good nutrition is critical for patients with osteopetrosis, especially for those who have hypocalcemia and require calcium and vitamin D supplements[7]. Hematopoietic stem cell transplantation is reserved for osteopetrosis that is malignant[4]. The majority of ADO patients have a better prognosis. Nonetheless, for mild osteopetrosis, it is critical to monitor the disease status and progression by the affected organs.
A case of rare ADO-II was accidentally diagnosed in a late-onset patient and her daughter based on increased BMD, classic radiographic features, and a CLCN7 gene mutation. It was suggested that genetic testing be used to identify precision classifications of osteopetrosis and to provide useful information for therapeutic decisions and prognosis.
We thank the patient and her relatives for kindly contributing to this study.
| 1. | Stark Z, Savarirayan R. Osteopetrosis. Orphanet J Rare Dis. 2009;4:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 320] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 2. | Albers-Schönberg HE. Röntgenbilder einer seltenen Knockenerkrankung. Munch. Med. Wochenschr. 1904;51:365-368. |
| 3. | Del Fattore A, Cappariello A, Teti A. Genetics, pathogenesis and complications of osteopetrosis. Bone. 2008;42:19-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 174] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | Bailey JR, Tapscott DC. Osteopetrosis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2022. [PubMed] |
| 5. | Palagano E, Menale C, Sobacchi C, Villa A. Genetics of Osteopetrosis. Curr Osteoporos Rep. 2018;16:13-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | Sobacchi C, Schulz A, Coxon FP, Villa A, Helfrich MH. Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat Rev Endocrinol. 2013;9:522-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 413] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 7. | Wu CC, Econs MJ, DiMeglio LA, Insogna KL, Levine MA, Orchard PJ, Miller WP, Petryk A, Rush ET, Shoback DM, Ward LM, Polgreen LE. Diagnosis and Management of Osteopetrosis: Consensus Guidelines From the Osteopetrosis Working Group. J Clin Endocrinol Metab. 2017;102:3111-3123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 162] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 8. | de Baat P, Heijboer MP, de Baat C. Osteopetrosis. Classification, etiology, treatment options and implications for oral health. Ned Tijdschr Tandheelkd. 2005;112:497-503. [PubMed] |
| 9. | Bollerslev J, Andersen PE Jr. Radiological, biochemical and hereditary evidence of two types of autosomal dominant osteopetrosis. Bone. 1988;9:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 111] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Carolino J, Perez JA, Popa A. Osteopetrosis. Am Fam Physician. 1998;57:1293-1296. [PubMed] |
| 11. | Del Fattore A, Peruzzi B, Rucci N, Recchia I, Cappariello A, Longo M, Fortunati D, Ballanti P, Iacobini M, Luciani M, Devito R, Pinto R, Caniglia M, Lanino E, Messina C, Cesaro S, Letizia C, Bianchini G, Fryssira H, Grabowski P, Shaw N, Bishop N, Hughes D, Kapur RP, Datta HK, Taranta A, Fornari R, Migliaccio S, Teti A. Clinical, genetic, and cellular analysis of 49 osteopetrotic patients: implications for diagnosis and treatment. J Med Genet. 2006;43:315-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 131] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Sobacchi C, Villa A, Schulz A, Kornak U. CLCN7-Related Osteopetrosis. In: GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle, 1993. [PubMed] |
| 13. | Kornak U, Kasper D, Bösl MR, Kaiser E, Schweizer M, Schulz A, Friedrich W, Delling G, Jentsch TJ. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell. 2001;104:205-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 762] [Cited by in RCA: 736] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 14. | Kasper D, Planells-Cases R, Fuhrmann JC, Scheel O, Zeitz O, Ruether K, Schmitt A, Poët M, Steinfeld R, Schweizer M, Kornak U, Jentsch TJ. Loss of the chloride channel ClC-7 leads to lysosomal storage disease and neurodegeneration. EMBO J. 2005;24:1079-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 284] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 15. | Yang Y, Ye W, Guo J, Zhao L, Tu M, Zheng Y, Li L. CLCN7 and TCIRG1 mutations in a single family: Evidence for digenic inheritance of osteopetrosis. Mol Med Rep. 2019;19:595-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Chu K, Koller DL, Snyder R, Fishburn T, Lai D, Waguespack SG, Foroud T, Econs MJ. Analysis of variation in expression of autosomal dominant osteopetrosis type 2: searching for modifier genes. Bone. 2005;37:655-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Pangrazio A, Pusch M, Caldana E, Frattini A, Lanino E, Tamhankar PM, Phadke S, Lopez AG, Orchard P, Mihci E, Abinun M, Wright M, Vettenranta K, Bariae I, Melis D, Tezcan I, Baumann C, Locatelli F, Zecca M, Horwitz E, Mansour LS, Van Roij M, Vezzoni P, Villa A, Sobacchi C. Molecular and clinical heterogeneity in CLCN7-dependent osteopetrosis: report of 20 novel mutations. Hum Mutat. 2010;31:E1071-E1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Pang Q, Chi Y, Zhao Z, Xing X, Li M, Wang O, Jiang Y, Liao R, Sun Y, Dong J, Xia W. Novel mutations of CLCN7 cause autosomal dominant osteopetrosis type II (ADO-II) and intermediate autosomal recessive osteopetrosis (IARO) in Chinese patients. Osteoporos Int. 2016;27:1047-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Pangrazio A, Caldana ME, Lo Iacono N, Mantero S, Vezzoni P, Villa A, Sobacchi C. Autosomal recessive osteopetrosis: report of 41 novel mutations in the TCIRG1 gene and diagnostic implications. Osteoporos Int. 2012;23:2713-2718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Matsumoto N, Daido S, Sun-Wada GH, Wada Y, Futai M, Nakanishi-Matsui M. Diversity of proton pumps in osteoclasts: V-ATPase with a3 and d2 isoforms is a major form in osteoclasts. Biochim Biophys Acta. 2014;1837:744-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Yu T, Yu Y, Wang J, Yin L, Zhou Y, Ying D, Huang R, Chen H, Wu S, Shen Y, Fu Q, Chen F. Identification of TCIRG1 and CLCN7 gene mutations in a patient with autosomal recessive osteopetrosis. Mol Med Rep. 2014;9:1191-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu X, China; Park J, United States S-Editor: Wang LL L-Editor: A P-Editor: Wang LL